J Clin Aesthet Dermatol. 2025;18(5–6 Suppl 1):20–22.
by Melodie Young, MSN, A/GNP-c
Ms. Young is with Mindful Dermatology and Modern Research Associates, Dallas, Texas.
Funding: Medical writing assistance was provided by Arcutis Biotherapeutics, Inc.
Disclosures: Ms. Young has been an investigator for AbbVie, Alumis, Amgen, AnaptysBio, ASLAN Pharmaceuticals, Boehringer Ingelheim, Cara Therapeutics, Incyte, Janssen, Lilly, Novartis, Pfizer, Sanofi, Takeda, and UCB; received consulting fees from Arcutis, Dermavant, Janssen, LEO Pharma, Novartis, Sanofi, Sun Pharma, and UCB; and received honoraria for speakers bureaus from Amgen, Arcutis, Boehringer Ingelheim, Dermavant, Janssen, LEO Pharma, Lilly, Sun Pharma, and UCB.
ABSTRACT: Despite the development of advanced targeted topical treatments for chronic inflammatory skin diseases and recent calls for caution when prescribing topical corticosteroids (TCS), TCS continue to be the most prescribed dermatologic agent. Yet the perception that adverse effects are uncommon with TCS use persists. To combat this misperception, I selected examples of patients from my practice that illustrate the real-world risks associated with TCS use. This case series provides several key clinical pearls for managing TCS use in everyday clinical practice including: 1) Even using TCS for short periods and as prescribed is not without risks. 2) Long-term TCS use (even intermittent use) can result in permanent skin changes that can even outlast the disease the TCS was used to treat. 3) TCS overuse can have serious adverse effects resulting in hospitalization, including adrenal suppression, infection, and even sepsis. 4) Patients who are dissatisfied with older TCS-sparing treatments might continue to seek more potent TCS, resulting in doctor/clinic hopping and potential TCS misuse. 5) Patients might repurpose TCS prescriptions for other body areas, leading to high-potency TCS being applied to thin-skinned areas and irreversible skin damage. KEYWORDS: Complications, adverse effect, topical corticosteroid
Introduction
Despite the development of advanced targeted topical treatments for chronic inflammatory skin diseases, topical corticosteroids (TCS) continue to be the most prescribed dermatologic agent1 due to their low cost, ease of access through insurance, and broad mechanism of action, including their anti-inflammatory, antiproliferative, and immunosuppressive activity.2 A recent consensus statement called for a cautious approach to prescribing TCS for chronic inflammatory skin diseases recommending that TCS, especially high- and very high–potency TCS, be used only in an acute setting for inflammatory diseases, and not maintained as long-term therapy.3 This consensus panel publication follows recent statements from regulatory bodies and patient advocacy groups calling to limit the use of TCS due to well-documented risks and safety concerns associated with their use.4–9 The adverse events associated with TCS use include both cutaneous (eg, atrophy, telangiectasia, striae, purpura) and systemic effects (eg, glaucoma, hyperglycemia, diabetes mellitus, decreased bone density, osteoporosis, increased risk of bone fracture, cardiovascular effects, Cushing syndrome).2,3,10–14 While the risk of cutaneous and systemic adverse effects with TCS use have been extensively documented and are routinely discussed between patients and clinicians, these events are systematically underreported by patients and healthcare professionals alike and might not be observed by or shared with the prescribing clinician, thereby impairing their ability to accurately weigh the relative benefits and risks of TCS treatment. In conversations with colleagues and peers, the perception that adverse effects are uncommon with TCS use is often raised. To combat this misperception, I selected examples of patients with notable adverse events related to TCS use from my practice, along with some clinical pearls I have learned along the way.
