Updates in Psoriasis Management 2020

Based on Selected Presentations from Maui Derm 2020
January 25–29, 2020, Maui, Hawaii

by Jo Ann LeQuang
Ms. Lequang is Owner of LeQ Medical in Angleton, Texas; Director of Scientific Communications at NEMA Research, Inc., in Naples, Florida; and Founding Director of No Baby Blisters in Colorado Springs, Colorado.
J Clin Aesthet Dermatol 2020;13(7):S7–S23
Based on presentations by
Andrew Blauvelt, MD, MBA
Dr. Blauvelt is with the Oregon Medical Research Center in Portland, Oregon.
Joel M. Gelfand, MD, MSCE, FAAD
Dr. Gelfand is with the University of Pennsylvania Perelman School of Medicine in Philadelphia, Pennsylvania.
Linda Stein Gold, MD
Dr. Stein Gold is with Henry Ford Health System in Detroit, Michigan.
Arthur Kavanaugh, MD
Dr. Kavanaugh is Professor of Medicine and Director of the Center for Innovative Therapy (CIT) at University of California, San Diego in San Diego, California.
Craig L. Leonardi, MD
Dr. Leonardi is Associate Clinical Professor of Dermatology with the St. Louis University Medical School in St. Louis, Missouri.
Funding: Funding for this supplement was provided by Sun Pharmaceutical Industries Ltd., Princeton, New Jersey.
Disclosures: Dr. Blauvelt has served as a scientific adviser, clinical study investigator, or paid speaker for AbbVie, Aclaris, Akros, Allergan,Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavant, Dermira, Inc., Eli Lilly and Company, Galderma, Genentech/Roche, GlaxoSmithKline, Janssen, Leo, Meiji, Merck, Sharp & Dohme, Novartis, Pfizer, Purdue Pharma, Regeneron, Revance, Sandoz, Sanofi, Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB Pharma, Valeant, and Vidac. Dr. Gelfand served as a consultant for Abcentra, BMS, Boehringer Ingelheim, Cara (DSMB), GSK, Lilly (DMC), Janssen Biologics, Novartis Corp, UCB (DSMB), Neuroderm (DSMB), Dr. Reddy’s Labs, Pfizer Inc., and Sun Pharma, receiving honoraria; receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Boehringer Ingelheim, Janssen, Novartis Corp , Celgene, Ortho Dermatologics, and Pfizer Inc.; received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly, Ortho Dermatologics and Novartis; is a co-patent holder of 4/04 resiquimod for treatment of cutaneous T cell lymphoma; is a Deputy Editor for the Journal of Investigative Dermatology, receiving honoraria from the Society for Investigative Dermatology; and is a member of the Board of Directors for the International Psoriasis Council, receiving no honoraria. Dr. Stein Gold has served as a speaker, consultant, or advisory board member for Leo Pharma, Mayne Pharma, Pfizer Inc., Sun, Arcutis, Dermavant and Valeant.. Dr. Kavanaugh has conducted research studies for AbbVie , Amgen , Astra-Zeneca, BMS, Celgene, Corrona, Galapagos/Gilead, Genentech/Roche , ITN , Janssen , LCTC, LIAI, NIH, Novartis , Pfizer, Regeneron/Sanofi , TREG, and UCB . Ms. LeQuang has no conflicts of interest relative to the content of this supplement. Dr. Leonardi has served as a consultant, speaker, advisory board member, or investigator for Abbvie, Allergan, Amgen, Celgene, Coherus, Dermira, Eli-Lilly, Galderma, Glaxo Smith Kline, Janssen, Leo, Merck, Merck-Serono, Novartis, Pfizer, Sandoz, Sienna, UCB, and Vitae. Dr. Martin has served as a speaker, consultant, or advisory board member for Aclaris, Aqua, Celgene, DUSA/Sun Pharma, Janssen, Pfizer, Ortho/Valeant, UCB, Aclaris, Celgene, Pfizer, and UCB.
A Message from the Program Director and Guest Editor

George Martin, MD
Dr. Martin is Guest Editor and Program Director of MauiDerm 2020 and with Dermatology Associates in Khei, Maui, Hawaii.
Dear Colleagues:
Each year, Maui Derm seeks to bring to the podium the most up-to-date
and cutting-edge scientific and clinical developments in the field of dermatology. Maui Derm 2020 was no exception—our outstanding faculty delivered an amazing amount of clinical information in multiple areas of dermatology. One therapeutic area in particular—psoriasis—continues to demonstrate a rapid expansion of new therapeutic options. In this supplement to The Journal of Clinical and Aesthetic Dermatology (JCAD), we’ve once again captured a select group of Maui Derm 2020 presentations we feel represents the key recent clinical developments in psoriasis management.
Here, we explore the pathogenesis of psoriasis and how our expanding knowledge of the disease is leading to new, innovative therapeutic discoveries. A better understanding of psoriasis pathogenesis also helps us better elucidate the relationships between psoriasis and other disease states, such as those involving the gums, liver, and heart, as well as certain cancers. There are also several exciting therapeutic developments in the treatment of psoriatic arthritis and moderate-to-severe psoriasis in adult and pediatric populations, as well as in topical therapies for psoriasis.
We hope the information presented in this supplement assists you in achieving optimal patient outcomes by expanding your knowledge of how these emerging therapies work. We also hope you can join us January 25–29, 2021, at the Grand Wailean in Maui, Hawaii, for what promises to be another outstanding educational event. If your schedule prevents you from joining us in 2021, however, we hope to once again provide you with highlights from some of the key presentations from our meeting faculty.
With aloha,
George Martin, MD
Introduction
Each year, the Maui Derm for Dermatologists symposium in Maui, Hawaii, offers lectures, panel discussions, and workshops that are designed to provide in-depth, cutting edge material on an extensive range of medical, cosmetic, coding/billing, and CLIA certification topics for practicing dermatologists. Among numerous other dermatological topics presented during the 16th annual 2020 Maui Derm event, several key opinion leaders presented information on the latest psoriasis treatment options, clinical trial results (including some important new head-to-head studies) in psoriasis, and novel and established therapies for the treatment and prevention of psoriasis. This article highlights insights and data presented by the following experts in the field of psoriasis: Andrew Blauvelt, MD, MBA; Joel Gelfand, MD, MSCE, FAAD; Arthur Kavanaugh, MD; Linda Stein Gold, MD; and Craig L. Leonardi, MD.
Shedding Light on Psoriasis Pathogenesis
Based on a presentation by Andrew Blauvelt, MD, MBA, Oregon Medical Research Center, Portland, Oregon.
Koebner phenomenon. The Koebner phenomenon is defined as the occurrence of psoriatic lesions as a result of trauma or mechanical stress to the skin of the patient.1 Although we’ve known about the Koebner phenomenon for over a century, its pathogenesis has yet to be fully elucidated, and a better understanding of this phenomenon might help to expand our existing knowledge of the pathogenesis of psoriasis. Areas of the body where skin is subject to greater mechanical stress, such as the elbows and knees, appear more susceptible to psoriatic outbreaks. To shed better light on the relationship between mechanical stress and keratinocyte activity, researchers cultured human keratinocytes in dishes and subjected the dishes to flexion. Increased cellular proliferation and the production of proinflammatory cytokines, antibacterial peptides, and chemokines (CXCL1 and CCL20) were observed.2 In an animal study, an expander was implanted in the back of mice that subjected their skin to mechanical stress.3 This also resulted in significant epidermal hyperproliferation, damage to the skin barrier, and release of psoriasis-related cytokines. The epidermis around the expander thickened in these psoriatic mice, and highly expressed interleukin (IL)-1 alpha, IL-6, IL-23, tumor necrosis factor (TNF)-alpha, CXCL1, and CCR20. Additionally, when the mice with the implanted dilators were treated with imiquimod or IL-23, they developed an aggravated phenotype of psoriasis.3 Taken together, these studies2,3 suggest that mechanical stress worsens the severity of psoriatic lesions due to cell hyperproliferation and increased production of proinflammatory cytokines by keratinocytes.
Human microbiome. Another emerging area of research in our attempt to better understand the pathogenesis of psoriasis is the relationship between the disease and the human skin and gut microbiomes. Microbial dysbiosis in inflammatory disease is not well understood, but research suggests that the skin microbiome of a person with psoriasis or atopic dermatitis (AD) is different from the skin microbiome of a healthy individual. Staphylococcus aureus is the predominant microbe on the skin microbiome of a person with AD, and enrichment of associated disease-relevant genes promotes barrier dysfunction and immune-system activation.4 By contrast, the skin microbiome of a person with psoriasis comprises co-occurring microbial colonies that have weaker associations with disease-related gene expression.4 The interactions between the host and its healthy microbes is crucial to cutaneous homeostasis, and, logically, aberrations in this balance might drive disease.5,6
In a randomized, double-blind, placebo-controlled, 12-week study,7 90 adults, between the ages of 18 and 70 years, with plaque psoriasis were randomized to either receive probiotic supplementation or placebo. After 12 weeks of treatment, patients in the probiotic group showed a greater reduction in the Psoriasis Area and Severity Index (PASI) of up to 75 percent, compared to patients who received placebo (75% vs. 49%, respectively; p<0.05). Additionally, more patients in the probiotic group demonstrated clinically relevant improvements in the Physician’s Global Assessment (PGA) scores (49% vs. 32%, respectively).7 These results support the theory that a healthy microbiome in the skin and gut of the human body is critical to its overall homeostasis.
Systemic inflammation. Delving deeper into the pathogenesis of psoriasis, there is yet another question: What comes first—skin symptoms or systemic inflammation? Emerging evidence suggests that when psoriasis therapy is withdrawn in a patient, skin disease recurs before IL-17A, IL-17F, and IL-22 levels can be detected in the blood, indicating a cutaneous source of circulating proinflammatory cytokines. The VOYAGE-2 study enrolled patients with psoriasis who were considered responders in the preceding 28 weeks (improvement was defined as a 90-percent or greater improvement in the PASI over baseline). In the VOYAGE-2 study, the enrolled participants were randomized to receive either placebo (n=182) or continue receiving guselkumab (n=193), an IL-23 blocker, for an additional 44 weeks.8 Patients maintained on guselkumab had durable results through Week 72, but those who were switched to placebo experienced decreased PASIs (PASI-90: 86.0% guselkumab versus 11.5% placebo). Patients in the placebo group were re-administered guselkumab at Week 72 or when they had lost at least 50 percent of the improvement they had achieved at Week 28 of the first portion of the study. Maintenance of therapeutic response was associated with the suppression of IL-17A, IL-17F, and IL-22, but cytokine levels tended to lag behind skin disease recurrence, and thus were not considered strongly predictive of psoriasis recurrence. Most of the patients who lost clinical response while on placebo during the second portion of the study were able to regain a therapeutic effect when the drug was reinstated.8 The bottom line is that this study showed that skin inflammation occurred prior to systemic inflammation when skin disease recurred.
Tissue-resident memory T-cells (Trm). A new area of interest in psoriasis is Trm, a group of memory T-cells that maintain stable and long-term residence in lymphoid and nonlymphoid organs without entering the blood. These non-recirculating Trms have been detected in the skin, lungs, salivary glands, brain, liver, and other organs. They protect against viral, fungal, and bacterial infections and might play a role in controlling tumors.9 Trms appear to develop in a pathogen-specific fashion in the peripheral immune system, and their longevity helps to guard the body against potential re-infection by the original pathogen. Trms have also been identified in the healed skin of patients with psoriasis, vitiligo, and fixed drug-reaction. When Trms are reactivated, they amplify the body’s immune response by recruiting supplemental immune cells from the circulatory system. Trms can persist in healed psoriatic lesions and continue producing cytokines associated with psoriasis pathogenesis.10 In a study of ex-vivo skin biopsies from 10 patients with psoriasis vulgaris, specimens subjected to skin-infiltrating T-cells using IL-2 and anti-CD3/CB28 antibody-coated microbeads were frozen and defrosted, and then were analyzed using either low cytometry or immunofluorescence staining for CD103 and other markers.11 The samples revealed that Trm cells, CD8+CD103+, which produce IL-17A, were present in psoriatic skin, suggesting that Trm cells play an important role in the clinical relapses of psoriasis.11
Biologics and cardiovascular disease. Finally, the relationship between cardiovascular disease and biologic therapies is increasingly being scrutinized. Early evidence suggests biologics can decrease cardiovascular disease risk. These findings upend the previous belief that biologics increased cardiovascular risk in patients, and potentially could create a paradigm shift in treatment of psoriatis. Anti-inflammatory effects of biologic therapy for psoriasis might modify coronary plaque morphology in a favorable way. In a study of patients with psoriasis who received biologic therapy or placebo, 107 consecutive participants underwent computed tomography angiography (CTA) at baseline and one year. The study showed a reduction in the noncalcified plaque burden in the biologic-treated patients, compared to the control group (1.12 ± 0.46mm2 vs. 1.04 ± 0.40mm2; p<0.001).12 Even when adjusted for other cardiovascular risk factors, such as age, body mass index (BMI), and statin use, patients in the biologic therapy group showed improvement in coronary plaque morphology (Figure 1).13 While noncalcified coronary artery plaques were less frequent in general among those patients treated with biologics, the level of improvements varied among the different biologic treatments used in the study. Patients treated with an IL-17 blocker experienced a 12-percent reduction in noncalcified coronary artery plaques, while those treated with TNF-alpha blockers experienced a five-percent reduction and those administered an IL-12/IL-23 blocker saw a two-percent reduction.13 This raises the important and controversial question as to whether biologics might specifically decrease the risk of major adverse cardiac events (MACE). In short-term studies of TNF-alpha, IL-17, and IL-23 blockers, there was no decrease in MACE when compared to placebo; however, more sophisticated analytical methods, combined with more extensive clinical experience with these biologics, might reveal that biologics can reduce the risk of MACE over the long-term.
