 J Clin Aesthet Dermatol. 2021;14(4):14–22.
J Clin Aesthet Dermatol. 2021;14(4):14–22.
by George Monks, MD; Ryan Rivera-Oyola, MS; Mark Lebwohl, MD
Dr. Monks is with the Department of Dermatology, University of Oklahoma College of Medicine in Oklahoma City, Oklahoma. Mr. Rovera-Oyola and Dr. Lebwohl are with the Department of Dermatology, Icahn School of Medicine at Mt. Sinai Hospital in New York, New York.
FUNDING: No funding was provided for this article.
DISCLOSURES: Dr. Monks is a speaker for Abbvie, Pfizer, Novartis, Eli Lilly, Sun Pharmaceuticals, Genetech, and Celgene. Dr. Lebwohl is an employee of Mount Sinai and receives research funds from Abbvie, Amgen, Arcutis, AstraZeneca, Boehringer Ingelheim, Celgene, Clinuvel, Eli Lilly, Incyte, Janssen Research & Development, LLC, Kadmon Corp., LLC, Leo Pharmaceutucals, Medimmune, Novartis, Ortho Dermatologics, Pfizer, Sciderm, UCB, Inc., and ViDac and is a consultant for Allergan, Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr. Reddy’s Laboratories, Theravance, and Verrica Mr. Rivera-Oyola reports no conflicts of interest relevant to the content of this article.
ABSTRACT: Psoriasis, an inflammatory disorder of the skin, is associated with an increased risk of systemic diseases, such as psoriatic arthritis, psychiatric disorders, malignancy, and cardiometabolic and inflammatory bowel diseases. Careful consideration of the presence of these comorbidities should guide selection of appropriate therapy. The evolution of therapeutic targets for the treatment of psoriasis has significantly advanced available treatment options, potentially leading to uncertainty when selecting the optimal treatment for each patient. In this article, we review evidence-based guidelines for the use of psoriasis treatments in patients with distinct comorbidities, and group appropriate therapeutic options into a visual aid. An easy-to-use visual tool incorporating treatment options best suited for specific comorbidities can increase physicians’ confidence when selecting the most appropriate treatment on an individualized basis.
Keywords: Psoriasis, comorbidities, multiple sclerosis, inflammatory bowel disease, depression, pregnancy, psoriatic arthritis, liver disease, kidney disease, biologics, secukinumab, ixekizumab, brodalumab, ustekinumab, guselkumab, tildrakizumab, risankizumab, methotrexate, cyclosporine, acitretin
Psoriasis is a chronic, inflammatory skin disorder known to affect 2 to 4 percent of adults in the United States.1 The association between psoriasis and comorbid conditions, such as cardiometabolic, gastrointestinal, and kidney disorders, is well established.2 In addition, malignancy, infection, inflammatory arthritis, and mood disorders are prevalent among patients with psoriasis.2 The evolution of therapeutic targets for the treatment of psoriasis has significantly advanced available treatment options. Presently, there are 10 biologic medications approved by the United States Food and Drug Administration for use in the treatment of mild-to-moderate psoriasis, as well as several oral small-molecule and systemic therapies. With the increase in therapeutic options available, many dermatologists experience uncertainty when deciding which psoriasis therapy is appropriate to achieve optimal patient outcomes on an individualized basis. Here, we introduce the Psoriasis Decision Tree, a useful tool for navigating through the complicated sea of psoriasis treatments. The tree was developed as a visual aid to guide the busy clinician in selecting the most appropriate treatment for each individual patient. We reviewed select comorbidities associated with psoriasis and designated each branch individually, highlighting optimal treatment options using evidence-based guidelines. The recommendations made are not absolute, as therapeutic options should be considered on a case-by-case basis.
Biologic Agents
The decision-making tree for the selection of biologic agents for psoriasis is illustrated in Figure 1.