Review of cases
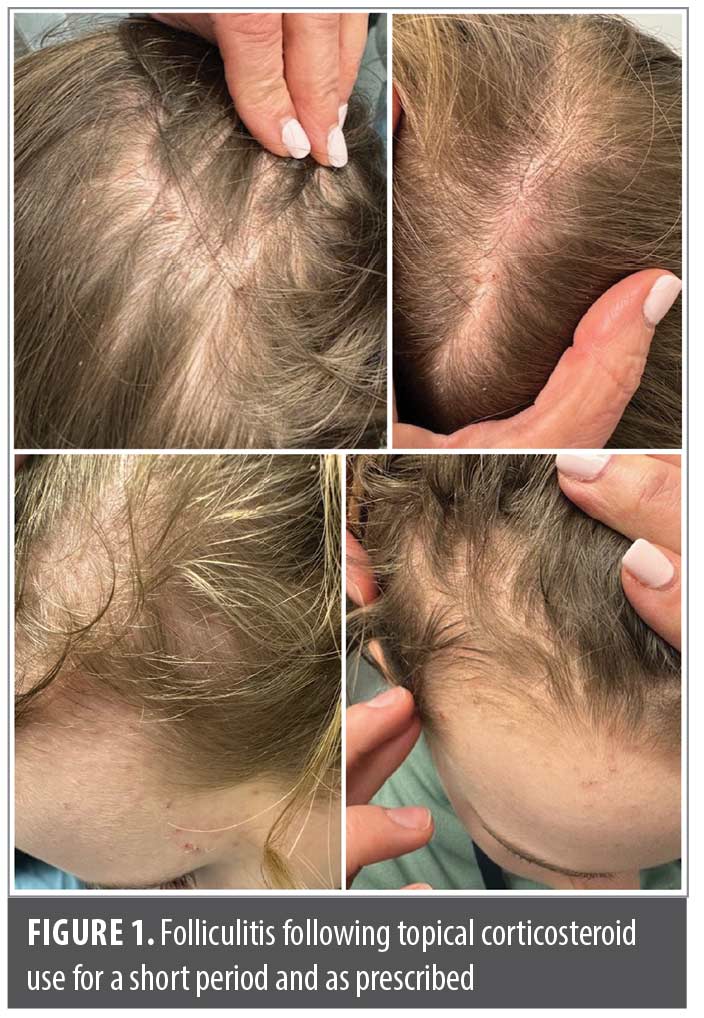
Case 1. A 12-year-old girl presented for treatment of extensive pruritic seborrheic dermatitis on her scalp. There was no evidence of other skin conditions, including acne, at initial presentation. In this case, my first-choice treatment was roflumilast foam 0.3%; however, her insurance required her to “step through” treatment with TCS prior to approving coverage. The patient was started on topical mometasone solution, which she applied as directed. She achieved improvement in scale, but there was no reduction in pruritus. Within two weeks, she returned to my office with wide-spread scalp folliculitis that had become secondarily infected and required oral doxycycline to resolve (Figure 1). Subsequently, her insurance company approved topical roflumilast, which has controlled her seborrheic dermatitis well.
Clinical pearl: Even using TCS for short periods and as prescribed is not without risks.