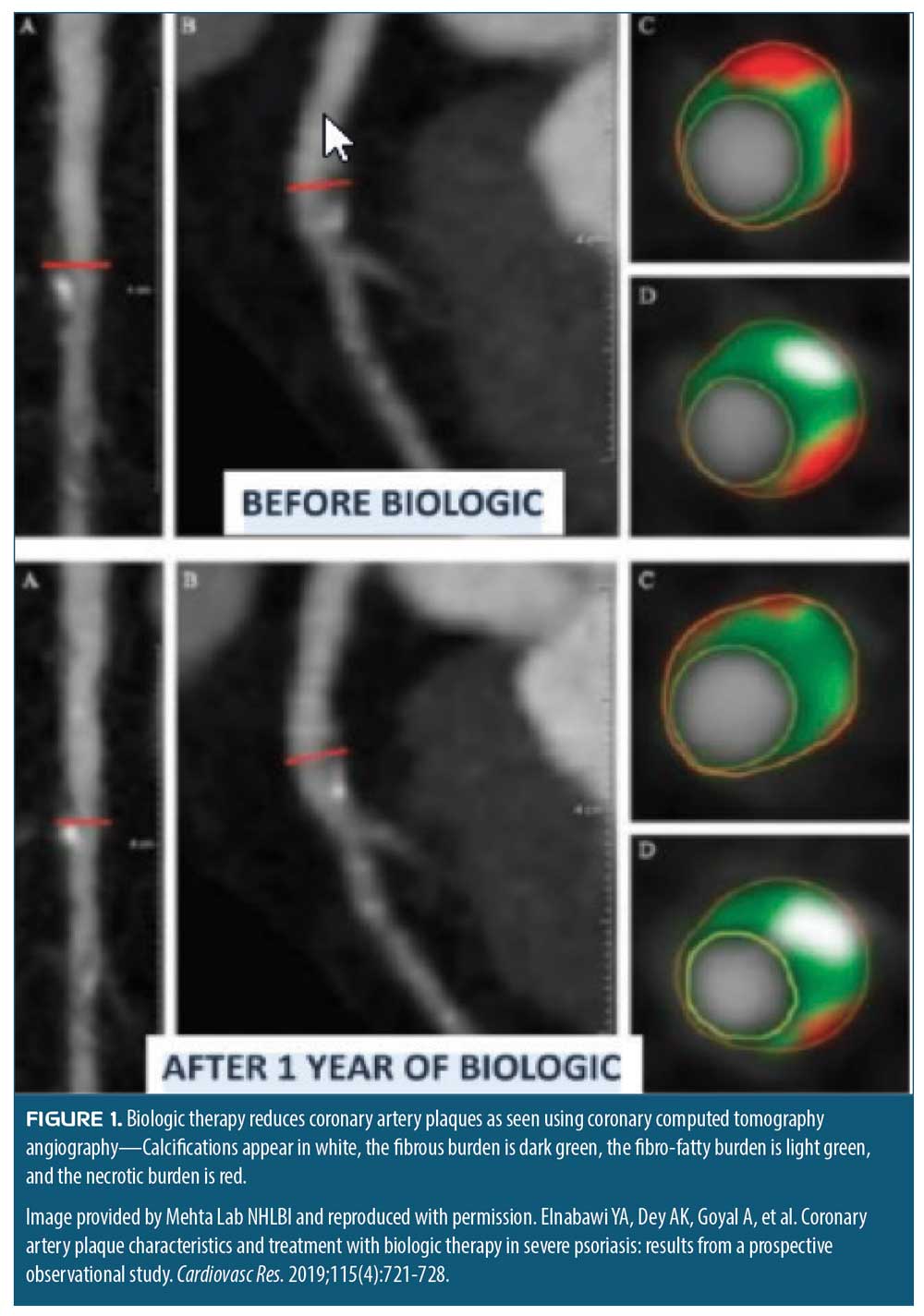
Understanding Psoriasis Comorbidities
Based on a presentation by Dr. Joel Gelfand, MD, MSCE, FAAD, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania
There is considerable interest and a wealth of new publications on the topic of psoriasis-related comorbidities, making it a challenge for busy clinicians to keep up to date on the latest findings. Psoriasis involves both genetic and environmental factors. Its pathophysiology appears to be mediated by Th1/17 inflammatory processes, which overlap with CV risk pathways, such as promoting thrombosis, atherosclerosis, and abnormal lipid metabolism; epidermal proliferation, which could increase uric acid levels, resulting in endothelial damage and oxidative stress; and upregulated angiogenesis, which could result in vascular damage.14
Psoriasis and gum disease. From a recent meta-analysis (n=8 studies, 812 patients), people with psoriasis have worse periodontal health than those without psoriasis, according to a variety of measures, including plaque index, gingival index, missing teeth, and bleeding on probing. In particular, patients with psoriasis tend to have worse gingival inflammation, more alveolar bone loss, and more missing teeth.15 Psoriasis, as an immune-mediated inflammatory disease, involves a dysregulation between innate and adaptive immunity, which work together to maintain tissue homeostasis. Adaptive immunity involves Th1 and Th2 cells, as well as Th17 cells and the cytokines IL-17 and IL-23. The inflammation produced by an unbalanced system can cause psoriasis as well as periodontitis and other inflammatory diseases, such as rheumatoid arthritis.16
In a randomized controlled study of 92 patients with psoriasis vulgaris and untreated periodontal disease, patients were randomized into an immediate-treatment group (n=46) and a delayed-treatment group (n=46). The immediate-treatment group received nonsurgical periodontal therapy while the delayed-treatment group were observed. At eight weeks, the immediate-treatment patients showed a significant decrease in IL-2 and IL-6 levels and improved PASI scores, suggesting that the periodontal therapy had improved their psoriasis.17
Psoriasis and liver disease. The hepatic comorbidities associated with psoriasis are important dermatological considerations. A cohort study of patients with psoriasis, psoriatic arthritis (PsA), or rheumatoid arthritis and matched controls found that the adjusted hazard ratios for any liver disease among people with psoriasis without systemic therapy was 1.37, for those taking systemic therapy it was 1.97, and the risk was 1.38 for people with PsA without systemic therapy and 1.67 for the same group on systemic therapy.18 The models were adjusted for age, sex, smoking status, drinking, BMI, oral glucocorticoid use, and the use of nonsteroidal anti-inflammatory drugs (NSAIDs). Nonalcoholic fatty liver disease (NAFLD) was more prevalent in patients with psoriasis on systemic therapy (2.23) and patients with PsA without systemic therapy (2.11). The risk for cirrhosis was greatest among patients with psoriasis on systemic therapy (2.62) and patients with PsA without systemic therapy (3.15). A significant trend (p<0.001) was observed showing that the greater the body area affected by psoriasis, the higher the prevalence of liver disease or cirrhosis. The association with liver disease was more pronounced in patients with psoriasis and/or PsA than with rheumatoid arthritis.18
Liver complications have been reported with long-term methotrexate therapy, used to treat both psoriasis and PsA. It is not clear whether patients with psoriasis and/or PsA are more vulnerable to methotrexate toxicity than other patients, such as those with rheumatoid arthritis who also take methotrexate. A retrospective database study of individuals taking methotrexate (n=5,687 patients with psoriasis, n=6,520 patients with PsA, n=28,030 patients with rheumatoid arthritis) was conducted.19 While the average weekly dose of methotrexate was similar among groups, the cumulative dose of methotrexate was highest in patients with rheumatoid arthritis. Compared to patients with rheumatoid arthritis taking methotrexate, patients with psoriasis on methotrexate therapy had a significantly higher risk of mild liver disease (hazard ratio [HR]: 2.22, 95% confidence interval (CI): 1.81–2.72), a higher risk for moderate-to-severe liver disease (HR: 1.56, 95% CI: 1.05–2.31), a greater likelihood of cirrhosis (HR: 3.38, 95% CI: 2.44–4.68), and a greater risk for a cirrhosis-associated hospitalization (HR: 2.25, 95% CI: 1.37–3.69). Patients with PsA also had a greater chance of mild liver disease, compared to patients with rheumatoid arthritis (HR: 1.27, 95% CI: 1.01–1.60) or those with cirrhosis (HR: 1.63, 95% CI: 1.10–2.42).19 This study suggests that patients with psoriasis and/or PsA who take methotrexate have a greater risk of liver complications than patients with rheumatoid arthritis on similar methotrexate therapy. The reasons for this disparity are not known but the finding emphasizes that dermatologists should monitor patients with psoriasis or PsA who are on long-term methotrexate therapy for liver conditions.