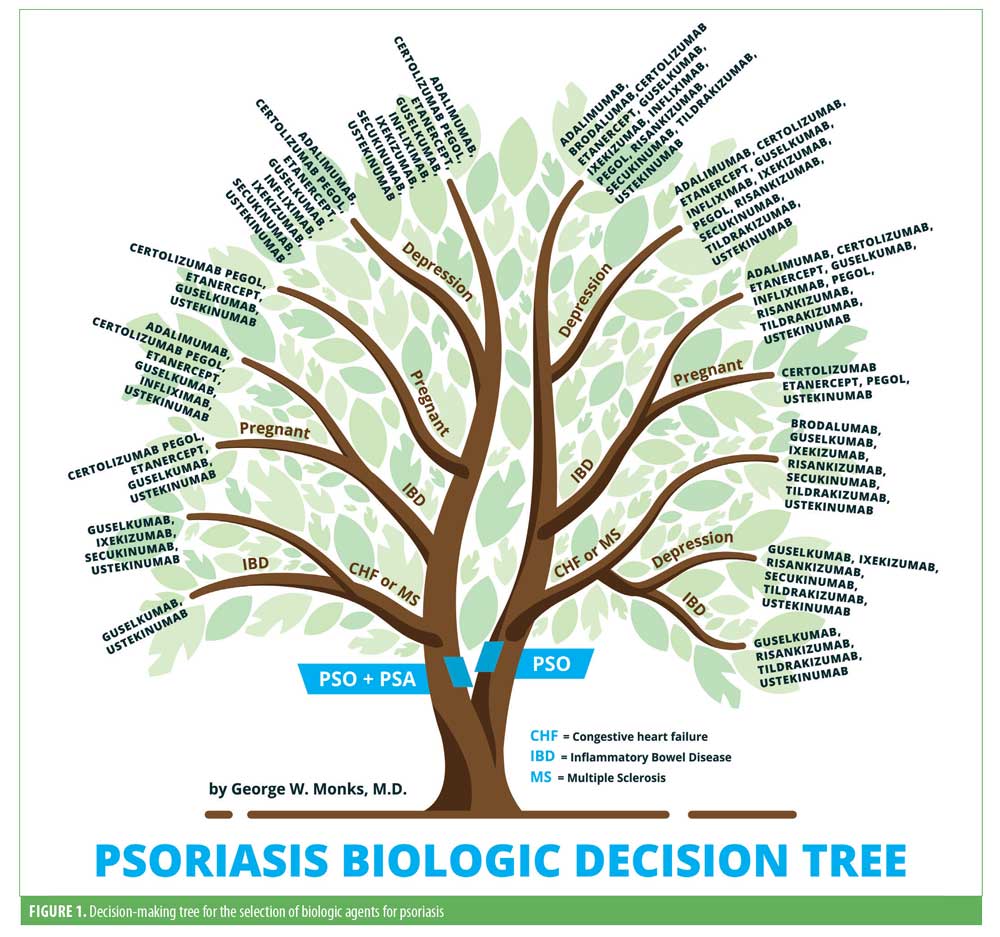
Congestive heart failure. According to a nationwide cohort study, psoriasis was associated with a disease severity-dependent increased risk of new-onset heart failure.3 The ATTACH trial evaluated the efficacy and safety of infliximab in patients with moderate-to-severe congestive heart failure (CHF). The combined risk of death from any cause or hospitalization for heart failure through 28 weeks was increased in patients receiving high doses (10mg/kg) of infliximab (hazard ratio: 2.84, 95% confidence interval: 1.01–7.97; p<0.043) relative to the placebo group.4 In addition, there are multiple case reports demonstrating both new-onset heart failure and exacerbations after initiation of tumor necrosis factor (TNF) antagonist therapy.5 Differently, a study with etanercept in patients with advanced heart failure resulted in a significant dose-dependent improvement in left ventricular structure and function.6 Long-term safety data on both ustekinumab and secukinumab did not report any cases of heart failure.7,8 Additional data are limited regarding the safety of other interleukin (IL)-23 and IL-17 blockers in patients with heart failure, although no cases have been reported in the trials.
Based on the evidence, ustekinumab and secukinumab are the first-line treatment options, followed by the other IL-17 and IL-23 blockers in patients with psoriasis with concomitant heart failure. TNF inhibitors should be avoided in patients with moderate-to-severe heart failure, but can be used with caution in mild disease.
Multiple sclerosis. Both psoriasis and multiple sclerosis are immune-mediated diseases of the body. Although the mechanism is unclear, a significant association has recently been observed in a retrospective cross-sectional study.9 TNF inhibitor use has been associated with increased incidence of both exacerbations and new-onset multiple sclerosis (MS).10,11 Multiple case reports and one Phase II study have reported no worsening neurologic disease after treatment with ustekinumab.12,13 Although limited, there are data suggesting secukinumab and its IL-17 blockade in patients with psoriasis and MS is associated with a reduction in the number of lesions seen with magnetic resonance imaging.14 The effect that IL-23 inhibitors have on patients with MS is yet to be studied.
Ustekinumab and secukinumab can be considered first-line treatment options, followed by the other IL-17 and IL-23 blockers, as no neurological adverse events have been reported. TNF inhibitors should be avoided completely.