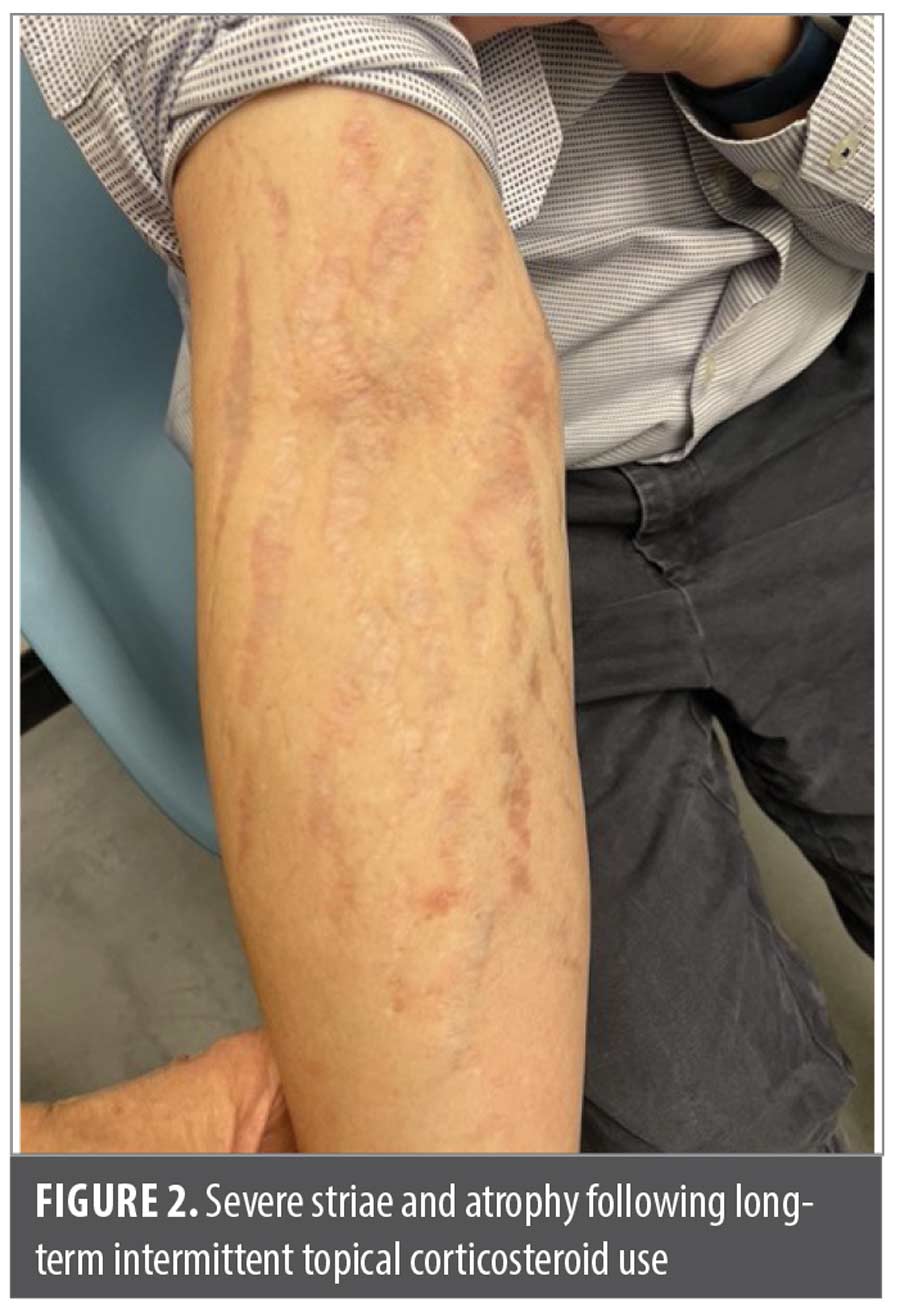
Case 2. A 37-year-old Asian man presented at my office asking for a refill of clobetasol, which he had been using intermittently for many years to treat his atopic dermatitis (AD). Upon examination, extensive striae and skin atrophy were noted in each of the areas he had applied TCS. Because of the extent and duration of disease he reported, I opted to prescribe an interleukin (IL)-13 inhibitor, which nearly cleared his AD. The patient returned to the clinic seeking a refill of his IL-13 inhibitor as well as TCS for episodic treatment of small flares. Upon re-examination, I again noted extensive striae (Figure 2), which were even more prominent now that his AD had been largely controlled. His AD remains well controlled with injectable treatment. Unfortunately, however, the TCS-induced damage to his skin is permanent.
Clinical pearl: Long-term TCS use (even intermittent use) can result in permanent skin changes that can even outlast the disease the TCS was used to treat.