Psoriasis and cancer. Ongoing concern remains regarding the association between psoriasis and cancer. A systematic review of the literature (58 studies) was conducted to help generate pooled relative risk (RR) estimates for cancer rates and cancer mortality in cohorts of people with or without psoriasis. Data revealed an increased risk of cancer overall in patients with psoriasis of any severity (RR: 1.18, 95% CI: 1.06–1.31), with more pronounced rates among those with severe psoriasis (RR: 1.22, 95% CI: 1.08–1.39).20 Specific cancers were studied (Table 1). The cancer mortality risk was greatest in those with severe psoriasis, and most pronounced for liver cancer (RR: 1.43, 95% CI: 1.09–1.88), esophageal cancer (RR: 2.53, 95% CI: 1.87–3.41), and pancreatic cancer (RR: 1.31, 95% CI: 1.02–1.69). Cancer risks were also elevated among individuals who smoked, consumed alcohol, or were obese.20
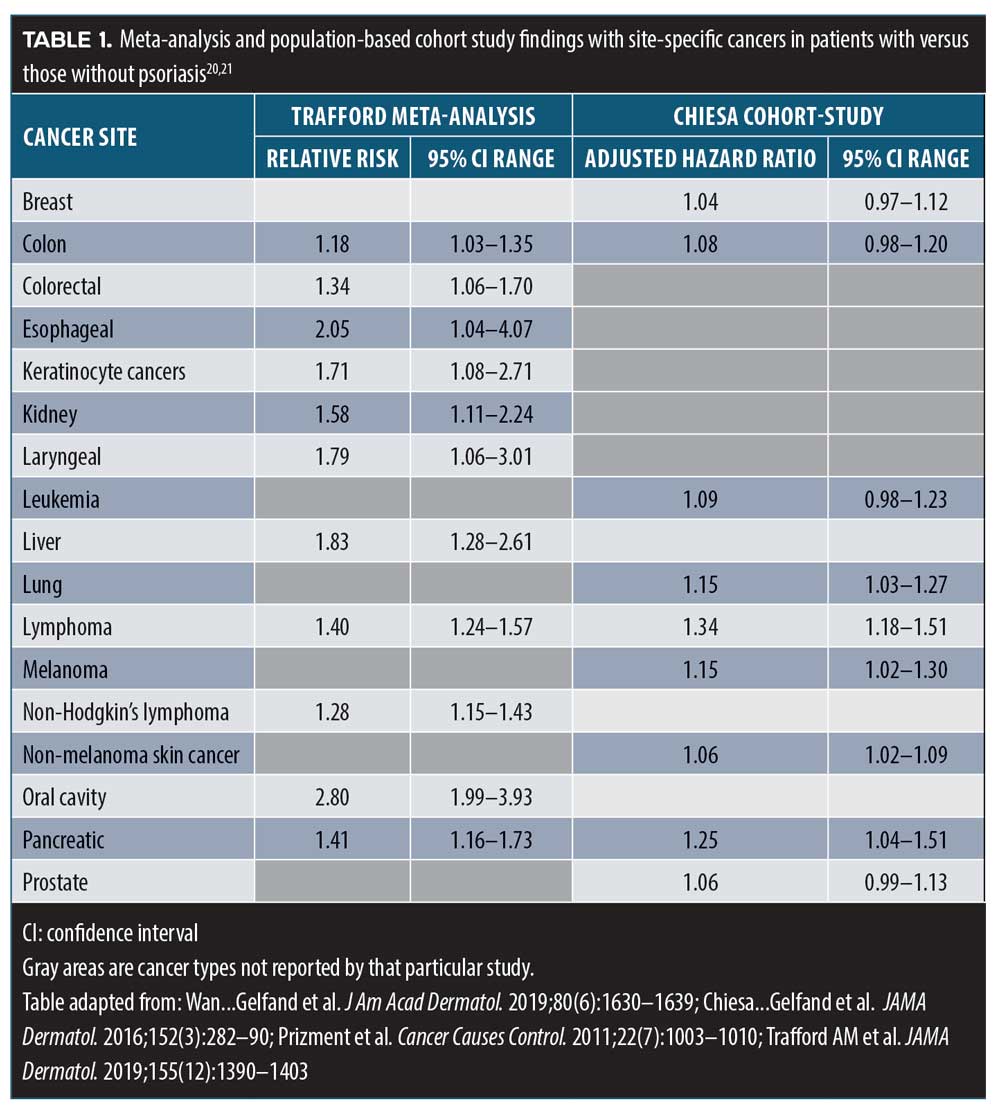
A study from Sweden (n=599) found that the incidence of psoriasis and psoriasis vulgaris was slightly elevated in patients with breast cancer (HR: 1.17 and 1.33, respectively; 95% CI: 1.07–1.52 and 1.17–1.52, respectively) but lifestyle, genetic, and treatment factors might have played roles in these results.22 A large population-based cohort study (n=937,716 control patients without psoriasis matched with 198,366 patients with psoriasis) reported a small association between psoriasis and cancer but saw no significant association between breast cancer, colon cancer, prostate cancer, or leukemia and psoriasis.21 These intriguing data suggest that patients with psoriasis are at a somewhat increased risk for cancer and cancer mortality, particularly certain site-specific cancers, but it is unclear as to what extent lifestyle factors, psoriasis treatments, and the inflammatory cascade associated with psoriasis might play in this higher risk. Clinicians should encourage patients with psoriasis and/or PsA to stay current with age-appropriate cancer screenings, such as the Pap smears, mammograms, and colonoscopies and low-dose computed tomography (CT) screenings for certain smokers. Patients with psoriasis and atypical disease characteristics or those who are unresponsive to psoriasis treatments should be considered for biopsy.
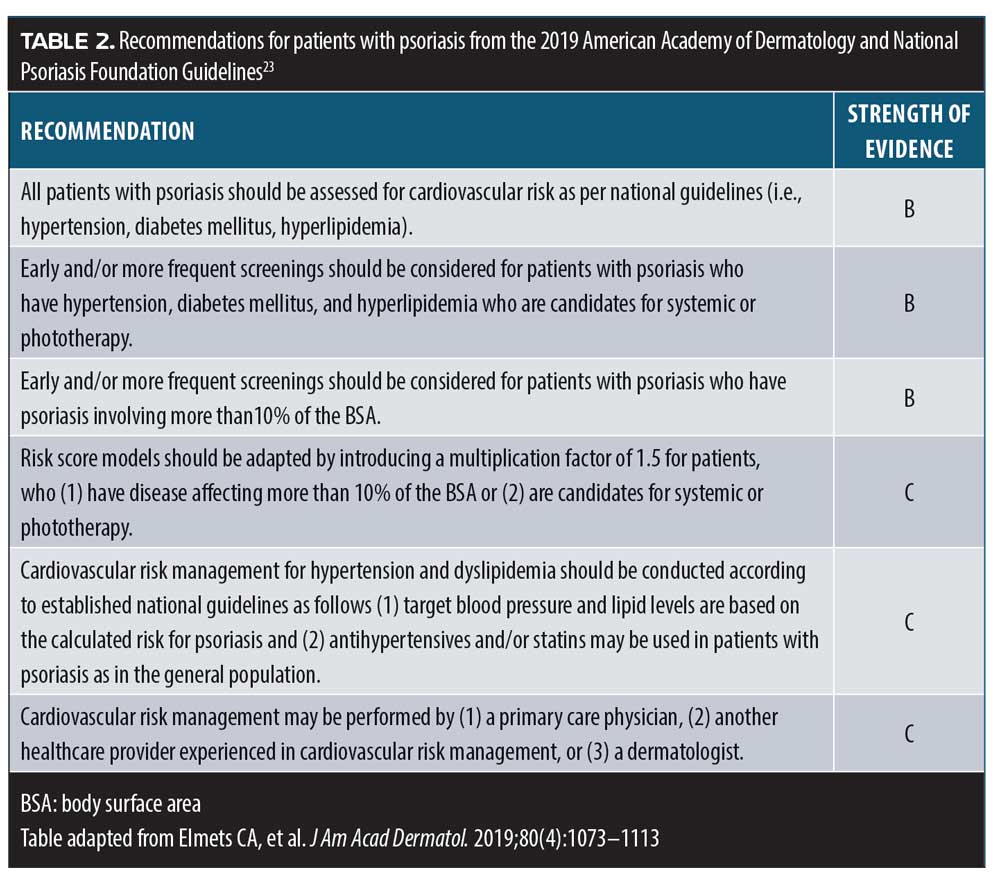
Psoriasis and cardiovascular disease. The new guidelines from the American Academy of Dermatology and the National Psoriasis Foundation provide dermatologists with clear recommendations regarding screenings for heart disease in their patients with psoriasis.23 Dermatologists on the front lines who care for patients with psoriasis have the opportunity to take the lead in assuring that patients are properly screened for cardiovascular comorbidities. Many patients under the care of a dermatologist do not regularly see their primary doctor (Table 2). Furthermore, the 2018 American Heart Association/American College of Cardiology Guideline on the Management of Blood Cholesterol specifically lists psoriasis as a risk-enhancing factor for cardiovascular disease, noting that psoriasis is among several conditions that promote inflammation, which increases risk for atherosclerosis. This guidance states that patients with advanced psoriasis are at increased risk for cardiovascular disease.24 Statins are recommended for people between the ages of 40 and 75 years, based on their 10-year risk of a CV event, and it is now recommended that those with psoriasis start statins earlier than people without psoriasis.24 However, dyslipidemia and hypertension are often underdiagnosed and undertreated in people with psoriasis or PsA.25 In a study of 2,254 patients (58.9% with PsA and 41.1% with psoriasis) from Canada, the United States, and Israel, 87.6 percent had at least one modifiable risk factor for cardiovascular disease (49.4% dyslipidemia, 45.1% hypertension). Additionally, more than half of patients with hypertension (59.2%) and dyslipidemia (65.6%) were not being appropriately treated for these conditions.25
The mortality in patients with psoriasis, stratified by straightforward, physician-assessed, objective measures of disease severity and with adjustments made for risk factors for high mortality, such as obesity, was evaluated.26 Using The Health Improvement Network (THIN) database in the United Kingdom, a nested cohort study was created of patients with psoriasis followed prospectively as part of the Incident Health Outcomes and Psoriasis Events (iHOPE) study.27 The study included 8,760 adults with psoriasis and 87,600 without psoriasis.26 The psoriasis group had higher rates of chronic kidney disease, chronic obstructive pulmonary disease (COPD), diabetes mellitus, and a history of myocardial infarction. Mortality among patients with psoriasis was 3.35 deaths per 1,000 person-years (95% CI: 2.81–3.99), compared to 3.24 deaths per 1,000 person-years (95% CI: 3.06–3.43) among people without psoriasis. Note that in the two cohorts, the average age was similar, although the psoriasis group had a slightly higher average BMI score and a greater proportion of men than the nonpsoriasis group. However, when physician-reported body surface area (BSA) was used to stratify patients with psoriasis into three groups (<3%, 3–10%, and >10%), using age- and sex-adjusted models, those with the most extensive psoriasis (BSA >10%) had a statistically significantly increased risk of death (HR: 2.12, 95% CI: 1.46–3.07). Thus, after controlling for baseline predictors of mortality and matching controls for age and sex, patients with psoriasis affecting a BSA of more than 10 percent had a 1.79 times increased risk of death.26
Similarly, the association of diabetes mellitus with psoriasis was further confirmed in a study by Wan et al.28 Using the THIN database from the United Kingdom, a population-based cohort study compared 8,124 adults with psoriasis to 76,599 patients without psoriasis and followed these patients for approximately four years. There was a rate of 3.44 percent incident cases of diabetes among patients with psoriasis, compared to 2.44 percent in those without psoriasis. Adjusting for age, sex, BMI, and BSA, the hazard ratios for patients with psoriasis developing diabetes were 1.21 ( 95% CI: 1.01–1.44) for those with 2% or less of the BSA affected, 1.01 ( 95% CI: 0.81–1.26) for those with 3 to 10% of the BSA affected, and 1.64 ( 95% CI: 1.23–2.18) for those with more than 10% of the BSA affected.28
This raises an important question for dermatologists in particular and our health care system at large: Should psoriasis be more aggressively treated to reduce the morbidity and mortality rates associated with cardiovascular disease? Biologic therapy in patients with inflammatory arthritis, including but not limited to PsA, reported a risk reduction in major cardiovascular events.29 In an analysis, risks of cardiovascular events associated with the use of ustekinumab, etanercept, adalimumab, and methotrexate, respectively, were similar among the agents.30 Observational studies have provided evidence of the cardiovascular and other benefits of biologic therapy in patients with psoriasis but have been limited by their design, short-term nature, imprecise estimates, and the fact that many do not include mortality as an endpoint.31,32 Of particular concern in observational studies is the potential bias that might be introduced due to what is called “the healthy user effect”—that is, studies might favor the enrollment of healthier patients.32
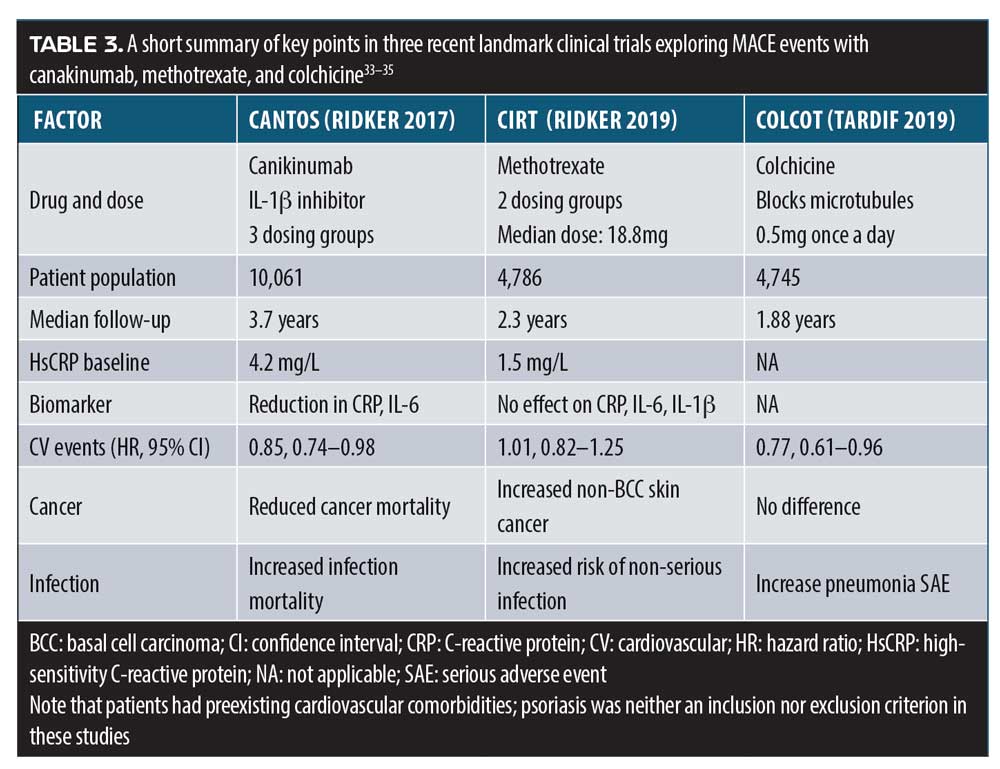
Major adverse cardiovascular events (MACE) have been evaluated in three landmark clinical trials in the past two years. CANTOS was a randomized, double-blind study of the monoclonal antibody canakinumab, which targets IL-1beta.33 The study population consisted of myocardial infarction survivors with a high-sensitivity C-reactive protein level of 2mg/L or greater. Doses of 50, 150, and 300mg of subcutaneous canakinumab every three months were evaluated. There was no difference in the all-cause mortality rate between any dose of canakinumab and placebo (0.94, 95% CI: 0.83–1.06; p=0.31), although canakinumab was associated with a higher risk for a fatal infection, compared to placebo. Subcutaneous canakinumab 150mg every three months was associated with a lower risk of recurrent cardiovascular events, compared with the placebo, independent of lowering lipid levels. Canakinumab significantly reduced high-sensitivity C-reactive protein levels, compared to baseline, without lowering low-density lipid levels.33
On the other hand, in the Cardiovascular Inflammation Reduction Trial (CIRT), low-dose methotrexate (15 or 20mg weekly) did not lead to a cardiovascular benefit in a randomized, double-blind, placebo-controlled study of 4,786 patients who had either survived a heart attack or had multivessel coronary disease and Type 2 diabetes or metabolic syndrome.34 The CIRT trial was halted early due to lack of evidence of benefit. Methotrexate (MTX) was not observed to lower IL-1beta, IL-6, or C-reactive protein levels more than the placebo and was associated with higher liver-enzyme levels, lower leukocyte counts, and reduced hematocrit levels.34
Colchicine, a medication indicated for gout and pericarditis, was evaluated in a randomized, double-blind study of 4,745 patients who had a history of recent myocardial infarction (within the past 30 days).35 Patients were randomized to treatment with low-dose colchicine (0.5mg once a day) or placebo. Compared to placebo, colchicine was associated with a significantly reduced risk of ischemic cardiovascular events, although colchicine-treated patients also showed a significantly higher risk for pneumonia (0.9% vs. 0.4%; p=0.03).35 These “three faces of MACE” show that, in patients with cardiovascular comorbidities, the safety and efficacy profiles of canakinumab, methotrexate, and colchicine are quite different, but are proof of principle that immune-targeted treatments can lower the risk of CV events (Table 3).