Inflammatory bowel disease. Psoriasis is associated with both ulcerative colitis (UC) and Crohn’s disease (CD), collectively referred to as inflammatory bowel disease (IBD).15 A Danish nationwide cohort study reported both a psoriasis severity-dependent increased risk of CD and UC and an increased risk of psoriasis in patients with IBD.16 TNF inhibitors are effective in the treatment of psoriasis and IBD.17 Aside from being safe, etanercept demonstrated insufficient efficacy in the treatment of CD.18 Long-term safety data suggest that infliximab is a safe treatment option for managing IBD.19 It has also proved to be highly efficacious in the treatment of IBD, as evidenced by two key trials (ACCENT-1 and -2).20,21 In a randomized, controlled trial (RCT), adalimumab induced remission with greater frequency than placebo in patients with active CD despite them previously being treated with infliximab or an intolerance to it.22 Efficacy of certolizumab was demonstrated in a Phase III RCT where induction and maintenance therapy were associated with modest improvements in response rates relative to the placebo.23 Ustekinumab has been approved for the treatment of CD based on favorable outcomes of three Phase III induction and maintenance trials (UNIT-1 and -2 and IM-UNIT1).24
In a proof-of-concept trial, secukinumab’s blockade of IL-17 was both ineffective and led to greater rates of adverse events relative to the placebo.25 A careful analysis of 10 Phase II and III clinical trials for secukinumab reported the exposure-adjusted incidence rates for both CD and UC to be 0.11 and 0.15, respectively.26 Data from an integrated database of seven ixekizumab trials proposed that CD and UC cases were uncommon (<1%).27 Only one case of IBD was reported during one of three Phase III trials for brodalumab in the treatment of plaque psoriasis (AMAGINE-1, -2, and -3).28,29 Importantly, during a Phase II RCT evaluating brodalumab’s efficacy in treating CD, 30 percent of patients exposed to brodalumab developed exacerbations, which led to it being contraindicated in CD.30 In a short-term Phase II study, significantly more patients with CD achieved clinical remission with risankizumab relative to the placebo.31
Infliximab, adalimumab, certolizumab, and ustekinumab are indicated for the treatment of CD. Although safe, etanercept has limited efficacy. Secukinumab and ixekizumab can be used with caution. Brodalumab is contraindicated in CD. Risankizumab and IL-23 have demonstrated encouraging data, although more are needed.
Depression. Recent studies have reported that individuals with psoriasis are 2 to 3 times more likely to develop depression than the general population.32,33 In addition, studies have shown that, if left untreated, depression in patients with psoriasis or psoriatic arthritis can lead to higher rates of suicidality and self-harm.34,35 During the pivotal trials for etanercept, adalimumab, infliximab, and ustekinumab, depressive symptoms were decreased.31,36 Similarly, Strober et al37 found adalimumab to have the strongest association with a lower risk of developing depression when analyzing data from patients in the Psoriasis Longitudinal Assessment and Registry (PSOLAR). Importantly, patients on ustekinumab and infliximab also trended toward a lower risk of depression but did not reach statistical significance.37
Strober et al38 analyzed pooled data from 10 clinical studies of secukinumab and reported no elevated risk for depression, anxiety, or suicidality in patients with psoriasis. Similarly, ixekizumab therapy resulted in the remission of depression for approximately 40 percent of patients in an integrated analysis of three Phase III clinical studies.39 The safety of brodalumab has been a controversial topic due to the suicidal ideation and behaviors (SIB) events observed in the brodalumab trials. In-depth analysis of the trials by Lebwohl et al40 revealed no causal association between brodalumab therapy and SIB events. Although four completed suicides occurred during the studies and long-term follow-up, there was no increase in the rates of suicidal behavior or completed suicides in the brodalumab treatment group.40 In a Phase III RCT (VOYAGE 2), guselkumab treatment was associated with greater improvements in symptoms of anxiety and depression in patients with psoriasis compared to the placebo and adalimumab.41 An analysis of pivotal trials for tildrakizumab and rizankizumab revealed no adverse psychiatric events.42,43
Etanercept, adalimumab, infliximab, and ustekinumb reduce depressive symptoms in patients with psoriasis. No increased risk for depression has been reported for secukinumab or ixekizumab. Brodalumab should increase physicians’ awareness of psychiatric comorbidities, but can be used with caution. Guselkumab appears to improve depressive symptoms. Risankizumab and tildrakizumab have not been shown to increase depression risk.
Pregnancy. Pregnancy generally improves psoriatic symptoms, yet some patients actually experience clinical deterioration.44 Patients with mild psoriasis have the option of discontinuing treatment. However, in cases of moderate-to-severe psoriasis, continuation of therapy might sometimes be necessary.45 Limited data exist on proper management of psoriasis in pregnant patients because of the ethical concerns of including this patient population in clinical trials and is limited to case reports and small case series, among others.46
A prospective comparator study suggested that TNF inhibitor treatment does not pose a major teratogenic risk in humans, although the conclusion was based on a relatively small number of pregnancies.47 Differently, a large prospective multicenter cohort study concluded that TNF inhibitors might carry a risk of adverse pregnancy outcomes, as evidenced by a statistically significant increase in risk of major birth defects, preterm birth, and low birth weight compared to the placebo.48 The use of etanercept in pregnancy has generally been well tolerated, as evidenced by multiple studies.49,50 Contrarily, one study suggested an association between etanercept and VACTERL syndrome, although this has since been largely rejected.51,52 Adalimumab has triggered no increase in the rate in miscarriage, malformations, or preterm birth, although data are limited to case reports and letters.53,54 Recent data from the TREAT Safety Registry reported no increase in adverse pregnancy outcomes for patients being treated with infliximab.55 Alarmingly, a case of neonatal death after being exposed to infliximab in utero following an injection of the Bacillus Calmette-Guérin vaccine at the age of three months has been reported, although a study of pregnant women enrolled in Crohn’s and Colitis Foundation PIANO registry found that routine vaccination in infants exposed to biologic therapy in utero did not affect their ability to mount an immune response.56,57 Certolizumab has been shown to cause minimal placental transfer from mother to infant due to its PEGylated structure and absence of an Fc portion, as highlighted by CRIB, a prospective pharmacokinetic study.58 Additionally, an analysis of 339 pregnancies exposed to certolizumab suggested a lack of harmful effects on pregnancy outcomes.59
In a small cohort study of 10 pregnant patients with plaque psoriasis, ustekinumab exposure during conception and pregnancy was not associated with any adverse events.60 Additionally, multiple case reports have been published where no adverse pregnancy outcomes occurred, with the exception of one report of a spontaneous abortion.61–64 Data regarding exposure to IL-17 and IL-23 during pregnancy are limited. No cases have been reported in the literature.
