J Clin Aesthet Dermatol. 2025;18(7):24–25.
by Erik Domingues, MD
Dr. Domingues is with Modern Dermatology of Massachusetts in Fall River, Massachusetts and the Department of Dermatology at the University of Massachusetts Chan Medical School in Worcester, Massachusetts.
FUNDING: No funding was provided for this article.
DISCLOSURES: Writing support was provided by Arcutis Biotherapeutics Inc.
Abstract: Scalp psoriasis is a chronic immune skin disease affecting up to 80 percent of patients with psoriasis. Treatments include topical steroids, keratolytics, tar, anthralin, vitamin D analogs, and retinoids. However, treatment can be difficult due to the presence of hair. Roflumilast 0.3% cream was approved in 2022 for the treatment of chronic plaque psoriasis, including intertriginous areas. Here, we present the case of a 28-year-old female patient with scalp psoriasis, who had previously trialed both topical and systemic treatments for her disease without success. Use of once daily roflumilast 0.3% cream resulted in rapid scalp psoriasis clearance, with results seen as early as Day 3 and complete clearance by Day 5 of treatment without adverse events or tolerability concerns. Keywords: Scalp psoriasis, roflumilast cream, phosphodiesterase-4 inhibitor
Introduction
Scalp psoriasis is a chronic immune skin disease with variable phenotypes and is expected to affect almost 80 percent of patients who suffer from psoriasis.1,2 Undertreated scalp psoriasis can result in a significant impact on a patient’s quality of life.3 Management of scalp psoriasis can be challenging despite numerous topical and systemic treatments available, underscoring a persistent unmet need for safe and effective therapies.2 Treatment of scalp psoriasis can be arduous due to the presence of hair, which makes topical application difficult and can affect efficacy as well as treatment adherence.1,2 Topical treatments for scalp psoriasis include topical corticosteroids, keratolytics, tar, anthralin, vitamin D analogs, and retinoids. Systemic agents include methotrexate, cyclosporine, oral retinoids, injectable biologics, and oral small molecules.1 Roflumilast cream 0.3% is a non-steroidal, highly selective, and potent topical phosphodiesterase-4 (PDE4) inhibitor approved in 2022 by the United States Food and Drug Administration (FDA) for the treatment of chronic plaque psoriasis, including intertriginous areas and in 2023 in a foam formulation for the treatment for seborrheic dermatitis.4,5 A Phase III study investigating once daily treatment with roflumilast foam 0.3% in patients with scalp and body psoriasis showed significant clearance of affected areas including scalp and rapid improvement in pruritus and was well tolerated.6 Roflumilast has demonstrated a higher affinity for binding to PDE4 relative to other approved PDE4 inhibitors, resulting in greater potency.7,8 Here, we report the case of a 28-year-old female patient with a 10-year history of recalcitrant scalp psoriasis successfully treated with topical roflumilast cream 0.3% once daily, with rapid improvement in five days.
Case Report
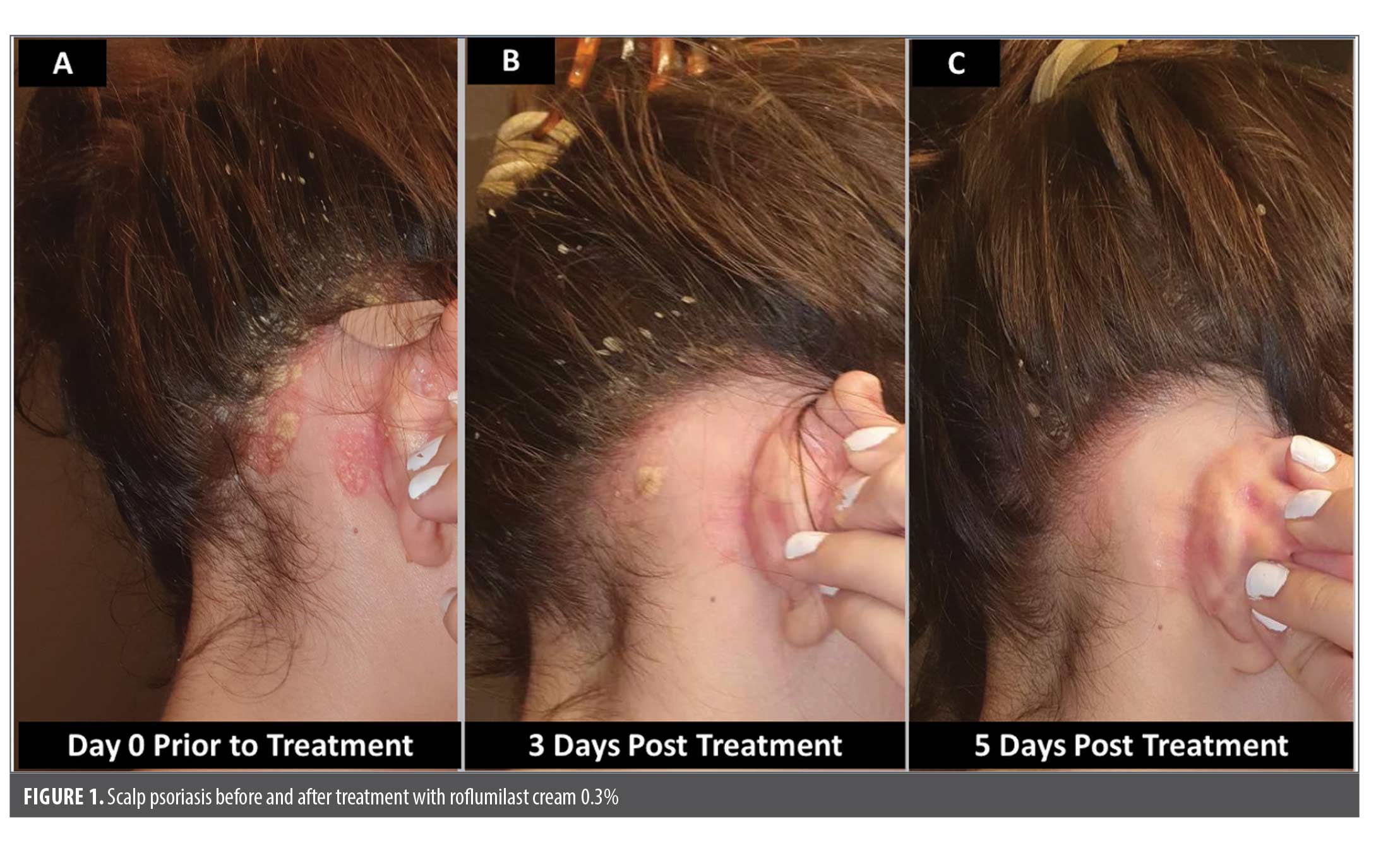