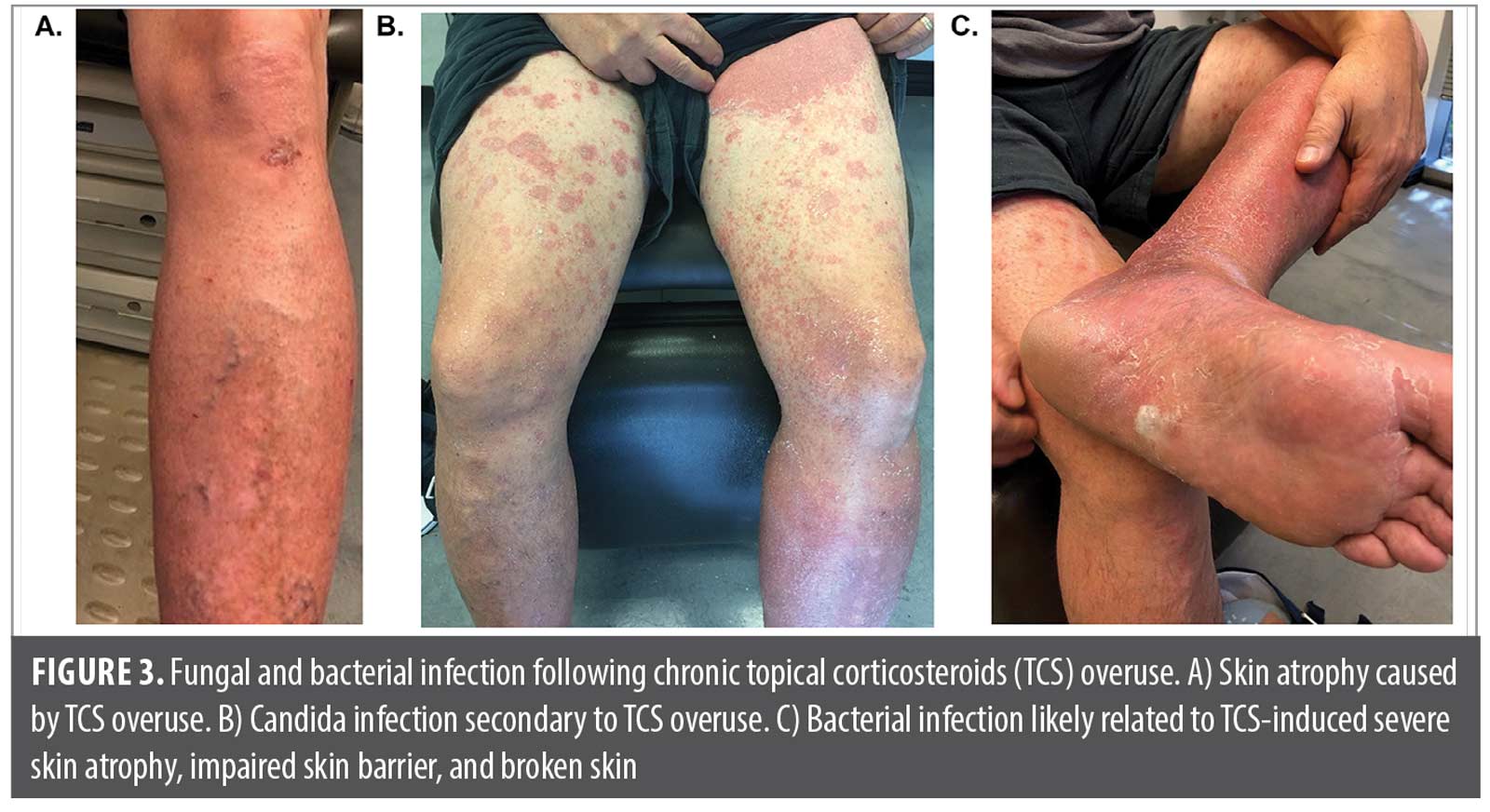
Case 3. A middle-aged man with extensive psoriasis was referred to me following hospitalization for bacterial sepsis. The referring infectious disease specialist had a high index of suspicion that the infection was a direct result of chronic overuse of clobetasol. The patient’s long history of clobetasol use had led to extensive skin atrophy and systemic immunosuppression (Figure 3A) as well as fungal (Figure 3B) and bacterial (Figure 3C) colonization. The resulting impaired skin barrier and broken skin likely allowed the introduction of the bacteria that led to sepsis. Following resolution of sepsis, the patient was given a biologic to treat his psoriasis and promote skin barrier repair, thereby reducing future risk of bacterial infection. The patient’s psoriasis is somewhat controlled with an IL-12/23 inhibitor, and there has been no recurrence of systemic infection. The extensive skin atrophy he experienced as a result of TCS overuse has, unfortunately, contributed to substantial permanent stasis changes in his legs (ie, vascular dilation) that are unusual for his age and medical history.