Certolizumab pegol should be the first-line therapy used in pregnant patients or in those of childbearing potential; existing data do not point to any birth defects associated with etanercept, adalimumab, or infliximab. Ustekinumab is a safe and effective alternative to TNF inhibitors. There are limited data for IL-17 and -23 inhibitors.
Psoriatic arthritis. Estimates of the prevalence of patients with psoriasis with associated psoriatic arthritis (PsA) range from 6 to 39 percent.65–67 As many as 15 percent of patients with psoriasis have undiagnosed PsA. Early intervention and treatment of PsA is critical to prevent deforming, irreversible joint damage.
The role of TNF inhibitors in the treatment of PsA has been the subject of multiple studies. In a Phase III RCT, etanercept was significantly more effective in improving multiple study measures compared to the placebo.68 Adalimumab was shown to significantly improve skin and joint symptoms, inhibit structural changes on radiographs, and improve quality of life compared to the placebo.69 Similarly, infliximab and certolizumab have both been shown to significantly improve signs and symptoms of PsA and currently have approval for treatment.70,71
Secukinumab and ixekizumab have both been shown to be superior to placebo in improving signs and symptoms of PsA throughout multiple Phase III RCTs.72,73 Although brodalumab is not currently approved for treatment of PsA, there have been Phase II studies that suggest significant and rapid clinically meaningful improvement in active PsA at week 24.74
Significant improvement in joint disease and delayed radiographic progression was observed in ustekinumab’s two Phase III RCTs for the treatment of PsA.75 Guselkumab has demonstrated its superior efficacy in the treatment of PsA in both Phase II and III RCTs.76–78 Risankizumab and tildrakizumab currently have promising Phase II data for PsA, but larger Phase III studies assessing their efficacy are still needed.
Etanercept, adalimumab, infliximab, certolizumab, secukinumab, ixekizumab, and guselkumab are indicated for the treatment of PsA. Brodalumab, although not approved, has shown efficacy in treatment. In patients with severe skin disease and mild arthritis, ustekinumab may be considered. Limited data exist for other IL-23 inhibitors.
Oral Agents
The decision-making tree for the selection of oral agents for psoriasis is illustrated in Figure 2.
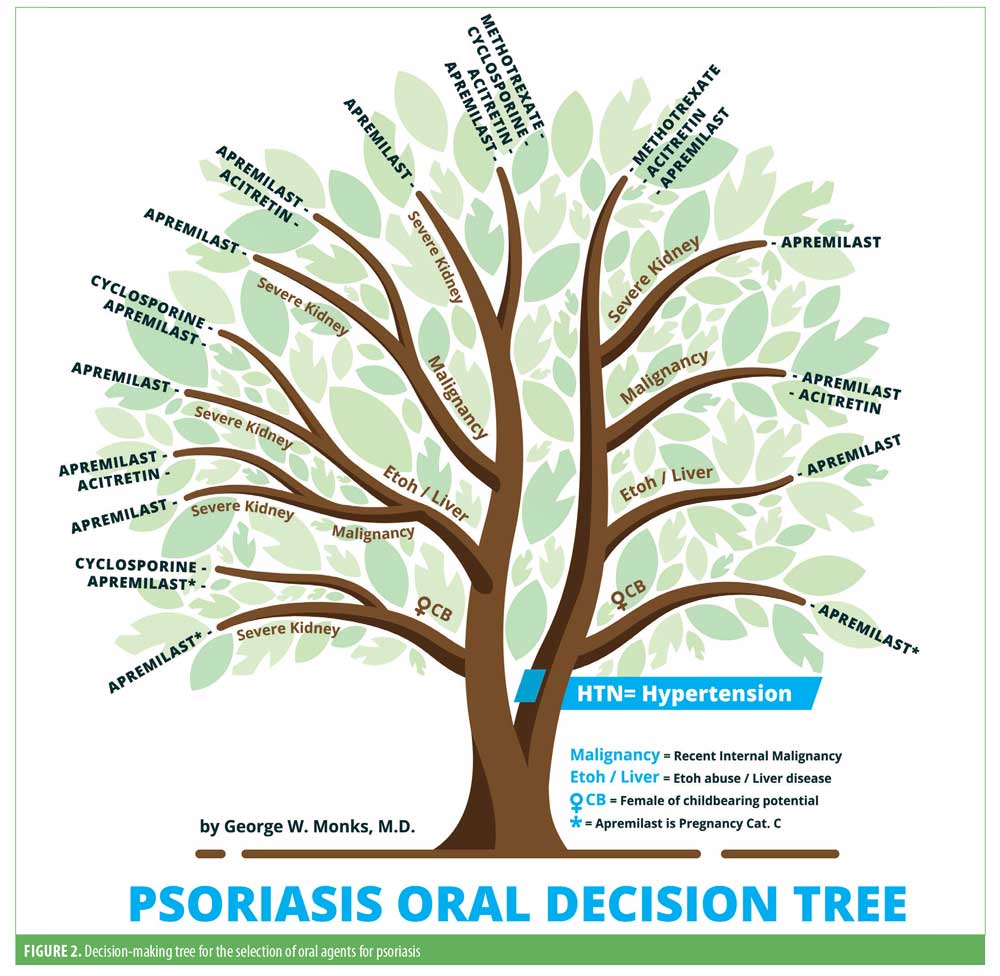
Hypertension/cardiac disease. Hypertension has been observed to be more prevalent in patients with psoriasis relative to those without. Several cohort studies have shown psoriasis is associated with an increased risk of incident hypertension.79 Similarly, the pooled odds ratio for the association between psoriasis and hypertension was found to be significantly increased (1.58; 95% confidence interval: 1.42–1.76) in a large meta-analysis.80
