 J Clin Aesthet Dermatol. 2021;14(12):24-28.
J Clin Aesthet Dermatol. 2021;14(12):24-28.
by Rachel L. Marsh, MD; Sean Kelly, MD; Khalid Mumtaz, MBBS; and Jessica Kaffenberger, MD
Drs. Marsh and Kaffenberger are with the Dpartment of Internal Medicine, Division of Dermatology at The Ohio State University Wexner Medical Center in Columbus, Ohio. Drs. Kelly and Mumtaz are with the Department of Internal Medicine, Division of Gastroenterology, Hepatology, and Nutrition at The Ohio State University Wexner Medical Center in Columbus, Ohio.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. Psoriasis is associated with hepatic steatosis, fibrosis, and methotrexate-associated liver injury. There is a need for reliable methods to monitor liver disease in psoriasis. Transient elastography (TE) is a validated non-invasive method for assessing hepatic steatosis and fibrosis. Psoriasis-specific TE studies have been limited until recently. Here, we review the utility and limitations of TE to detect and monitor liver disease in the context of psoriasis.
Methods. A comprehensive search using OVID, PubMed, and gray literature was conducted (2005–November 2019) to identify studies of TE use in psoriasis for assessment of hepatic steatosis and fibrosis.
Results. Fifteen studies met inclusion criteria. A total of 1,536 patients with psoriasis or psoriatic arthritis were represented. TE-detected liver fibrosis is associated with age, diabetes, obesity, and severity of psoriasis. TE successfully evaluates hepatic steatosis and fibrosis. Elastography has a high negative predictive value and specificity in the context of methotrexate-associated liver fibrosis in psoriasis; however, reported associations between abnormal elastography results and cumulative methotrexate dose varied significantly despite methotrexate’s association with hepatotoxicity and fibrosis. The presence of central adiposity is associated with increased TE failure rate.
Limitation. The TE studies included in this review date from 2007 to 2019, which could contribute to publication bias, as the technique of TE has improved over this time period.
Conclusion. TE is a useful and non-invasive modality to detect hepatic steatosis and fibrosis in psoriasis. Dermatologists might consider TE in psoriatic patients and concomitant risk factors for fibrosis with the understanding that failure rates may be higher in patients with central adiposity.
Key Words. Transient elastography, psoriasis, liver disease, hepatic fibrosis, hepatic steatosis, methotrexate, nonalcoholic fatty liver disease, Fibroscan, hepatotoxicity
Psoriatic skin disease is associated with increased risk of hepatic injury, including steatosis and or fibrosis, contributing to significant morbidity and mortality. Chronic inflammation, systemic medications, and comorbidities (including obesity, hyperlipidemia, and diabetes mellitus) mediate hepatic steatosis/fibrosis in psoriatic patients.1–3 Early identification of asymptomatic patients with hepatic steatosis/fibrosis is necessary to guide therapeutic management and combat liver-related morbidity and mortality. Liver function test (LFT) abnormalities are nonspecific and unreliable in detecting psoriasis-associated hepatic steatosis/fibrosis. Liver biopsy has been considered the gold standard for diagnosis of hepatic steatosis/fibrosis in psoriasis, but it is invasive, expensive, and can be subject to sampling errors.4 Patients might remain asymptomatic until the onset of advanced fibrosis, contributing to a divergence between clinical evaluations and pathologic findings of liver disease on biopsy. Additionally, the prevalence of methotrexate (MTX)-associated hepatotoxicity in psoriasis might vary based on co-existing risk factors. There is a pressing need for reliable non-invasive methods to detect hepatic steatosis/fibrosis in psoriasis.