Clinical pearl: TCS overuse can have serious adverse effects resulting in hospitalization, including infection, adrenal suppression, and even sepsis.
Case 4. A young woman with a history of sebo-psoriasis around her ears and hairline presented to my clinic for a TCS refill. She has been using fluticasone cream BID for more than one year (with few breaks) to prevent scale and itch even when her skin was clear. Upon examination, I noted mild postauricular skin atrophy (Figure 4A) and advised her to discontinue TCS. The patient was prescribed a topical calcineurin inhibitor (this was prior to the recent availability of additional alternative topical treatments), which she did not tolerate because of greasiness and application-site stinging. The patient initiated calcipotriene cream and twice-weekly (BIW) TCS, but she was unable to assimilate the rotating schedule into her routine and felt that the combination therapy was not effective as the TCS monotherapy she had used previously. Eventually, the patient was lost to follow-up because of her frustration with the treatment options available to her.
Clinical pearl: Patients who are dissatisfied with older TCS-sparing treatment might continue to seek more potent TCS, resulting in doctor/clinic hopping and potential TCS misuse.
Case 5. An elderly woman presented to my clinic for lesions in her axilla. She had been prescribed betamethasone cream to treat stasis dermatitis on her legs. Some time later, she noted itching in her axilla and applied the “left over” betamethasone cream to her axilla. This mismatch between steroid potency and application site, in addition to the patient’s advanced age, resulting in moderate skin atrophy and purpura (Figure 4B). The patient was instructed to discontinue use of the potent TCS on her axilla, and was prescribed fluticasone cream to be used intermittently, and only on specified areas.
Clinical pearl: Patients can repurpose TCS prescriptions for other body areas, leading to high-potency TCS being applied to thin-skinned areas and irreversible skin damage.
Conclusion
This case series illustrates the real-world risks associated with TCS use and provides some key clinical pearls for managing TCS use in everyday clinical practice (Figure 5).
In cases where TCS are appropriate or necessary, it is recommended that treatment with low- to mid-potency TCS should not exceed 1 to 4 weeks, and superpotent TCS such as clobetasol propionate should not be used for more than two weeks.2,3 To avoid cutaneous and systemic adverse events, a cumulative corticosteroid load should be limited across all modalities, including topical, oral, ophthalmic, nasal, inhaled, intravenous, intramuscular, intra-articular, and intralesional routes.15 When prescribing TCS, proper patient counseling to ensure understanding of the importance of applying TCS exactly as prescribed (ie, application location, frequency, and duration) is critical to minimize the risks of adverse events. Unfortunately, the complicated treatment regimens that involve multiple TCS that are needed for patients with extensive involvement or chronic disease lead to nonadherence and reduced treatment satisfaction. Unfortunately, even with counseling, most patients exceed the recommended duration and extent of TCS application.15 Importantly, providing a follow-up appointment or additional patient monitoring is recommended to ensure proper use of TCS and assess the ongoing risk of adverse events.