The available evidence suggests that methotrexate might have beneficial effects on vascular homeostasis and blood pressure.81 A five-year cohort study reported that treatment with methotrexate was associated with significantly lower rates of cardiometabolic events when compared with other therapies.82 However, cyclosporine was found to increase the risk for developing hypertension, hyperlipidemia, and diabetes in a prospective cohort study, in addition to increasing the incidence of new-onset hypertension.83,84 Hyperlipidemia has been associated with acitretin in patients with psoriasis.83 Differently, several studies have shown a slowing in the progression of atherosclerotic disease in patients on acitretin therapy.85,86 There was no increased risk of any major cardiovascular event in patients treated with apremilast when analyzing the safety data of two Phase III RCTs.87
Methotrexate can be used due to demonstrated cardioprotective benefits, while cyclosporine and acitretin should be avoided due to the elevated risk of hypertension and hyperlipidemia. Additional long-term data are required for apremilast.
Pregnancy. Most of the data on the adverse effects of treatment with methotrexate and cyclosporine during pregnancy have been obtained from patients undergoing therapy for cancer and organ transplant, respectively. Methotrexate is teratogenic and mutagenic, as well as an abortifactant. Therefore, it is contraindicated in pregnancy.88 Although cyclosporine crosses the placental–blood barrier, there is no evidence of teratogenicity in humans, and it has been demonstrated to be a safe option for treating pregnant patients with psoriasis.89–91 In a meta-analysis, the prevalence rate for malformations in cyclosporine-exposed live births was three percent, similar to in the general population.92 In contrast, in a large retrospective case series, preterm deliveries and low birth weight were reported in cyclosporine-exposed individuals.93 In-utero exposure to acitretin is associated with high risk of fetal malformations, involving craniofacial, thymic, cardiac and central nervous system structures.94 Its use is therefore contraindicated in pregnant women. Although the risk of gastrointestinal upset may be a considerable nuisance for pregnant women already dealing with nausea and vomiting, there are no reported cases of apremilast-induced embryonal abnormalities.
Methotrexate and acitretin are contraindicated in pregnancy. Cyclosporine can be used if the potential benefit justifies the potential risk to the fetus. There are limited data for IL-17 and IL-23 inhibitors and apremilast.
Liver disease. Chronic liver diseases are a major cause of morbidity and mortality worldwide. The incidence of nonalcoholic fatty liver disease (NAFLD) has increased steadily in recent years.95 Associations between psoriasis, NAFLD, chronic hepatitis, and alcoholic liver disease have been reported.94 Methotrexate use in patients with preexisting liver pathology has been associated with an increased risk of methotrexate-induced hepatotoxicity across multiple studies.97,98 For patients with psoriasis, an association with hepatotoxicity and liver injury is noted in the cyclosporine package insert. Although acitretin-induced hepatoxicity is rare, a study found that cholestasis might be more common than previously thought.99 In a Phase III RCT, there were no hepatic adverse events in the apremilast-treated group.100
In the presence of preexisting liver disease, methotrexate, cyclosporine, and acitretin have potential disadvantages. Apremilast might be a suitable option.