Based on the knowledge that psoriasis is comorbid with dyslipidemia, cardiovascular events, and mortality, the Vascular Inflammation in Psoriasis (VIP) study sought to determine the role played by vascular inflammation in these comorbidities.36 The double-blind, randomized trial compared adalimumab (a TNF-alpha inhibitor), phototherapy, and placebo over 12 weeks, at which point all patients crossed over to adalimumab for a total of 52 weeks. Ninety-seven patients were randomized: 92 completed the randomized clinical trial portion and 61 completed the 52 weeks. Vascular inflammation was assessed by 18F-fluorodeoxyglucose positron emission tomography/CT, biomarkers for inflammation, insulin resistance, and levels of lipoproteins. Study results indicated that adalimumab had neither a positive or negative effect on vascular inflammation.36 These results align with findings from other studies (Table 4).
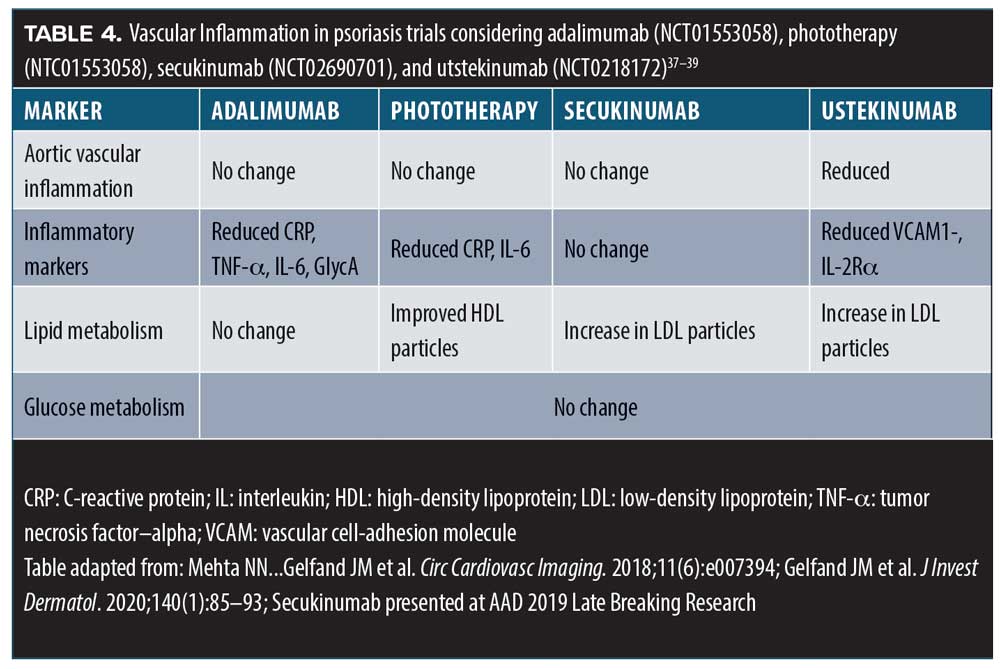
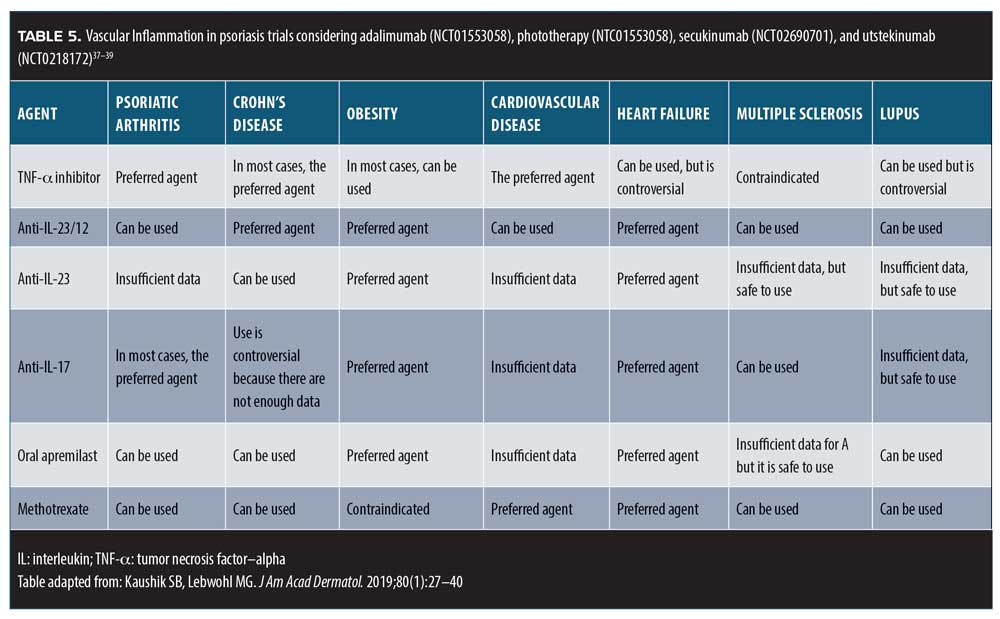
Practice pearls and pitfalls. When selecting psoriasis treatments, it is crucial to consider the patient’s comorbidities (Table 5). Many patients under the care of a dermatologist might not be seeing other specialists, and thus dermatologists should take the lead in the early identification of such comorbid conditions.
New Developments in the Treatment of Moderate-to-severe Psoriasis
Based on a presentation by Craig Leonardi, MD, Adjunct Professor of Dermatology, Saint Louis University, St. Louis, Missouri
Tremendous changes in the care provided to patients with moderate-to-severe psoriasis have occurred following the advent of biologic therapy and landmark studies about the use of various agents in general and pediatric populations.
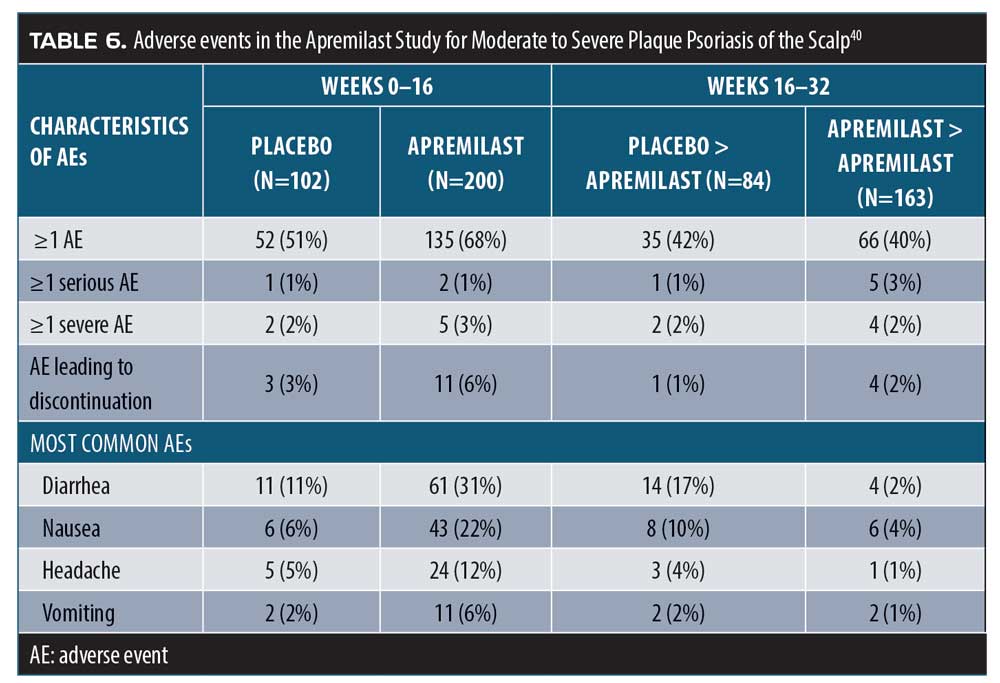
Apremilast for scalp psoriasis. Scalp psoriasis can be especially troubling to patients, due to its visibility, accompanying symptoms of pruritus, and challenges in effective treatment. Topical therapies might be effective but are also messy and make the hair seem greasy. A randomized, double-blind, placebo-controlled study of apremilast 30mg twice a day was conducted over 16 weeks, whereupon all patients crossed over to apremilast for 16 more weeks (32 weeks in total).40 The patients (n=303) had moderate-to-severe scalp psoriasis scores of three points or higher on the static Psoriasis PGA (ScPGA) scale, with a psoriasis-involved scalp surface area of 20 percent or more. Patients also had moderate-to-severe plaque psoriasis (PASI 12 points or greater, 10% or greater BSA involvement, and sPGA 3 points or greater). All enrolled patients had failed to respond to and/or had not tolerated at least one prior topical treatment of scalp psoriasis. At the end of the 16-week randomized portion of the study, apremilast patients achieved significantly improved scores in ScPGA using the last observation carried forward (LOCF), compared to placebo patients (40.3% vs. 13.7%; p<0.001). Moreover, when placebo patients crossed over to apremilast at 16 weeks, they improved markedly and, at Week 24, the placebo-apremilast patients had better LOCF scores than the original apremilast group. Nonresponder imputation (NRI) analysis at Week 16 was 38.8 percent for apremilast, compared to10.8 percent for placebo (p<0.001).40 Adverse events are reported in Table 6.