Transient elastography (TE) using FibroScan® (Echosens; Paris, France) is a rapid ultrasound-based imaging technique that evaluates liver stiffness measurement (LSM) to assess severity of hepatic disease in outpatient setting. TE measures the velocity of propagating ultrasound waves to determine LSM, measured as the median of at least 10 measurements and recorded in kilopascal (kPa) units. Transmission of vibrations increases with hepatic fibrosis, resulting in higher kPa values.5 TE assesses fibrosis with high accuracy and has reproducibility in patients with chronic liver diseases, including viral hepatitis, alcoholic liver damage, and non-alcoholic fatty liver disease (NAFLD).5–7 TE also assesses hepatic steatosis using controlled attenuation parameter (CAPTM) to measure fatty changes based on attenuation of ultrasound signal acquired through TE. Healthy hepatic tissue attenuates ultrasound signal at lower levels (150–200 dB/m) than fatty liver tissue (300–400 dB/m).8 There is clinical interest in utilizing TE in psoriasis as a diagnostic alternative to biopsy, but, until recently, there have been limited studies on the efficacy of TE in liver disease assessment in psoriasis. As a result, TE is not yet a well-established tool in dermatologic clinical practice.
Here, we review the utility and limitations of TE in monitoring hepatic steatosis/fibrosis in psoriasis.
Methods
We conducted a comprehensive search strategy using the PRISMA checklist as a guide. A systematic literature search was performed using the following search terms: “psoriasis” AND (“transient elastography” OR “fibroscan” OR “liver stiffness”) which resulted in 1,170 potential studies screened for retrieval (160 OVID articles, 24 PubMed articles, 986 articles on gray literature Google Scholar search). The search covered articles from November 2005 through November 2019. Studies were individually reviewed for the following selection criteria:
- Adult participants (i.e., at least 18 years of age)
- TE results reported in patients with psoriasis or psoriatic arthritis independently of other conditions
- Evaluation of hepatic tissue
- Use of English language
Exclusion criteria were:
- Non-peer reviewed papers
- Review articles, corresponding letters, or editorials that did not report their own results
- Abstracts with data that have been published as full-length articles
- Exclusion of psoriasis patients
- Did not utilize transient elastography
- Did not evaluate hepatic tissue
Titles and abstracts from search results were screened, and full articles that met the selection criteria were obtained for review. Studies were assessed using the Quality Rating Scheme, which uses a four-point scale, as follows:
- A score of 1 indicates properly powered and conducted randomized clinical trial or systematic review with meta-analysis.
- A score of 2 indicates well-designed controlled trial without randomization or prospective comparative cohort trial.
- A score of 3 indicates case-control studies or retrospective cohort study.
- A score of 4 indicates case series with or without intervention or cross-sectional study.
- A score of 5 indicates the opinion of respected authorities or case reports.
TE values in psoriasis patients have been compared with two different histopathologic scoring systems: METAVIR stages (F0–F4) and Roenigk classification (1–4). METAVIR scoring was initially designed and validated for patients with hepatitis C and is predominantly used to measure liver fibrosis with scores F greater than equal to 2 indicative of fibrosis. The Roenigk classification for psoriasis was developed by the Psoriasis Task Force as a histopathologic reference to measure methotrexate hepatotoxicity (greater than or equal to 3a) in psoriasis patients (Table 1).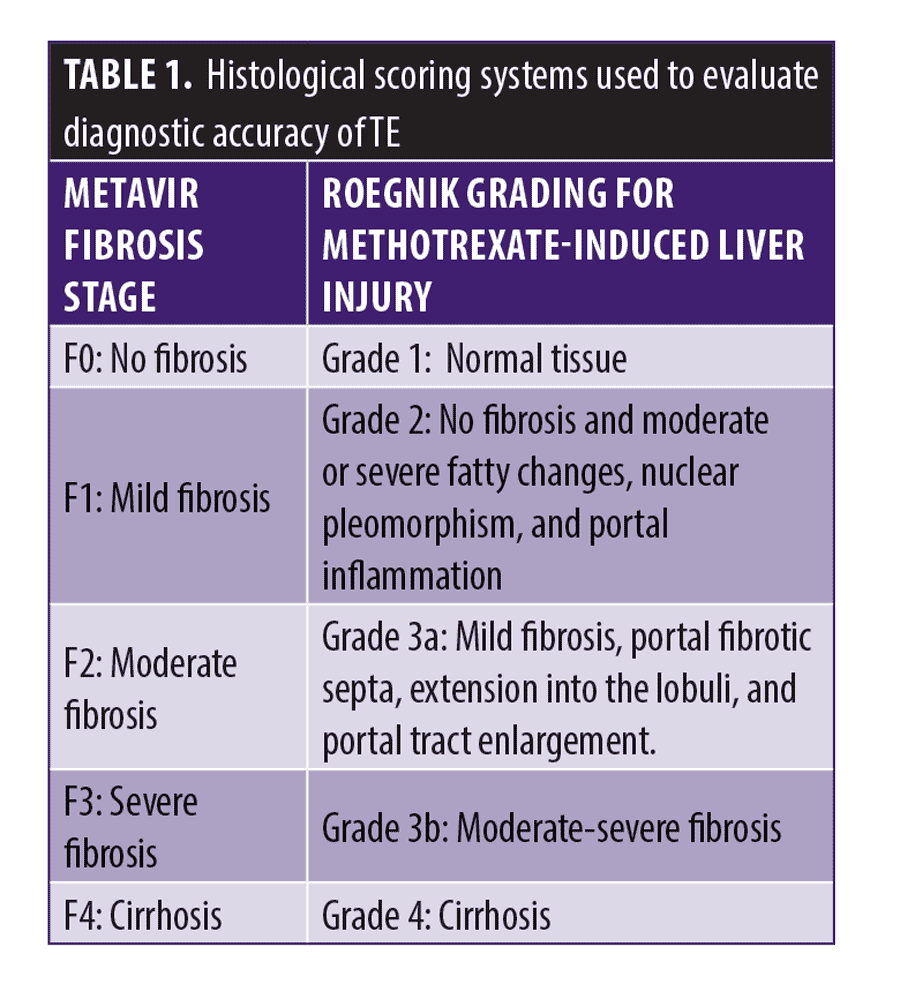
Results
The initial search identified 1,170 study references, and 93 full-text articles were assessed for eligibility. A total of fifteen studies comprising 11 cross-sectional studies and four case-control studies were included in the final analysis, with 1,536 patients with psoriasis or psoriatic represented (Table 2).
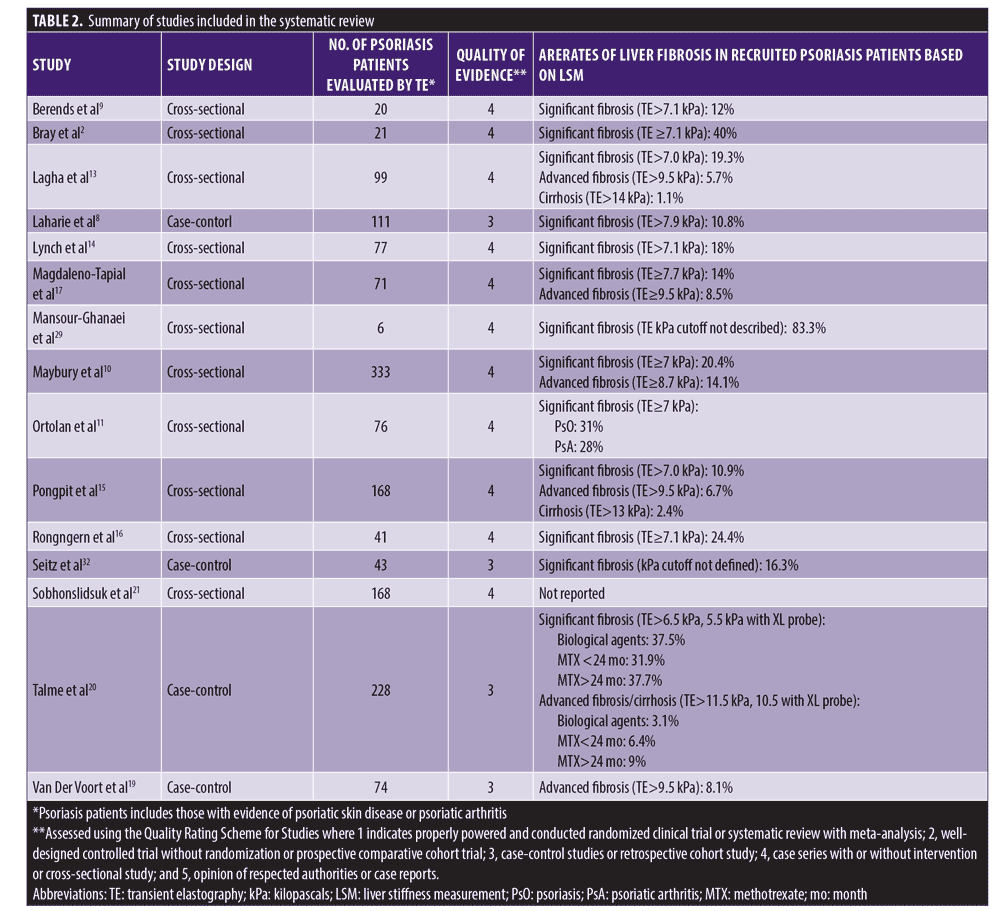
TE-detected hepatic fibrosis and MTX-associated hepatic fibrosis based on METAVIR and Roenigk scoring. During the study period, rates of TE-detected significant fibrosis (F greater than or equal to 2) in psoriasis were reported using various cutoff scores (Table 2) and were validated by METAVIR scoring of histopathology. Significant fibrosis was reported in 12 to 31 percent of recruited psoriatic patients using a cutoff at greater than or equal to 7.0 kPa and 10.9 to 40 percent using a cutoff at greater than or equal to greater than or equal to 7.1 kPa.9–16 Higher cutoffs at greater than or equal to 7.7 kPa and > 7.9 kPa yielded rates of 14 percent and 10.8 percent, respectively.17,18 TE detected advanced liver fibrosis in 14.1 percent of recruited patients with psoriasis using a cutoff of greater than or equal to 8.7 kPa, an estimated seven-fold increase compared to the advanced fibrosis in the general population.10 Advanced fibrosis defined by cutoff at 9.5 kPa yielded rates of 5.7 percent, 6.1 percent, and 8.5 percent in studied psoriasis patients aged 18 years or older, and 8.1 percent of studied psoriasis patients aged 55 years or older.13,15,17,19 TE-detected cirrhosis was significantly less, reported in only two studies in 1.1 to 2.4 percent of patients.3,15 Factors associated with TE-detected liver fibrosis included age, insulin resistance or diabetes, obesity, steatosis, and severity of psoriasis.10,11,13–15,19,20