Malignancy. The risk of malignancy attributable to psoriasis remains unclear. A meta-analysis evaluating the risk of malignancy in patients with psoriasis demonstrated and increased overall risk.101 Specifically, patients with psoriasis were observed to have a persistently increased risk of lymphoma.102 Interestingly, however, those being treated for severe psoriasis were at the greatest risk of developing malignancy.103 Methotrexate’s association with the development of malignancy in patients with psoriasis remains unclear, as studies have reported mixed results. Recent data showed that patients with psoriasis treated with low-dose methotrexate monotherapy were not at an increased risk for developing malignancy compared to the placebo.104 Differently, a study found an elevated risk of lymphoma in patients treated with methotrexate for longer than 36 months relative to the general population (incidence rate ratio: 3.65; 95% confidence interval: 1.34–9.90).105 In a prospective cohort study, there was a sixfold increase in the incidence of nonmelanoma skin malignancies, particularly squamous cell carcinoma, in patients with psoriasis treated with cyclosporine compared to in the general population. This was especially true in patients with a history of more than 200 psoralen and ultraviolet A radiation treatments.106,107 With the exception of lymphoma, the incidence of internal malignancies in patients with psoriasis treated with cyclosporine was not significantly increased compared to the general population (1.7; 95% confidence interval: 0.7–3.5 vs. 1.2; 95% confidence interval: 0.7–1.9). In a nested cohort study, system retinoid use significantly reduced the risk of developing squamous cell carcinoma in patients with psoriasis treated with psoralen and ultraviolet A radiation.108 Similarly, acitretin therapy decreased the incidence of actinic keratoses in renal transplant recipients in a randomized trial.109 Although a case of melanoma recurrence was reported during treatment with apremilast, no causality was established.
Methotrexate and cyclosporine should be avoided, while acitretin is protective against skin cancer. Limited data regarding apremilast.
Kidney disease. Moderate-to-severe psoriasis might be an independent risk factor for chronic kidney disease and end-stage renal disease.2 Numerous studies have suggested an increased risk of death from kidney disease in patients with psoriasis.110,111 Unsurprisingly, psoriasis was found to be a possible independent risk factor for chronic kidney disease as well as end-stage renal disease.112 After six months of treatment, significant decreases in renal clearance and creatinine clearance were observed in patients treated with methotrexate in a pharmacokinetic testing study.113 Nephrotoxicity is a well-known side-effect of cyclosporine use. Studies have demonstrated an increased risk of renal dysfunction in patients with psoriasis and concomitant renal disease.17,114 The package insert advises against the use of acitretin in patients with kidney disease. Apremilast has been reported to exhibit changes in kidney function only in patients with severe renal disease. Conversely, no decrease in renal function was observed in those with mild renal impairment.115
Methotrexate should be used with caution in patients with renal disease. Cyclosporine and acitretin should be avoided in patients with significant renal impairment. Apremilast can be used, although dose reduction in patients with moderate-to-severe renal dysfunction is recommended.
Conclusion
In conclusion, when determining the optimal treatment for patients on an individual basis, the numerous comorbidities associated with psoriasis should be taken into consideration. A recent survey performed by the National Psoriasis Foundation concluded that undertreatment and nontreatment of psoriasis and PsA remain significant issues in the United States.116 Another survey performed by Lebwohl et al117 found physicians reporting the treatment and management of patients with psoriasis and/or PsA to be particularly complex and time-consuming, possibly contributing to undertreatment.Many factors must be considered when electing the optimal therapy for psoriasis patients.118,119 The development of highly efficacious agents for treating psoriasis has transformed the way psoriasis is managed today. We hope our Psoriasis Decision Tree can help aid physicians in selecting the most appropriate treatment options in a time-efficient manner while taking into consideration the presence or absence of comorbidities, ultimately increasing their confidence when prescribing systemic therapies to a heterogeneous patient population.
References
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390.
- Khalid U, Ahlehoff O, Gislason GH, et al. Psoriasis and risk of heart failure: a nationwide cohort study. Eur J Heart Fail. 2014;16(7):743–748.
- Chung ES, Packer M, Lo KH, et al. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107(25):3133–3140.
- Kwon HJ, Cote TR, Cuffe MS, et al. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138(10):807–811.
- Bozkurt B, Torre-Amione G, Warren MS, et al. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001;103(8):1044–1047.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168(4):844–854.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32(9):1507–1514.
- Guido N, Cices A, Ibler E, et al. Multiple sclerosis association with psoriasis: a large U.S. population, single centre, retrospective cross-sectional study. J Eur Acad Dermatol Venereol. 2017;31(9):e397–e398.
- Kaltsonoudis E, Voulgari PV, Konitsiotis S, Drosos AA. Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun Rev. 2014;13(1):54–58.
- TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology. 1999;53(3):457–465.
- Segal BM, Constantinescu CS, Raychaudhuri A, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7(9): 796–804.
- Chang S, Chambers CJ, Liu FT, Armstrong AW. Successful treatment of psoriasis with ustekinumab in patients with multiple sclerosis. Dermatol Online J. 2015;21(7).
- Havrdova E, Belova A, Goloborodko A, et al. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J Neurol. 2016;263(7):1287–1295.
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–2078.
- Egeberg A, Mallbris L, Warren RB, et al. Association between psoriasis and inflammatory bowel disease: a Danish nationwide cohort study. Br J Dermatol. 2016;175(3):487–492.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850.
- Sandborn WJ, Hanauer SB, Katz S, et al. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121(5):1088–1094.
- Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58(4):501–508.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1519.
- Sands BE, Blank MA, Patel K, van Deventer SJ. Long-term treatment of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II Study. Clin Gastroenterol Hepatol. 2004;2(10): 912–920.
- Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146(12):829–838.
- Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357(3):228–238.
- Armuzzi A, Ardizzone S, Biancone L, et al. Ustekinumab in the management of Crohn’s disease: expert opinion. Dig Liver Dis. 2018;50(7):653–660.
- Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–1700.
- van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(1):83–98.e4.
- Reich K, Leonardi C, Langley RG, et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: a presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol. 2017;76(3):441–448.e2.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286.
- Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol. 2016;111(11):1599–1607.
- Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389(10080):1699–1709.
- Egeberg A, Thyssen JP, Wu JJ, Skov L. Risk of first-time and recurrent depression in patients with psoriasis: a population-based cohort study. Br J Dermatol. 2019;180(1):116–121.
- Wu JJ, Penfold RB, Primatesta P, et al. The risk of depression, suicidal ideation and suicide attempt in patients with psoriasis, psoriatic arthritis or ankylosing spondylitis. J Eur Acad Dermatol Venereol. 2017;31(7):1168–1175.
- Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895.
- Pompili M, Innamorati M, Trovarelli S, et al. Suicide risk and psychiatric comorbidity in patients with psoriasis. J Int Med Res. 2016;44(1 suppl):61–66.
- Fleming P, Roubille C, Richer V, et al. Effect of biologics on depressive symptoms in patients with psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2015;29(6):1063–1070.
- Strober B, Gooderham M, de Jong E, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78(1):70–80.
- Strober BE, Langley RGB, Menter A, et al. No elevated risk for depression, anxiety or suicidality with secukinumab in a pooled analysis of data from 10 clinical studies in moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178(2):e105–e107.
- Griffiths CEM, Fava M, Miller AH, et al. Impact of ixekizumab treatment on depressive symptoms and systemic inflammation in patients with moderate-to-severe psoriasis: an integrated analysis of three phase 3 clinical studies. Psychother Psychosom. 2017;86(5):260–267.
- Lebwohl MG, Papp KA, Marangell LB, et al. Psychiatric adverse events during treatment with brodalumab: analysis of psoriasis clinical trials. J Am Acad Dermatol. 2018;78(1):81–89.e5.
- Gordon KB, Armstrong AW, Han C, et al. Anxiety and depression in patients with moderate-to-severe psoriasis and comparison of change from baseline after treatment with guselkumab vs. adalimumab: results from the Phase 3 VOYAGE 2 study. J Eur Acad Dermatol Venereol. 2018;32(11):1940–1949.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288.
- Tauscher AE, Fleischer AB, Jr., Phelps KC, Feldman SR. Psoriasis and pregnancy. J Cutan Med Surg. 2002;6(6):561–570.
- Babalola O, Strober BE. Management of psoriasis in pregnancy. Dermatol Ther. 2013;26(4):285–292.
- Lam J, Polifka JE, Dohil MA. Safety of dermatologic drugs used in pregnant patients with psoriasis and other inflammatory skin diseases. J Am Acad Dermatol. 2008;59(2):295–315.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, Ornoy A. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78–84.
- Weber-Schoendorfer C, Oppermann M, Wacker E, et al. Pregnancy outcome after TNF-alpha inhibitor therapy during the first trimester: a prospective multicentre cohort study. Br J Clin Pharmacol. 2015;80(4):727–739.
- Berthelot JM, De Bandt M, Goupille P, et al. Exposition to anti-TNF drugs during pregnancy: outcome of 15 cases and review of the literature. Joint Bone Spine. 2009;76(1):28–34.
- Rump JA, Schonborn H. [Conception and course of eight pregnancies in five women on TNF blocker etanercept treatment]. Z Rheumatol. 2010;69(10):903–909. Article in German.
- Carter JD, Valeriano J, Vasey FB. Tumor necrosis factor-alpha inhibition and VATER association: a causal relationship. J Rheumatol. 2006;33(5): 1014–1017.