Pediatric patients. While there is great interest in the potential use of biologics to treat pediatric psoriasis, dosing remains a challenge for this population. A Phase II, multicenter, open-label study of 63 children between the ages of six and 17 years with moderate-to-severe plaque psoriasis found that weight-based dosing of apremilast 20mg twice daily in children (6–11 years) or apremilast 20 to 30mg twice daily in adolescents (12–17 years) was comparable to adult dosing of 30mg twice daily based on the area under the concentration-time curve from 0 to 12 hours after the dose.41 The study was restricted to patients weighing more than 20kg. Eligible subjects had previously experienced an inadequate response to topical therapy and were considered appropriate candidates for systemic therapy or phototherapy. Most participants did not object to the taste of the tablet, and PASI scores overall revealed 68-percent improvement over baseline in adolescents and 79-percent improvement over baseline in children.41
In the IXORA-PEDS study, a Phase III study of patients, aged 6 to 17 years, with moderate-to-severe plaque psoriasis, participants were randomized to weight-based dosing of ixekizumab every four weeks or placebo for 12 weeks followed by crossover to open-label ixekizumab Q4 weeks for an additional 12 weeks.42 All participants were deemed appropriate candidates for systemic therapy or phototherapy and could not previously have been treated with an IL-17 blocker or etanercept. Ixekizumab was administered every four weeks; weight-based dosing stipulated that patients who weighed under 25mg receive 40mg of ixekizumab at Week 0, transitioning to 20mg; those who weighed 25 to 40kg receive 80mg of ixekizumab at Week 0, then 40mg; and those weighing over 50kg receive 150mg of ixekizumab at Week 0 and then 80mg. The primary endpoint was the proportion of patients who achieved PASI-75 or greater at 12 weeks and a static PGA score of zero or one point(s). Study results indicated that ixekizumab achieved significantly superior results to placebo for PASI-75 (89% vs. 25%) and for static PGA (81% vs. 11%), and the responses at 12 weeks remained durable to 48 weeks. Patients in this study were permitted to use concomitant treatments, such as topical steroids, special shampoos, moisturizers, oils, and oatmeal baths. Adverse events were reported at Week 12 and affected 45 percent of the placebo group and 56 percent of ixekizumab group. No deaths occurred, one serious adverse event occurred (ixekizumab group), and one patient discontinued treatment because of adverse events (placebo group).42
Head-to-head clinical trials for moderate-to-severe psoriasis. Large randomized, clinical trials making head-to-head comparisons among biologics continue to provide excellent insight to aid in prescribing choices by dermatologists. The IXORA-R study was a head-to-head, randomized, double-blind, clinical trial comparing two biologics (ixekizumab and guselkumab) in 1,027 adults with moderate-to-severe plaque psoriasis (sPGA 3 points or greater, PASI 12 points or greater, 10% or greater BSA involvement).43 Dosing for ixekizumab was 160mg at Week 0, administered as two injections of 80mg each and then 80mg every four weeks thereafter; dosing of guselkumab was 100mg at Weeks 0 and 4 and then 100mg every eight weeks thereafter. The primary endpoint, measured at 12 weeks, was PASI-100. Significantly more patients in the ixekizumab group, compared to those in the guselkumab group, achieved this endpoint (41% vs. 25%; p<0.001), with the frequency of adverse events being three percent in both groups.43
The IXORA-R study should be viewed in context with the ECLIPSE trial, which compared guselkumab to secukinumab and found that guselkumab provided superior efficacy at 48 weeks, compared to secukinumab. In the ECLIPSE trial (n=1,048), PASI-90 at 48 weeks was the primary endpoint.44
Pustular psoriasis. Pustular psoriasis is a rare and potentially life-threatening cutaneous disease characterized by recurring flares and erythematous pustular rashes. The IL-36 pathway has been implicated in the pathogenesis of pustular psoriasis, and a new IL-36 blocker, BI 655130, has been developed. Results from a Phase I study in seven patients with acute generalized pustular psoriasis have been reported. Patients in this study had an average Generalized Pustular Psoriasis PGA (GPPGA) score of three points upon entering the study. After a drug infusion of 10mg/kg body weight, the mean improvement in GPPGA at one week over baseline was 59.0 percent; at two weeks, it increased to 73.2 percent, and was 79.8 percent at Week 4.45 Note that this Phase I study excluded patients with life-threatening flares or patients requiring intensive care. Adverse events were reported in all patients (100%), with the most commonly reported ones being eosinophilia (57.1%), vomiting (28.6%), and urinary or respiratory tract infections (28.6%).45
Tyrosine-kinase 2 (TYK2) inhibition. Tyrosine-kinase 2 (TYK2) is an intracellular signaling enzyme that mediates cytokine signaling and has been implicated in psoriasis pathogenesis. TYK2 activates signal transducer and activator of transcription (STAT)-dependent gene expression and the responses of IL-12, IL-23, and Types I and III interferon receptors. BMS-986165 is a novel TYK2 inhibitor and was evaluated in adults with moderate-to-severe psoriasis (n=267) in a Phase II, double-blind, placebo-controlled trial.46 Patients were assigned to placebo or one of the following various dosing groups of BMS-986165: 3mg every other day, 3mg every day, 3mg twice a day, 6mg twice a day, and 12mg a day. The primary endpoint of the study was PASI-75 or better at 12 weeks. At doses of 3mg a day or greater, BMS-986165 promoted significantly better psoriasis clearance than the placebo.46 Adverse events were reported in 51 percent of placebo patients, compared to 59 percent (3mg every other day), 55 percent (3mg daily), 64 percent (3mg twice daily), 80 percent (6mg twice daily), and 77 percent (12mg daily) of actively treated patients, while serious adverse events affected two percent or less of all groups. Acne developed in nine percent of patients taking 12mg of BMS-986165 but not in placebo patients (0%).46
Bimekizumab. The IL-17 family of cytokines includes IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (also known as IL-25), and IL-17F. Of these, IL-17A is the most thoroughly studied to date. Ixekizumab and secukinumab inhibit the IL-17A pathway and brodalumab inhibits all five IL-17 isoforms (IL17A, IL17B, IL17C, IL17D, IL17F). Despite the close relationship between IL-17A and IL-17F, IL-17F has not been as thoroughly studied as IL-17A. Both IL-17A and IL-17F are proinflammatory cytokines with a somewhat overlapping area of function, and animal studies suggest that both play a crucial role in the pathogenesis of psoriasis and PsA. Dual inhibition of IL-17A and IL-17F occur with bimekizumab in contrast with other agents that block IL-17A alone.47 In a double-blind, placebo-controlled, Phase IIb, dose-ranging study (BE ABLE 1), patients were randomized to one of six groups: placebo or subcutaneous bimekizumab every four weeks at doses of 64mg, 160mg, or 160mg after a loading dose of 320mg, 320mg, or 480mg.47 The primary endpoint was PASI-90 at 12 weeks, which was achieved significantly more often in all bimekizumab groups (46.2%–79.1%) than in the placebo group (0%) (p<0.0001 for all doses). At 12 weeks, 27.9 to 60.0 percent of the bimekizumab groups achieved PASI-100, compared to zero percent in the placebo group (p less than or equal to 0.0002 for all doses). At 12 weeks, 60.0 percent took a 320mg loading dose of bimekizumab, followed by a dose of 160mg. Adverse events occurred more frequently in bimekizumab patients (61%) than placebo patients (36%). This study did not include active comparators, but the results exceeded those of IL-23 inhibitors, and further research is warranted.47
Netakimab. Netakimab, a novel monoclonal antibody that targets IL-17A, is being evaluated for its potential role in the treatment of moderate-to-severe plaque psoriasis. Netakimab is approved in Russia for this indication, but this novel agent is far less well-known in the United States. The PLANETA trial evaluated 213 patients with plaque psoriasis in a multicenter, Phase III study in Russia and Belarus over 12 weeks. Patients were included if they had a PASI score of 10 points or higher, a sPGA score of three points or higher, and at least 10-percent BSA involvement.48 Patients were randomized to one of three groups: placebo or 120mg of netakimab every two or four weeks. The primary endpoint was PASI-75 at 12 weeks, which was achieved by 83.3 percent and 77.7 percent of the netakimab two- and four-week patient groups, respectively, compared to zero percent of patients in the placebo group (p<0.0001), with adverse events occurring in 17.7 percent and 16.7 percent of netakimab two and four-week patients, respectively, versus 18.2 percent of placebo patients. Thirty-three percent of patients achieved complete clearance. The study concluded that 120mg of netakimab every four weeks was not inferior to the every-two-week dosing schedule.48
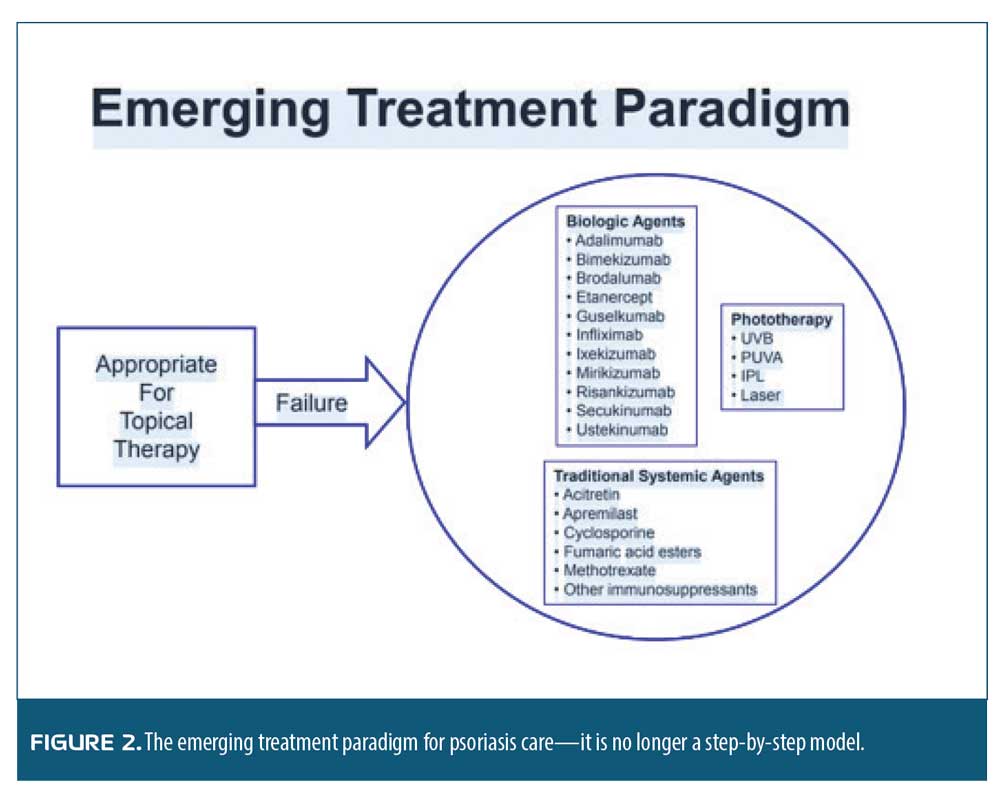
Practice pearls and pitfalls. Our incremental elucidation of the pathophysiology of psoriasis, along with the availability of novel biologic agents, has changed the paradigm of care for patients with psoriasis. Not so long ago—and, indeed, perhaps to some extent still true today—the psoriasis care paradigm recommended treating psoriasis in a stepwise progression, starting with over-the-counter products and then advancing to prescription topicals, phototherapy, and finally systemic treatments. Patients had to move step-by-step through the model and could not skip steps. In other words, they could not advance to the next therapy until they had tried and failed the prior one. What we have learned about psoriasis today is that the appropriate treatment paradigm is not progressing step-by-step through a series of treatments but rather having patients begin with topical therapy. If that fails, they may then move to traditional systemics (e.g., methotrexate, acitretin, apremilast), biologics, or phototherapy. There is not a particular progression pattern that patients must follow. The therapeutic choice should be based on the individual patient’s characteristics. While effective, this new paradigm can appear more challenging to adopt among clinicians who must individualize care for each patient holistically. However, this new paradigm has the potential to provide much better results, ultimately benefiting patients, clinicians, and our healthcare system (Figure 2).
What’s New in Psoriatic Arthritis Treatments?
Based on a presentation by Arthur Kavanaugh, MD, Professor of Medicine with the University of California, San Diego in San Diego, California.
In a study of 2,617 PsA patients from the Corrona Registry, the occurrence of three domains was most frequent (28.0%) among patients taking biologics (n=354), while five, six, or one domain(s) were less frequent (6.2%, 0.8%, and 12.7%, respectively).49 This study included six PsA domains and did not include irritable bowel syndrome or iritis. Data from 1996 to 2012 indicate that joint surgery was about twice as frequent in patients with psoriatic arthritis (PsA) relative to the general population. When followed for 15 years, about 30 percent of patients with PsA underwent some type of joint surgery. In fact, a younger patient with PsA (18–40 years of age) has a higher risk for joint surgery than the general population of people older than 60 years.50 This points to an important and an under-recognized need in the PsA population.