Overall TE accuracy rates when validated by the METAVIR histology scoring for fibrosis were similar across studies at 70 to 76 percent. High negative predictive values (NPV) of 86 to 100 percent and low positive predictive values (PPV) of 10 to 33 percent were also reported. TE was reported to be 50 to 100 percent sensitive and 50 to 88 percent specific in detecting liver fibrosis compared to METAVIR scores on liver biopsy.9,12,15,16,18 Two psoriasis-specific studies concluded that 7 of 21 and 3 of 5 liver biopsies could have been avoided based on normal TE findings in conjunction with serum levels of procollagen III peptide, a biomarker of collagen turnover to assess hepatic fibrosis in patients on long-term MTX.12,14
Four studies evaluated the diagnostic accuracy of TE-detected MTX-associated liver injury based on Roenigk staging. A cutoff TE value of 7.1 kPa was 50- to 100-percent sensitive and 66.7- to 84-percent specific with a 75.6-percent accuracy level. Studies also reported high NPVs (83.9–100%) and lower PPVs (25–50%).12,16 Talme et al20 reported liver fibrosis (Roenigk greater than or equal to 3a) confirmed on biopsy in 4 of 6 patients with TE-detected severe liver fibrosis (greater than or equal to 11.5 kPa) and a history of MTX exposure longer than 24 months.20 In another study, five patients on MTX underwent liver biopsy; two had fibrosis on biopsy (Roenigk greater than or equal to 3a), one of which had TE-detected fibrosis (TE greater than or equal to 7.1 kPa), and TE scan was invalid in the other (body mass index [BMI] of 34.7 kg/m2). Of three patients without evidence of MTX-associated fibrosis on biopsy (Roenigk < 3a), TE results were normal in two patients and TE was not performed on the third patient.14
Prevalence and diagnostic accuracy of TE-detected NAFLD. One study investigated the utility of TE-detected NAFLD using controlled attenuation parameter (CAP) which measures liver fat from ultrasound signals of TE.21 NAFLD was reported in 63.6 percent of patients with psoriasis, with an odds ratio of 1.05 between CAP and NAFLD after adjusting for BMI and hypertension. This method was 79-percent sensitive and 82-percent specific in detecting mild fatty liver (>238 dB/m) and demonstrated higher sensitivity (95%) and specificity (88.3%) in diagnosing severe NAFLD (>315 dB/m) compared to ultrasonography.