Despite growing safety concerns, tolerability limitations, and risk of hypersensitivity to corticosteroids, most insurers place TCS as the first step in treatment of many inflammatory skin diseases because of their low cost. However, TCS use, especially long-term use and misuse, is associated with potentially costly adverse effects, including those that might lead to hospitalization like infection, septicemia, and adrenal suppression as well as chronic conditions like osteoporosis, diabetes mellitus, and glaucoma.2,3,10–14 Additionally, treatment guidelines for the topical treatment of psoriasis and AD have not yet been updated to include newer advanced targeted topical treatments, which further limits patient access to these treatments via insurance coverage or placement in the treatment algorithm covered by insurance. In response, clinicians have called on insurers to consider all topical treatments as first line therapy to support decision-making between clinicians and the patients/caregivers.
References
- Dermatological agents: total prescriptions in 2022. ClinCalc DrugStats Database version 2024.08. ClinCalc LLC. Accessed March 26, 2025. https://clincalc.com/DrugStats/TC/DermatologicalAgents.
- Gabros S, Nessel TA, Zito PM. Topical corticosteroids. StatPearls Publishing; 2025. Accessed March 26, 2025. https://www.ncbi.nlm.nih.gov/books/NBK532940/weeks.
- Burshtein J, Chovatiya R, Golant A, et al. Risks of topical corticosteroid therapy and role for advanced targeted topical treatments for inflammatory skin diseases: an expert consensus panel. Dermatol Online J. 2025;31(1):10.5070/D331164978.
- Drucker AM, Eyerich K, de Bruin-Weller MS, et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol. 2018;178(3):768–775.
- Health Canada. Safety Brief: Topical Corticosteroids and the Risk of Topical Withdrawal Reactions. Her Majesty the Queen in Right of Canada. July 2022. Accessed March 18, 2025. https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/health-product-infowatch/july-2022.html.
- Medicines and Healthcare Products Regulatory Agency. MHRA Public Assessment Report: Topical Steroid Withdrawal Reactions: A Review of the Evidence. September 15, 2021. Accessed March 18, 2025. https://www.gov.uk/government/publications/topical-steroid-withdrawal-reactions-a-review-of-the-evidence/topical-steroid-withdrawal-reactions-a-review-of-the-evidence
- National Eczema Association. Education Announcement: Use of Topical Steroids for eczema. July 17, 2021. Accessed March 18, 2025. https://nationaleczema.org/blog/warnings-for-topical-steroids-eczema/.
- National Eczema Society, British Dermatological Nursing Group, British Association of Dermatologists. Topical Steroid Withdrawal: A Joint Statement by National Eczema Society, the British Dermatological Nursing Group, and the British Association of Dermatologists. February 2024. Accessed March 18, 2025. https://cdn.bad.org.uk/uploads/2024/02/22095550/Topical-Steroid-Withdrawal-Joint-Statement.pdf.
- Coondoo A. Topical corticosteroid misuse: the Indian scenario. Indian J Dermatol. 2014;59(5):451–455.
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1–15.
- Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5(4):416–425.
- Lam LH, Sugarman JL. Adrenal suppression with chronic topical corticosteroid use in psoriasis patients. J Drugs Dermatol. 2016;15(8):945–458.
- Koshi EJ, Young K, Mostales JC, et al. Complications of corticosteroid therapy: A comprehensive literature review. J Pharm Technol. 2022;38(6):360–367.
- DiRuggiero D, DiRuggiero M. Beyond skin deep: the systemic impact of topical corticosteroids in dermatology. J Clin Aesthet Dermatol. 2025;18(1–2 Suppl 1):S16–S20.
- Barta K, Fonacier LS, Hart M, et al. Corticosteroid exposure and cumulative effects in patients with eczema: results from a patient survey. Ann Allergy Asthma Immunol. 2023;130(1):93–99.