It has been established that about 30 percent of patients with psoriasis will develop PsA, which occurs subsequent to the skin disease in about 85 percent of individuals.51 In many cases, the time lag between cutaneous psoriasis and PsA is about one decade. Epigenetics could help elucidate how environmental factors impact the development of PsA and facilitate a timely diagnosis. A hypothesis was presented at the European League Against Rheumatism (EULAR) 2019 meeting in Madrid that the epigenetic deregulation of DNA methylation occurs very early on in the pathogenesis of PsA, even before symptoms develop, such that epigenetic markers might be useful to predict PsA. In a cohort study, 60 psoriasis converters were compared with 60 nonconverters for an epigenome-wide comparison of DNA methylation in baseline samples of whole blood. After adjusting for the heterogeneity of cell types, 68 individual CpG sites (cytosines following by guanine residues) were identified that were different between converters and nonconverters in terms of methylation. Differently methylated regions (DMRs) that contained at least four significant CpGs could be identified in genes, such as the FBXO27.51 Thus, changes in individual CpGs, DMRs, and inflammatory pathways were observed to differ between converters and nonconverters, supporting the hypothesis that DNA methylation changes occur very early on upstream in the pathogenesis of PsA. Thus, these biomarkers might have a predictive value in the early identification of patients with psoriasis who are likely to develop PsA.
Treatment options. The optimal therapy for PsA must be individualized for each patient. Disease-modifying drugs (DMARDs), biologics, jakinibs, and phosphodiesterase-4 inhibitors are mainstays of PsA treatment. Adjunctive treatments include analgesics, such as NSAIDs, steroids, topical products, and physical therapy, which might also be helpful. A variety of experimental treatments are currently in development, including some new jakinibs (filgotinib, upadacitinib, BMS-986165, and baricitinib), alpha-Il-23 monoclonal antibodies (guselkumab, risankizumab, and tildrakizumab), and other IL-17 blockers (brodalumab, bimekizumab).
Etanercept and methotrexate. In a double-blind, head-to-head study (SEAM-PsA), 851 patients with PsA were randomized to receive 20mg oral methotrexate plus subcutaneous placebo every week; subcutaneous etanercept 50mg and oral placebo every week; or subcutaneous etanercept 50mg and oral methotrexate 20mg every week.52 Thus, patients received methotrexate monotherapy, etanercept monotherapy, or a combination therapy of etanercept plus methotrexate. At 24 weeks, the American College of Rheumatology (ARC) 20-percent improvement (ACR20) response and Minimal Disease Activity (MDA) response were primary endpoints. At 24 weeks, ACR20, ACR50, and ACR70 were higher in the etanercept monotherapy or combination therapy than in the methotrexate monotherapy group, which also showed a higher rate of nausea as an adverse event (while other adverse events were similar across all three groups). Etanercept monotherapy and etanercept/methotrexate combination therapy were similarly effective, suggesting that methotrexate provided no synergistic benefits to etanercept monotherapy. In terms of skin psoriasis, the results of etanercept alone or etanercept plus methotrexate were only slightly improved over those of methotrexate monotherapy.52 However, adding methotrexate to etanercept treatment in patients with PsA might have positive results on skin endpoints. For instance, among patients who achieved a BSA reduction in skin psoriasis of up to three percent or up to one percent, respectively, combination therapy patients had significantly better scores.
Targeted therapies. The development of novel targeted therapies has been the source of significant progress in the effective treatment of PsA but autoimmune diseases are so complex that agents effective in one autoimmune disorder might have limited effectiveness in others; additionally, some agents have remarkably disparate effects on various domains and diseases. This can be challenging in the care of patients with PsA, a population with widely heterogeneous clinical characteristics. The advancing science in these targeted approaches sometimes results in unexpected observations. Thus, a “bedside to bench” approach is warranted in which clinical studies of specific, targeted therapies help to aid our understanding of the immunopathogenesis of PsA.53
Ustekinumab. The Enthesial Clearance in Psoriatic Arthritis (ELCLIPSA) study was a randomized, controlled, open-label study of patients with PsA and active enthesitis who were assigned to receive either ustekinumab or a TNF inhibitor for 24 weeks, with the primary endpoint of complete clearance of enthesitis, defined as a score of zero points on the Spondyloarthritis Research Consortium of Canada (SPARCC) index.54 ECLIPSA (n=51) found that 73.9 percent of ustekinumab and 41.7 percent of TNF inhibition patients achieved the endpoint (p=0.018). However, ustekinumab had superior results to TNF inhibition with respect to enthesitis (p=0.007) and skin psoriasis (p=0.030) but not PsA (p=0.95). While caution is reasonable for such a small study that allowed more than one kind of TNF inhibitor, the results suggest that ustekinumab (IL-12/IL-23 inhibition) might be more effective specifically in treatment of enthesitis.54 By the same token, Deodhar et al reported on three randomized, double-blind, multicenter trials of ustekinumab for axial spondyloarthritis and found that ustekinumab was not effective for this indication.55
Ustekinumab vs. etanercept. As drug development advances and our understanding of epigenetics increases, it becomes increasingly necessary to better understand how various agents can influence the pathological activation of specific genes. This is a complex new field but one that could open the door to disease normalization.56 In a genetic study of ustekinumab and etanercept, it was shown that ustekinumab reduced the expression on gene transcripts related to inflammation to a greater degree than etanercept, with less residual expression of gene products.56 It might be that “drug survival”—that is, the ability for a drug to exert durable positive effects over the long-term—relates to a lower expression of cytokines, chemokines, and inflammatory mediators; in other words, a more stable environment is created that is less likely to permit the reactivation of pathogenesis. Brodmerkl et al found that even in patients treated with different drugs who achieved similar results (PASI-75) there might be markedly distinct degrees of suppression of the disease profile at the molecular level and different molecular scars—that is, different qualitative suppression levels of inflammatory gene transcripts.56
Ixekizumab vs. adalimumab. In the SPIRIT-H2H study, investigators compared ixekizumab head-to-head against adalimumab in biologic-naïve patients with both PsA and skin psoriasis.57 Patients (n=566) were randomized to one of the two drugs in this open-label study with endpoints of ACR50 and PASI-100 at 24 weeks. Ixekizumab was the more effective agent, with 36 percent of patients meeting the primary endpoint, compared to 28 percent of adalimumab patients (p=0.036). Serious adverse events were reported in 3.5 percent of ixekizumab and 8.5 percent of adalimumab patients.57
Secukinumab. Results from a three-year study plus a one-year extension of the FUTURE-1 trial regarding the safety and efficacy of secukinumab in treating PsA were recently published. In FUTURE I, over the first two years, secukinumab demonstrated safety and effectiveness in the treatment of PsA.58 With three years of data, new findings indicate that secukinumab exhibited a low rate of disease progression on radiography and demonstrated consistent safety results, providing support for the long-term use of secukinumab to treat PsA. In the FUTURE-1 extension trial (n=460, including 308 patients who were originally randomized to secukinumab in the first part of the study), secukinumab showed a good tolerability profile, with no new emerging safety signals.58 The patient retention rate was high (95.2%), and improved quality of life scores were shown to be durable over three years.58
Guselkumab. Guselkumab is an effective agent with an acceptable risk-to-benefit ratio as a treatment for PsA, as demonstrated in a randomized, double-blind, multicenter, placebo-controlled study (n=624) with the primary endpoint of ACR20 at 24 weeks.59 Patients were assigned to one of three groups: guselkumab every four or eight weeks or placebo. The proportion of patients who achieved ACR20 was 59 percent (guselkumab every four weeks), 52 percent (guselkumab every eight weeks), and 22 percent (placebo). Both guselkumab groups were significantly superior to placebo (both p<0.0001). Serious adverse events occurred in four percent of placebo patients, three percent of patients taking guselkumab every eight weeks, and no patients (0%) taking guselkumab every four weeks.59 A PASI-75 score was a secondary endpoint of this study and occurred in 86.5 percent of patients taking guselkumab every four weeks, 75.6 percent taking guselkumab every eight weeks, and 14.1 percent of placebo patients. Guselkumab was shown to be effective in treating enthesitis and dactylitis as well.59
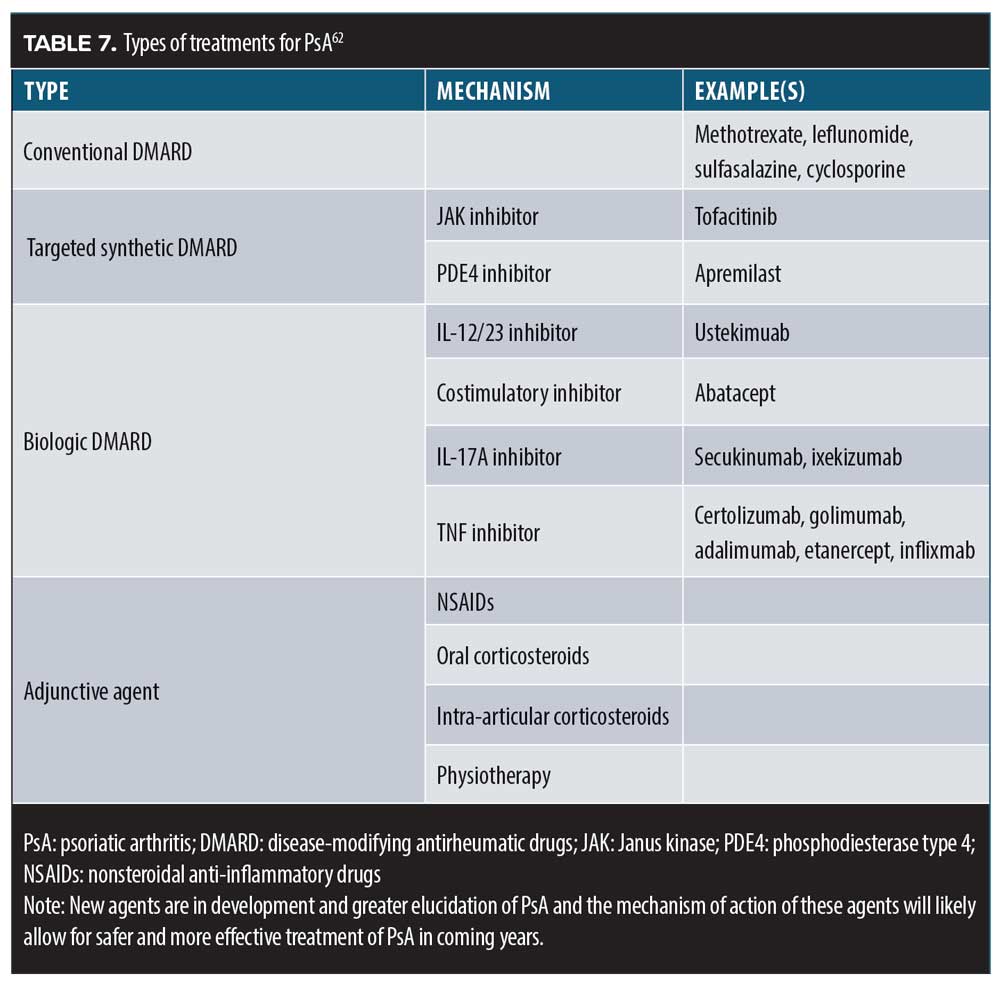
Tildrakizumab. Tildrakizumab is an IL-23p19 monoclonal antibody that was recently studied in patients with PsA (n=391) randomized to one of four dose regimens: 200mg every four weeks; 200mg every 12 weeks; 100mg every 12 weeks; and 20mg every 12 weeks.60 At 24 weeks, more tildrakizumab patients than placebo patients had achieved PASI-90 and ACR50. A dose-dependent response was observed but a shorter dose interval for 200mg (from every 12 weeks to every four weeks) did not improve results. Patients who were administered 200mg of tildrakizumab every 12 week achieved PASI-75 (79.6%) and PASI-90 (50%) significantly more often than placebo patients (16.7% and 7.1%, respectively; p<0.0001).60,61 Thus, the armamentarium for PsA treatment is large, continues to grow, and allows for individualized treatment approaches (Table 7).