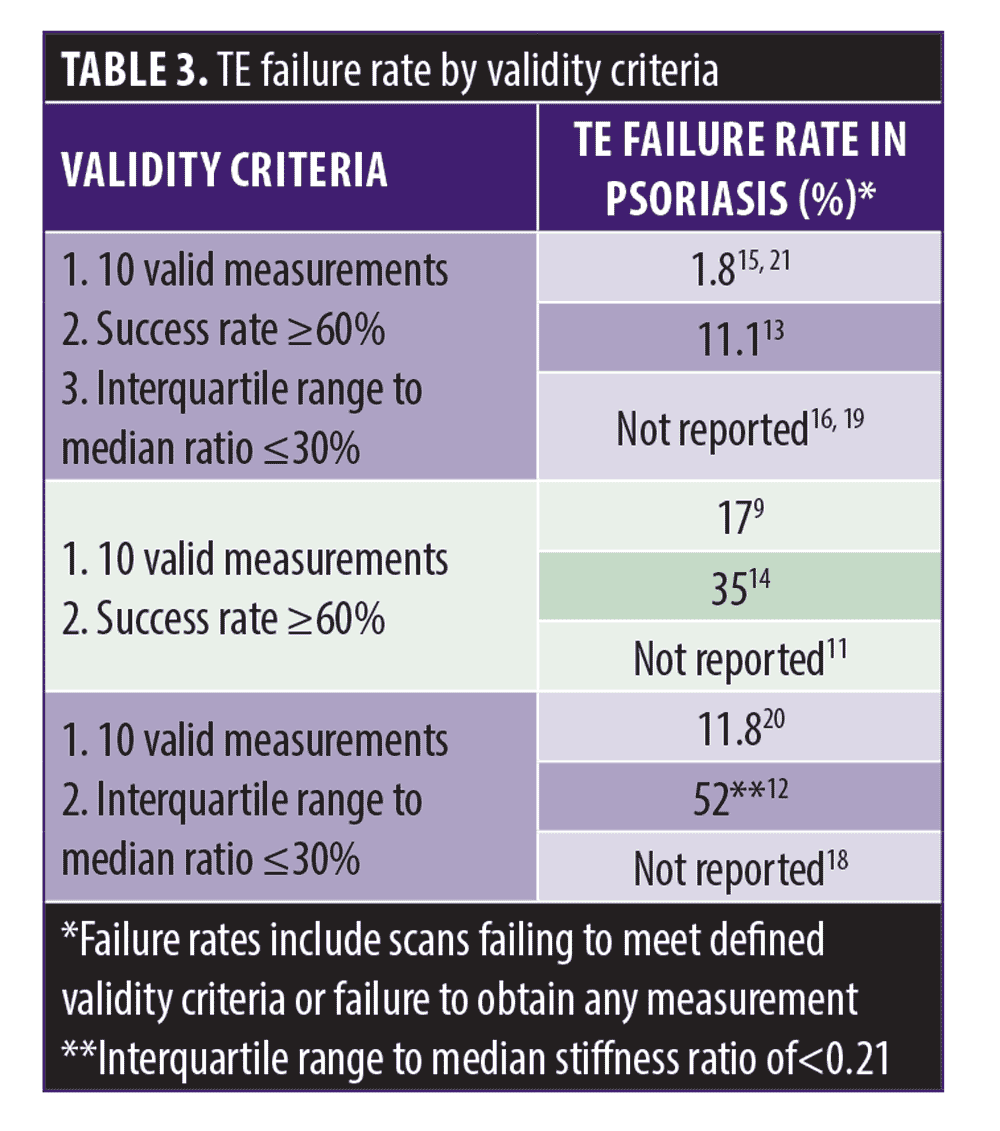
TE failure rate. Psoriasis studies utilized different validity criteria to characterize TE failure rates (Table 3). Failure rates included scans failing to meet defined validity criteria or inability to obtain any TE measurement. The most commonly cited reason for TE failure in psoriatic patients was obesity.9,12,14,20 Bray et al reported 52 percent of patients with invalid scans were due to obesity alone and BMI over 25 kg/m2 predicted an invalid TE result in a study by Lynch et al.12, 14 Similarly, Bray et al reported relative risk of four of scan failure for those with a BMI >30 kg/m2.12 Three studies utilized XL probes designed for adults with overweight with a skin to liver capsule distance exceeding 25mm, and two commented on limited success.12,19,20
Discussion
Methotrexate therapy in patients with psoriatic skin disease is associated with hepatic dysfunction in the form of hepatic steatosis/fibrosis, leading to morbidity and mortality. There is a pressing need for reliable non-invasive methods to detect hepatic changes in psoriasis, combat liver-related morbidity and mortality, and guide therapeutic management. Dermatologists should be aware of the clinical utility and limitations of TE as a non-invasive tool to monitor the presence and progression of liver disease in the context of psoriatic disease.
TE scans are deemed reliable if there at least 10 valid measurements at the same spot with one probe, the interquartile range/median (IQR/M) value less than or equal to 30 percent when final median stiffness exceeds 7.1 kPa, and/or the success ratio (SR, defined as the ratio of valid measurements to the total number of acquisitions) is at least 60 percent.22 Psoriasis-specific TE failure rates are reported in Table 3. Obesity was highly associated with TE failure, paralleling studies in non-psoriatic patients with chronic liver disease.23, 24 Success rate of TE scans is inversely