A 28-year-old female patient presented to clinic with a medical history of psoriasis involving scalp, nails, abdomen, and intertriginous areas. Her psoriasis was refractory and uncontrolled for 10 years despite treatments with multiple topical agents (Figure 1A). Prior topical treatments included calcipotriene and coal tar shampoo, both of which were ineffective, tacrolimus, which was discontinued due to stinging, and apremilast, which was discontinued due to night terrors and depression. The patient was reluctant to go on systemic therapy due to the severity of adverse effects from prior therapies.
Treatment options were discussed with the patient and the decision was made to start topical roflumilast cream 0.3% once daily due to its anti-inflammatory, non-steroidal mechanism of action. Rapid improvement was observed as early as three days after treatment, with clearance of scalp lesions noted by Day 5 (Figure 1B–C). Treatment with roflumilast 0.3% cream was well tolerated and no adverse reactions were noted. Upon clearance of the lesions, the patient discontinued application of roflumilast cream 0.3%. The lesions remained clear for several weeks, with the cream subsequently being applied for any recurrent lesions.
Conclusion
This case report of a 28-year-old female patient treated with roflumilast cream 0.3% once daily for recalcitrant scalp psoriasis resulted in rapid improvement within five days. Treatment with roflumilast cream 0.3% was well tolerated compared to discontinued topical therapies and improvement was documented as early as three days after treatment. This report demonstrates the significant potential of roflumilast cream 0.3% in addressing the inflammatory etiology of scalp psoriasis and suggests roflumilast cream 0.3% can be a safe and effective alternative topical treatment option for scalp psoriasis. Additional clinical research will be necessary to understand the efficacy and safety of roflumilast 0.3% cream for the treatment of scalp psoriasis.
References
- Wang TS, Tsai TF. Managing scalp psoriasis: an evidence-based review. Am J Clin Dermatol. 2017;18(1):17–43.
- Ghafoor R, Patil A, Yamauchi P, et al. Treatment of scalp psoriasis. J Drugs Dermatol. 2022;21(8):833–837.
- Blakely K, Gooderham M. Management of scalp psoriasis: current perspectives. Psoriasis (Auckl). 2016;6:33–40.
- ZORYVE (roflumilast) topical cream [prescribing information online]. Westlake Village, CA: Arcutis Biotherapeutics. July 2022. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- ZORYVE (roflumilast) topical foam [prescribing information online]. Westlake Village, CA: Arcutis Biotherapeutics. December 2023. https://www.arcutis.com/wp-content/uploads/zoryve-foam-pi-hcp.pdf
- Gooderham M, Alonso-Llamazares J, Bagel J, et al. Roflumilast foam 0.3% in patients with scalp and body psoriasis in the phase 3 ARRECTOR trial: Efficacy, patient-reported outcomes, and safety. Paper presented at: European Academy of Dermatology & Venereology Congress; October 11–14, 2023; Berlin, Germany.
- Wang J, Bunick CG. Clinically relevant differences in the chemical and structural mechanism of action of dermatological phosphodiesterase-4-inhibitors. J Invest Dermatol. 2023.143(5):S194.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358(3):413–422.