A recent study explored baseline features of patients on apremilast and/or biologic therapy for PsA using the Corrona Psoriatic Arthritis/Spondyloarthritis Registry in real-world clinical use.63 In the group of patients in the United Sates who were prescribed apremilast plus a biologic DMARD, there were more female patients, more people with depression, more patients with diabetes, and higher BMI scores. Most of these patients (90%) had previously been prescribed a biologic.63 This is real-world evidence that the combination approach of apremilast plus a biologic is often used in practice and appears to be well-tolerated.
Janus family of tyrosine kinases (JAKs). Recent scientific and research activity in the development of JAKs has resulted in prescribing challenges. When selecting an appropriate JAK agent for PsA treatment, it is important to consider in-vitro selectivity of the agent and its dosing, the isoforms it affects, in-vivo selectivity and dose effects, and each patient’s individual characteristics. Various cytokines employ various JAK pathways with some degree of overlap. For instance, IL-2, IL-4, IL-7, IL-9, IL-15 and others use JAK1; IL-6, IL-11, Il-3, and others use JAK2; JAK3 is used by IL-2, IL-4, IL-7, IL-9, and IL-15; and IL-6, Il-11, and IL-12/23 use TYK2.64,65 These lists are not exhaustive. Cytokine receptors appear to pair with different JAKs, which can catalyze the transfer of phosphate to various receptors, allowing the recruitment of the STAT signaling molecules of DNA binding proteins; STATs are a JAK substrate. Because JAKs are crucial to the cytokine signaling processes, they have emerged as important drug targets.64 Likewise, even subtle disruptions in these JAK pathways can lead to various diseases.66 JAK pathways and the various relevant cytokine pathways they affect likely also play roles in the inflammatory cascade, antiviral activity, antitumor activity, innate immunity, growth, and other biological functions.
JAK inhibitors, or jakinibs, play a role in many autoimmune diseases and are particularly important in dermatology, due to their observed effectiveness in treating psoriatic skin disease and PsA.53
Practice pearls and pitfalls. For better treatment of PsA, it is imperative that dermatologists develop better tools to predict which patients with skin psoriasis are at greatest risk to develop PsA. That will allow for earlier and plausibly more effective treatment. Personalized medicine might help to minimize toxicity and maximize effectiveness across different domains in treating patients with PsA. Earlier diagnosis, earlier appropriate intervention, and better treatment algorithms are necessary to combat PsA more efficiently. Combination therapies show potential, and a few promising new drugs are in development.
This raises a key clinical question: Is there any way to prevent PsA? If a patient has skin psoriasis and is known to be at elevated risk for PsA, could strategies be developed to mitigate this risk? A recent retrospective study from Argentina (n=797; 10,017 patient-years) concluded that treatment with biologics might lower the risk of developing PsA.67 Using Cox regression analysis and adjusting for sex, age, and BMI, the risk that skin psoriasis patients treated with biologics have for the development of PsA is HR: 0.1 (95% CI: 0.013–0.7; p=0.021).67 While this was a relatively small, single-center chart-review study, the results are intriguing and warrant further investigation.
Innovations in Topical Therapy for Psoriasis
Based on a presentation by Linda Stein Gold, MD, Henry Ford Health System, Detroit, Michigan
Topical treatments remain a crucial element in the armamentarium against psoriasis, and dermatologists, like other clinicians, must focus on improving outcomes in the pursuit of this art.
Combining agents. “Drug stacking,” which is the use of multiple topical formulations applied one on top of the other is commonly used in dermatology; however, mixing vehicles can affect efficacy by effecting drug penetration. Crisaborole is a novel phosphodiesterase-4 inhibitor for topical treatment of AD.68 In a study in which crisaborole was applied 15 minutes before an emollient, skin penetration was not affected. However, when crisaborole was applied right after emollient application and again 15 minutes later, there was decreased crisaborole penetration.69
Stacking or layering topicals must be carefully considered due to the risk of reducing effectiveness between incompatible agents. For example, calcipotriene is an effective topical agent that requires a pH higher than 7.5 and is unstable in an aqueous acidic environment. Betamethasone, on the other hand, is the opposite: it is unstable in an aqueous alkaline environment and prefers a pH of around 4. While these two drugs might be combined for the treatment of psoriasis, the two substances are incompatible with each other. The use of a polyoxypropylene-15 stearyl ether as a solvent offers a way to combine calcipotriol and betamethasone in such a way that they can both be delivered and work together.70
Vehicles. The role of the vehicle in topical products should not be underestimated. A new enhanced vehicle for halobetasol propionate and tazarotene lotion relies on a honeycomb network that facilitates the uniform distribution of both active ingredients and the hydrating ingredients within the same drop of oil; it enhances delivery to the skin, repairs the skin barrier, and promotes even distribution of active ingredients to the skin.71
Halobetasol propionate and tazarotene lotion. Several recent studies have evaluated the clinical efficacy of a fixed-dose combination lotion using halobetasol propionate and tazarotene for treating plaque psoriasis. A multicenter, randomized, double-blind, Phase II study (n=212) found that combination therapy was consistently more effective than monotherapeutic regimens or vehicles in treatment success at eight weeks, with similar safety data and no new safety signals.72,73 Results from a pair of multicenter, randomized, double-blind, Phase III studies in 418 patients with psoriasis found that, at eight weeks, 40.7 percent of patients using the topical combination halobetasol propionate/tazarotene achieved treatment success relative to 9.9 percent who achieved such using only the vehicle (p<0.001).74 Durable results were shown in a study that evaluated results four weeks after the conclusion of an eight-week study.75 In a report of two multicenter, randomized, double-blind, vehicle-controlled, Phase III studies of 418 patients with plaque psoriasis treated with halobetasol propionate and tazarotene lotion for eight weeks with four weeks of follow-up, the combination lotion was superior in reducing signs and symptoms of psoriasis and reducing the BSA affected by the disease (note that in this study no patients were included who had an affected BSA >12%).76
In a post-hoc analysis of 212 patients with moderate-to-severe plaque psoriasis who were treated with halobetasol propionate/tazarotene lotion, halobetasol alone, tazarotene alone, or vehicle over eight weeks, the combination lotions were statistically significantly superior at eight and 12 weeks.77 Treatment success was maintained in 33 percent of the patients in both studies at 12 weeks.77
With promising results from this fixed combination, these drugs are currently available as single agents. However, stacking diminishes the penetration of tazarotene. A fixed combination of calcipotriene plus betamethasone dipropionate in an aerosol foam formulation was cleared to market; it offers good local bioavailability and has been associated with favorable outcomes.78 From pooled results of three randomized, four-week Phase II and III studies in adults with mild-to-severe psoriasis vulgaris, the aerosol foam showed a positive ratio of benefit to risk, was more effective than the fixed combination ointment, and was well-tolerated by patients.79 Based on results from recent studies, the United States Food and Drug Administration (FDA) has approved the use of this foam in pediatric patients 12 years of age or older who have psoriasis of the body and/or scalp.80
Psoriasis in the anogenital, intertriginous, and facial regions have always posed a therapeutic challenge due to lack of effectiveness of topical treatments. A randomized, vehicle-controlled, double-blind study was conducted in 21 patients with such forms of psoriasis who were treated twice a day with a crisaborole 2% ointment or vehicle for four weeks.81 At the end of the study, crisaborole patients showed a 66-percent improvement in psoriasis, compared to a nine-percent improvement in the vehicle group (p=0.0011). During the open-label phase of the study, patients treated with crisaborole continued to improve, achieving an 81-percent improvement at eight weeks, with 71 percent of patients reporting clinical clearance. No adverse events were reported.
The vehicle of a topical product is crucial to the product’s effectiveness and success, which underscores the importance of developing better vehicles. The optimal vehicle would be one that patients like and enhances the effectiveness of the active ingredient(s). New technologies include the PAD™ vehicle, which helps to form a robust droplet of oil, stabilized by an aqueous film. This technology could enhance solubility which, in turn, could lead to better skin penetration. These products have low amounts of surfactants, compared to conventional topical products. Early studies have shown promising results but have not yet been published.
Tapinarof: a new small molecule for topicals. New molecules could potentially change topical psoriasis care in the near future. Tapinarof is a small, naturally derived molecule produced by bacterial symbionts of entomopathogenic nematodes. While its mechanism of action is not completely understood, it appears to be effective in treating patients with psoriasis and AD. Chemically, it is structurally similar to resveratrol, a plant-derived stilbenoid found in red grapes and other foods.82 Tapinarof modulates the aryl hydrocarbon receptor (AhR) and decreases inflammation (likely by reducing Th17 cytokines), reduces oxidative stress, promotes a healthy skin barrier, and decreases inflammation associated with AD (by reducing Th2 cytokines). In a Phase II, randomized, dose-finding study of tapinarof in the treatment of plaque psoriasis, treatment success, defined as PGA score of zero or one point(s) and a two-grade improvement at 12 weeks, occurred significantly more often in patients using tapinarof, compared to those using the vehicle only.83 Treatment success occurred in 65 percent of those receiving tapinarof 1% twice daily; 56 percent of those receiving of tapinarof 1% once daily; 46 percent of those receiving tapinarof 0.5% twice daily; and 36 percent of those receiving tapinarof 0.5% once daily), compared to the vehicle groups (11% twice daily, 5% once daily).83 The most frequently reported adverse events with tapinarof were folliculitis (10% tapinarof vs. 1% vehicle), contact dermatitis (3% all tapinarof), and headache (1% all tapinarof).83
Cannabidiol (CBD) in topicals. BTX1308 is a novel CBD agent for topical use in the treatment of plaque psoriasis. CBD is thought to act as an anti-inflammatory agent. Early anecdotal reports suggest that CBD ointment might provide soothing relief to a variety of cutaneous conditions.84 A wide range of CBD products are available to the consumer, but the BTX1308 product appears to deliver more CBD through the skin than other similar over-the-counter products, likely because of its vehicle.
Practice pearls and pitfalls. Topical therapy continues to be a mainstay in psoriasis treatment, but growing awareness of the importance of product vehicles is changing how topical products are developed. The optimal vehicle can enhance penetration, improve efficacy, meet patient expectations, and promote adherence. For topical steroids, vitamin D is an important addition because it complements product efficacy and might act to help counteract the adverse effects of steroids. Product stacking is a common practice but must be done carefully to preserve the effectiveness of the drug combination; fixed combination products might be a more effective and convenient option. Tazarotene is an older agent but still holds a crucial place in topical care for patients with psoriasis. Nevertheless, new developments in tapinarof and topical CBD offer some important future directions.
Conclusion
Much has been learned about psoriasis in the past years but more research is required. A greater appreciation of psoriasis comorbidities has aided treatment and helped to explain the complexities of this autoimmune disease, which manifests as a cutaneous condition and develops into PsA in about one-third of patients. New research into PsA suggests that clinicians might soon have better tools to predict which patients are more likely to develop PsA, with the idea that early diagnosis means earlier treatment and possibly better outcomes. Because of the many comorbid conditions linked to psoriasis, dermatologists should be at the forefront of pursuing cardiac and specialized care for their patients. New treatment models are evolving that dispense with the old step-by-step approach to psoriasis in favor of one that is more individualized.
References
- Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol. 2002;16(3):241–248.
- Qiao P, Wang G. 341 Mechanical stretch contributes to the pathogenesis of psoriasis by regulating the phenotype of keratinocytes. J Invest Dermatol. 2017;137(10 Suppl 2):S251.
- Qiao P, Guo W, Ke Y, et al. Mechanical stretch exacerbates psoriasis by stimulating keratinocyte proliferation and cytokine production. J Invest Dermatol. 2019;139(7):1470–1479.
- Fyhrquist N, Muirhead G, Prast-Nielsen S, et al. Microbe-host interplay in atopic dermatitis and psoriasis. Nat Commun. 2019;10(1):4703.
- Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–859.
- Alekseyenko AV, Perez-Perez GI, De Souza A, et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome. 2013;1(1):31.
- Navarro-López V, Martínez-Andrés A, Ramírez-Boscá A, et al. Efficacy and safety of oral administration of a mixture of probiotic strains in patients with psoriasis: a randomized controlled clinical trial. Acta Derm Venereol. 2019;99(12):
1078-1084. - Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal Is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 Study. J Invest Dermatol. 2019;139(12):2437–2446e1.
- Chu K-L, Batista NV, Girard M, Watts TH. Monocyte-derived cells in tissue-resident memory T cell formation. J Immunol. 2020;204(3):477–485.
- Shen Z. Tissue-resident memory T cells in psoriasis recurrence [097]. J Invest Dermatol. 2018;138(5):S17–S17.