correlated with central adiposity.23 Some speculate central obesity impacts TE failure more significantly than BMI because extra hepatic adipose tissue interferes with the transmission and measurement of propagating mechanical shear waves by transient elastography, thereby altering LSM.24 To improve TE reliability, special XL probes have been designed for adults with overweight. These contain a sensitive ultrasonic transducer that utilizes deeper focal length and larger vibration amplitude to enable deeper measurements below the skin surface.24 However, XL probes were utilized with limited success in psoriatic patients in 2 of 3 studies.12,19,20
TE was effective in excluding advanced stages of liver fibrosis (as measured by METAVIR scoring) in psoriatic patients as evidenced by high reported NPVs (86–100%) and accuracy rates (70–76%).9,12,16 Similarly, TE had high NPV (83.9–100%) and specificity (66.7-84%) when identifying methotrexate-associated liver injury via the Roenigk classification.12,16 Variable rates of fibrosis progression due to timing differences between TE scans and liver biopsy might explain differences in reported sensitivity and specificity across the psoriasis studies evaluated. Meta-analyses evaluating chronic liver diseases in non-psoriasis patients have reported pooled estimates of 70- to 72-percent sensitivity and 82- to 84-percent specificity in TE-detected liver fibrosis (F2) in the setting of chronic liver disease.25,26 These meta-analyses reported higher pooled sensitivity (84.5–87%) and specificity (91–95%) of TE-detected liver cirrhosis (F4), suggesting the degree of liver fibrosis might influence TE diagnostic accuracy.25,26 However, cirrhosis-specific (F4) sensitivity and specificity levels were not evaluated in TE psoriasis studies. Further, TE studies included in this review date from 2007 to 2019, contributing to publication bias as the technique of TE has improved over this time period.