- Kurihara K, Fujiyama T, Phadungsaksawasdi P, et al. Significance of IL-17A-producing CD8+CD103+ skin resident memory T cells in psoriasis lesion and their possible relationship to clinical course. J Dermatol Sci. 2019;95(1):21–27.
- Elnabawi AY, Varghese JN, Sanda EG, et al. Immunomodulatory therapy favorably modifies coronary plaque morphology in psoriasis [12596]. Circulation. 2018;138(Suppl 1):
A12596–A12596. - Elnabawi YA, Dey AK, Goyal A, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115(4):721–728.
- Azfar RS, Gelfand JM. Psoriasis and metabolic disease: epidemiology and pathophysiology. Curr Opin Rheumatol. 2008;20(4):416–422.
- Qiao P, Shi Q, Zhang R, et al. Psoriasis patients suffer from worse periodontal status—a meta-analysis. Front Med (Lausanne). 2019;6:212.
- Bunte K, Beikler T. Th17 Cells and the IL-23/IL-17 Axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20(14):3394.
- Ucan Yarkac F, Ogrum A, Gokturk O. Effects of non-surgical periodontal therapy on inflammatory markers of psoriasis: a randomized controlled trial. J Clin Periodontol. 2020;47(2):
193–201. - Ogdie A, Grewal SK, Noe MH, et al. Risk of incident liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis: a population-based study. J Invest Dermatol. 2018;138(4):760–767.
- Wan J, Zhang HJ, Ogdie A, et al. Risk of liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis treated with methotrexate. Abstract presented at the 35th International Conference on Pharmacoepidemiology & Therapeutic Risk Management, Philadelphia, PA: August 24–28, 2019. Pharmacoepidemiol Drug Saf. 2019;28(52):5–585.
- Trafford AM, Parisi R, Kontopantelis E, et al. Association of psoriasis with the risk of developing or dying of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2019;155(12):1390–1403.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, Gelfand JM. The risk of cancer in patients with psoriasis: a population-based cohort study in the Health Improvement Network. JAMA Dermatol. 2016;152(3):282–290.
- Yang H, Brand JS, Li J, et al. Risk and predictors of psoriasis in patients with breast cancer: a Swedish population-based cohort study. BMC Med. 2017;15(1):154.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):
e285–e350. - Eder L, Harvey P, Chandran V, et al. Gaps in diagnosis and treatment of cardiovascular risk factors in patients with psoriatic disease: an international multicenter study. J Rheumatol. 2018;45(3):378–384.
- Noe MH, Shin DB, Wan MT, Gelfand JM. Objective measures of psoriasis severity predict mortality: a prospective population-based cohort study. J Invest Dermatol. 2018;138(1):228–230.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179.
- Wan MT, Shin DB, Hubbard RA, et al. Psoriasis and the risk of diabetes: a prospective population-based cohort study. J Am Acad Dermatol. 2018;78(2):315–322.e311.
- Lee JL, Sinnathurai P, Buchbinder R, et al. Biologics and cardiovascular events in inflammatory arthritis: a prospective national cohort study. Arthritis Res Ther. 2018;20(1):171.
- Crowley JJ, Warren RB, Cather JC. Safety of selective IL-23p19 inhibitors for the treatment of psoriasis. J Eur Acad Dermatol Venereol. 2019;33(9):1676–1684.
- Gelfand JM. Commentary: Does biologic treatment of psoriasis lower the risk of cardiovascular events and mortality?: a critical question that we are only just beginning to answer. J Am Acad Dermatol. 2018;79(1):69–70.
- Horreau C, Pouplard C, Brenaut E, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27 (Suppl 3):12–29.
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131
- Ridker PM, Everett BM, Pradhan A, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–762.
- Tardif J-C, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–2505.
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11(6):e007394.
- Mehta NN, Yu Y, Saboury B, et al. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147(9):1031–1039.
- Gelfand JM, Shin DB, Duffin KC, et al. A randomized placebo-controlled trial of secukinumab on aortic vascular inflammation in moderate-to-severe plaque psoriasis (VIP-S). J Invest Dermatol. 2020 Feb 21. [Epub ahead of print].
- International Psoriasis Council. Focus on Psoriasis: a report from the 2019 annual meeting of the American Academy of Dermatology. Available at: https://www.psoriasiscouncil.org/2019_aad_congress_report.htm#Contents. Accessed April 25, 2020.
- Van Vorhees A, Stein Gold L, Lebwohl B, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3, multicenter, randomized, placebo-controlled, double-blind study [195]. Skin. 2019;3(2).
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82(2):389–397.
- Paller AS, Seyger MMB, Magarinos GA, et al. Efficacy and safety of ixekizumab in a phase 3, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020 Apr 21. [Epub ahead of print].
- Blauvelt A, Papp K, Gottlieb A, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2020;182(6):1348–1358.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839.
- Bachelez H, Choon SE, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380(10):981–983.
- Papp K, Gordon K, Thaci D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379(14):1313–1321.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79(2):277–286.e210.
- Bukulev A, Samtsov A, Artemeva A, et al. Netakimab: 12-week results from PLANETA study, a phase III trial of a novel IL-17 inhibitor in moderate-to-severe plaque psoriasis. Available at. https://www.wcd2019milan-dl.org/abstract-book/documents/late-breaking-abstracts/35-psoriasis/netakimab-12-week-results-from-287.pdf. Accessed April 25, 2020.
- Ogdie A, Hur P, Liu M, et al. Prevalence of disease domain presentations among patients with psoriatic arthritis: results from the Corrona Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) Registry [2475]. Ann Rheum Dis. 2019;78:922–923.
- Guldberg-Moller J, Cordtz RL, Kristensen LE, Dreyer L. Incidence and time trends of joint surgery in patients with psoriatic arthritis: a register-based time series and cohort study from Denmark. Ann Rheum Dis. 2019;78(11):1517–1523.
- Pollock R, Machhar R, Chandran V, Gladman D. Characterizing the epigenomic landscape of psoriasis patients destined to develop psoriatic arthritis [OP0203]. Ann Rheum Dis. 2019;78:177–178.
- Mease PJ, Gladman DD, Collier DH, et al. Etanercept and methotrexate as monotherapy or in combination for psoriatic arthritis: primary results from a randomized, controlled phase 3 trial. Arthritis Rheumatol. 2019;71(7):1112–1124.
- Bravo A, Kavanaugh A. Bedside to bench: defining the immunopathogenesis of psoriatic arthritis. Nat Rev Rheumatol. 2019;15(11):645–656.
- Araujo EG, Englbrecht M, Hoepken S, et al. Effects of ustekinumab versus tumor necrosis factor inhibition on enthesitis: Results from the enthesial clearance in psoriatic arthritis (ECLIPSA) study. Semin Arthritis Rheum. 2019;48(4):632–637.
- Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol. 2019;71(2):258–270.
- Brodmerkel C, Li K, Garcet S, et al. Modulation of inflammatory gene transcripts in psoriasis vulgaris: differences between ustekinumab and etanercept. J Allergy Clin Immunol. 2019;143(5):1965–1969.
- Mease P, Smolen J, Behren F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–131.
- Mease PJ, Kavanaugh A, Reimold A, et al. Secukinumab in the treatment of psoriatic arthritis: efficacy and safety results through 3 years from the year 1 extension of the randomised phase III FUTURE 1 trial. RMD Open. 2018;4(2):e000723.
- Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFalpha inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115–1125.
- Mease P, Chohan S, Fructoso F, et al. Randomized, double-blind, placebo-controlled, multiple-dose, phase 2b study to demonstrate the safety and effiacy of tildrakizumagb, a high-affinity anti-interluekin-23p19 monocolonal antibody, in patients with active psoriatic arthritis [2878]. Available at: https://acrabstracts.org/abstract/randomized-double-blind-placebo-controlled-multiple-dose-phase-2b-study-to-demonstrate-the-safety-and-efficacy-of-tildrakizumab-a-high-affinity-anti-interleukin-23p19-monoclonal-antibody/. Accessed April 26, 2020.
- Ozeri D. Phase 2B trial of tildrakizumab shows promise in psoriatic arthritis. Available at: https://www.rheumatologynetwork.com/psoriatic-arthritis/phase-2b-trial-tildrakizumab-shows-promise-psoriatic-arthritis. Accessed April 26, 2020.
- Chao R, Kavanaugh A. Psoriatic arthritis: newer and older therapies. Curr Rheumatol Rep. 2019;21(12):75.
- Ogdie A, Liu M, Glynn M, et al. Real-world use of apremilast in combination with biologic therapies in patients with psoriatic arthritis: findings from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry [1481]. Available at: https://acrabstracts.org/abstract/real-world-use-of-apremilast-in-combination-with-biologic-therapy-in-patients-with-psoriatic-arthritis-findings-from-the-corrona-psoriatic-arthritis-spondyloarthritis-registry/. Accessed April 26, 2020.
- O’Shea JJ, Kontzias A, Yamaoka K, et al. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72 (Suppl 2):ii111–115.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–287.
- O’Sullivan LA, Liongue C, Lewis RS, Stephenson SE, Ward AC. Cytokine receptor signaling through the Jak-Stat-Socs pathway in disease. Mol Immunol. 2007;44(10):2497–2506.
- Lo Giudice L, Acosta Flequer M, Mazzuoccolo L, et al. Can biologics “prevent” the development of psoriatic arthritis in psoriasis patients? [2505]. Available at: https://acrabstracts.org/abstract/can-biologics-prevent-the-development-of-psoriatic-arthritis-in-psoriasis-patients-data-from-a-large-university-hospital-cohort-in-argentina/. Accessed April 26, 2020.
- Hoy SM. Crisaborole Ointment 2%: a review in mild to moderate atopic dermatitis. Am J Clin Dermatol. 2017;18(6):837–843.
- Skin permeation and penetration of crisaborole when coapplied with emollients. J Am Acad Dermatol. 2019;81(4):AB125.
- Simonsen L, Hoy G, Didriksen E, et al. Development of a new formulation combining calcipotriol and betamethasone dipropionate in an ointment vehicle. Drug Dev Ind Pharm. 2004;30(10):1095–1102.
- Ramachandran V, Bertus B, Bashyam AM, Feldman SR. Treating psoriasis with halobetasol propionate and tazarotene combination: a review of phase II and III clinical trials. Ann Pharmacother. 2020 Mar 4. [Epub ahead of print].
- Sugarman JL, Gold LS, Lebwohl MG, et al. A Phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16(3):197–204.
- Blauvelt A, Green LJ, Lebwohl MG, et al. Efficacy of a once-daily fixed combination halobetasol (0.01%) and tazarotene (0.045%) lotion in the treatment of localized moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2019;18(3):297–299.
- Halobetasol 0.01%/Tazarotene 0.045% lotion in the treatment of moderate-to-severe plaque psoriasis: maintenance of therapeutic effect after cessation of therapy. J Drugs Dermatol. 2019;18(8):815–820.
- Pariser DM, Green LJ, Stein Gold L, et al. Halobetasol 0.01%/tazarotene 0.045% lotion in the treatment of moderate-to-severe plaque psoriasis: maintenance of therapeutic effect after cessation of therapy. J Drugs Dermatol. 2018;17(7):723–726.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79(2):287–293.
- Stein Gold L, Bagel J, Lebwohl M, et al. Halobetasol and tazarotene: further defining the role of a unique fixed combination topical lotion in moderate-to-severe plaque psoriasis. J Drugs Dermatol. 2018;17(12):1290–1296.
- Giovene GL, Giacomelli L. Calcipotriene plus betamethasone dipropionate in aerosol foam formulation: will this effective treatment for mild-to-moderate psoriasis change clinical practice? G Ital Dermatol Venereol. 2018;153(6):872–876.
- Menter A, Gold LS, Koo J, et al. Fixed-combination calcipotriene plus betamethasone dipropionate aerosol foam is well tolerated in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. Skinmed. 2017;15(2):119–124.
- United States Food and Drug Administration. Highlights of prescribing information: ENSTILAR (calcipotriene and betamethasone dipropionate) foam for topical use. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207589s005s006s007lbl.pdf. Accessed April 27, 2020.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: A double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82(2):360–365.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof Is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137(10):2110–2119.
- Robbins K, Bissonnette R, Maeda-Chubachi T, et al. Phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of plaque psoriasis. J Am Acad Dermatol. 2019;80(3):714–721.
- Palmieri B, Laurino C, Vadala M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin Ter. 2019;170(2):e93–e99.