Associations between abnormal TE results and cumulative dose or duration of MTX therapy varied significantly across studies despite methotrexate’s known hepatotoxic effects. Long-term methotrexate therapy has been correlated with the development of hepatic steatosis, fibrosis, and cirrhosis. While the exact mechanism is incompletely understood, it is hypothesized that MTX induces cellular arrest via inhibition of RNA and DNA synthesis to induce hepatic dysfunction.27 A positive association between LSM and cumulative methotrexate dose was reported in psoriasis and RA patients.13,28 In contrast, eight psoriasis studies reported no association between liver fibrosis and cumulative MTX dose.9,10,14–16,18,20,29 However, these studies might be limited by the number of patients with advanced fibrosis, minimal variability of MTX doses, and duration of MTX use. MTX-induced fibrosis and cirrhosis typically occurs after 2 to 10 years of treatment.27 A study in RA patients suggests significant differences in LSM are observed only when the cumulative dose of MTX exceeds 4,000mg.28 Patients on higher doses of oral MTX therapy and daily dosing regimens have a 20 percent cirrhosis risk after 5 to 10 years of treatment. In contrast, weekly dosing regimens of 5 to 15mg of oral MTX with folate supplementation are associated with improved clinical efficacy and reduced risk of liver disease, even with longitudinal MTX use.2,27,30
High BMI also correlates with the severity of TE-detected fibrosis in patients on methotrexate.14,20,29 Patients with psoriasis and concomitant obesity, diabetes, and metabolic syndrome have an increased risk of liver fibrosis in the setting of MTX, and evidence suggests MTX might accelerate the progression of steatohepatitis to fibrosis, especially in psoriatic patients with comorbidities.15,20,31 However, high BMI is correlated with increased risk of TE failure.9,12,14,20 Therefore, exclusion of patients with obesity who are more likely to have MTX-associated fibrosis might serve as a limitation in the studies correlating TE results with MTX usage. Nonetheless, these findings suggest MTX use is not the solitary factor mediating hepatic changes in psoriasis, and concurrent comorbidities likely compound the effects of MTX-associated liver damage. This is also evident as studied psoriasis patients 55 years and older with NAFLD had a four-fold risk of advanced liver fibrosis compared to a reference population independent of systemic anti-psoriatic drugs and other known risk factors associated with liver fibrosis.19
Dermatologists might therefore consider TE prior to initiating systemic MTX, particularly in psoriatic patients with concomitant risk factors for fibrosis, including obesity, insulin resistance, excessive alcohol use, and steatosis, who might not otherwise receive hepatic screening. Additionally, since TE has high NPV and specificity for liver fibrosis, it may be an excellent tool in excluding liver fibrosis before patients start MTX therapy.16
Conclusion
TE is a useful, non-invasive technique to identify hepatic steatosis and fibrosis in patients with psoriasis. TE has a high NPV and specificity in the context of MTX and liver fibrosis, and, thus, might be efficacious in monitoring patients before and during MTX therapy. Dermatologists might also consider TE in psoriatic patients with concomitant risk factors for fibrosis, including obesity, insulin resistance, and steatosis, who might not otherwise receive hepatic screening. However, TE failure rates and false positive findings might be higher in patients with psoriasis and obesity and central adiposity, thus, limiting the clinical applicability of TE. Additional high-quality psoriasis-specific prospective studies with large cohorts are warranted to further characterize the diagnostic accuracy of TE, improve TE efficacy in patients with obesity and formulate evidence-based guidelines for liver monitoring in psoriasis
References
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46(6):1111-8.
- Abedini R, Salehi M, Lajevardi V, Beygi S. Patients with psoriasis are at a higher risk of developing nonalcoholic fatty liver disease. Clin Exp Dermatol. 2015;40(7):722-7.
- Ganzetti G, Campanati A, Offidani A. Non-alcoholic fatty liver disease and psoriasis: So far, so near. World J Hepatol. 2015;7(3):315-26.
- Gilmore IT, Burroughs A, Murray-Lyon IM, et al. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36(3):437-41.
- Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835-47.
- Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134(4):960-74.
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56(7):968-73.
- Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29(7):1470-6.
- Berends MA, Snoek J, de Jong EM, et al. Biochemical and biophysical assessment of MTX-induced liver fibrosis in psoriasis patients: Fibrotest predicts the presence and Fibroscan predicts the absence of significant liver fibrosis. Liver Int. 2007;27(5):639-45.
- Maybury CM, Porter HF, Kloczko E, et al. Prevalence of Advanced Liver Fibrosis in Patients With Severe Psoriasis. JAMA Dermatol. 2019;155(9):1028-1032
- Ortolan A, Lorenzin M, Tadiotto G, et al. Metabolic syndrome, non-alcoholic fatty liver disease and liver stiffness in psoriatic arthritis and psoriasis patients. Clin Rheumatol. 2019;38(10):2843-2850.
- Bray AP, Barnova I, Przemioslo R, Kennedy CT. Liver fibrosis screening for patients with psoriasis taking methotrexate: a cross-sectional study comparing transient elastography and liver biopsy. Br J Dermatol. 2012;166(5):1125-7.
- Lagha IB, Jaber K, Rabhi F, et al. Psoriasis and liver fibrosis: an investigation using transient elastography in Tunisian patients with psoriasis. Br J Dermatol. 2019.
- Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150(8):856-62.
- Pongpit J, Porntharukchareon S, Kaewduang P, et al. Liver stiffness measurement in psoriasis: do metabolic or disease factors play the important role? Biomed Res Int. 2016:7963972.
- Rongngern P, Chularojanamontri L, Wongpraparut C, et al. Diagnostic performance of transient elastography for detection of methotrexate-induced liver injury using Roenigk classification in Asian patients with psoriasis: a retrospective study. Arch Dermatol Res. 2017;309(5):403-8.
- Magdaleno-Tapial J, Valenzuela-Onate C, Ortiz-Salvador JM, et al. Prevalence of non-alcoholic fatty liver and liver fibrosis in patients with moderate-severe psoriasis: A cross-sectional cohort study. Australas J Dermatol. 2019.
- Laharie D, Seneschal J, Schaeverbeke T, et al. Assessment of liver fibrosis with transient elastography and FibroTest in patients treated with methotrexate for chronic inflammatory diseases: a case-control study. J Hepatol. 2010;53(6):1035-40.
- van der Voort EA, Koehler EM, Nijsten T, et al. Increased prevalence of advanced liver fibrosis in patients with psoriasis: a cross-sectional analysis from the Rotterdam Study. Acta Derm Venereol. 2016;96(2):213-7.
- Talme T, Nikamo P, Rosenberg P, Stahle M. Transient elastography may improve detection of liver fibrosis in psoriasis patients treated with methotrexate. Acta Derm Venereol. 2017;97(8):952-4.
- Sobhonslidsuk A, Pongpit J, A. T, et al. The evaluation of non-alcoholic fatty liver disease (NAFLD) and its associate factors in psoriasis patients using ultrasonography and the controlled attenuation parameter (CAP) measured with transient elastography. J Hepatol. 2015;62:S732-S7333.
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97(10):2614-8.
- Kettaneh A, Marcellin P, Douvin C, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007;46(4):628-34.
- Myers RP, Pomier-Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55(1):199-208.
- Stebbing J, Farouk L, Panos G, et al. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010;44(3):214-9.
- Talwalkar JA, Kurtz DM, Schoenleber SJ, et al. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5(10):1214-20.
- Methotrexate. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD)2012.
- Arena U, Stasi C, Mannoni A, et al. Liver stiffness correlates with methotrexate cumulative dose in patients with rheumatoid arthritis. Dig Liver Dis. 2012;44(2):149-53.
- Mansour-Ghanaei F EA, Shafaghi A, Joukar F, Hajiabasi A, et al. Transient elastography in methotrexate administered patients. Hepatitis Monthly. 2017;17(8):e57917.
- Radmanesh M, Rafiei B, Moosavi ZB, Sina N. Weekly vs. daily administration of oral methotrexate (MTX) for generalized plaque psoriasis: a randomized controlled clinical trial. Int J Dermatol. 2011;50(10):1291-3.
- Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol. 2009;60(5):824-37.
- Seitz M, Reichenbach S, Moller B, et al. Hepatoprotective effect of tumour necrosis factor alpha blockade in psoriatic arthritis: a cross-sectional study. Ann Rheum Dis. 2010 Jun;69(6):1148-50