- Winger EE, Reed JL. Was risk properly assessed in Carter, et al’s safety assessment of tumor necrosis factor antagonists during pregnancy? J Rheumatol. 2009;36(9):2122; author reply 3.
- Chambers CD, Johnson DL. Emerging data on the use of anti-tumor necrosis factor-alpha medications in pregnancy. Birth Defects Res A Clin Mol Teratol. 2012;94(8):607–611.
- Mishkin DS, Van Deinse W, Becker JM, Farraye FA. Successful use of adalimumab (Humira) for Crohn’s disease in pregnancy. Inflamm Bowel Dis. 2006;12(8):827–828.
- Kane S. Trick or TREAT? More safety data of infliximab during pregnancy. Am J Gastroenterol. 2018;113(11):1592–1593.
- Beaulieu DB, Ananthakrishnan AN, Martin C, et al. Use of biologic therapy by pregnant women with inflammatory bowel disease does not affect infant response to vaccines. Clin Gastroenterol Hepatol. 2018;16(1):99–105.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4(5):603–605.
- Mariette X, Forger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77(2):228–233.
- Clowse ME, Wolf DC, Forger F, et al. Pregnancy outcomes in subjects exposed to certolizumab pegol. J Rheumatol. 2015;42(12):2270–2278.
- Watson N, Wu K, Farr P, et al. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180(1):195–196.
- Andrulonis R, Ferris LK. Treatment of severe psoriasis with ustekinumab during pregnancy. J Drugs Dermatol. 2012;11(10):1240.
- Cortes X, Borras-Blasco J, Antequera B, et al. Ustekinumab therapy for Crohn’s disease during pregnancy: a case report and review of the literature. J Clin Pharm Ther. 2017;42(2):234–236.
- Fotiadou C, Lazaridou E, Sotiriou E, Ioannides D. Spontaneous abortion during ustekinumab therapy. J Dermatol Case Rep. 2012;6(4):105–107.
- Rocha K, Piccinin MC, Kalache LF, et al. Pregnancy during ustekinumab treatment for severe psoriasis. Dermatology. 2015;231(2):103–104.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68(4):915–923.
- Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573.
- Leonard DG, O’Duffy JD, Rogers RS. Prospective analysis of psoriatic arthritis in patients hospitalized for psoriasis. Mayo Clin Proc. 1978;53(8):511–518.
- Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356(9227):385–390.
- Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52(10): 3279–3289.
- Kavanaugh A, Antoni CE, Gladman D, et al. The Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT): results of radiographic analyses after 1 year. Ann Rheum Dis. 2006;65(8):1038–1043.
- Mease PJ, Fleischmann R, Deodhar AA, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis. 2014;73(1):48–55.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–1146.
- Nash P, Kirkham B, Okada M, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389(10086):2317–2327.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306.
- Kavanaugh A, Ritchlin C, Rahman P, et al. Ustekinumab, an anti-IL-12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double-blind, placebo-controlled PSUMMIT-1 and PSUMMIT-2 trials. Ann Rheum Dis. 2014;73(6):1000–1006.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391(10136):2213–2224.
- Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNF? inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1115–1125.
- Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10230):1126–1136.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159(4):895–902.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31(3): 433–442; discussion 442–443.
- Mangoni AA, Tommasi S, Zinellu A, et al. Methotrexate and vasculoprotection: mechanistic insights and potential therapeutic applications in old age. Curr Pharm Des. 2019;25(39):4175–4184.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. 2015;29(6):1128–1134.
- Gisondi P, Cazzaniga S, Chimenti S, et al. Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: evidence from the Italian Psocare Registry. J Eur Acad Dermatol Venereol. 2013;27(1):e30–e41.
- Ho VC, Griffiths CE, Albrecht G, et al. Intermittent short courses of cyclosporin (Neoral(R)) for psoriasis unresponsive to topical therapy: a 1-year multicentre, randomized study. The PISCES Study Group. Br J Dermatol. 1999;141(2):283–291.
- Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18(10).
- Stern RS, Fitzgerald E, Ellis CN, et al. The safety of etretinate as long-term therapy for psoriasis: results of the etretinate follow-up study. J Am Acad Dermatol. 1995;33(1):44–52.
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/=156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77(2):310–317.e1.
- Temprano KK, Bandlamudi R, Moore TL. Antirheumatic drugs in pregnancy and lactation. Semin Arthritis Rheum. 2005;35(2):112–121.
- Lamarque V, Leleu MF, Monka C, Krupp P. Analysis of 629 pregnancy outcomes in transplant recipients treated with sandimmun. Transplant Proc. 1997;29(5):2480.
- Raddadi AA, Baker Damanhoury Z. Cyclosporin and pregnancy. Br J Dermatol. 1999;140(6):1197–1198.
- Wright S, Glover M, Baker H. Psoriasis, cyclosporine, and pregnancy. Arch Dermatol. 1991;127(3):426.
- Bar Oz B, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71(8):1051–1055.
- Sgro MD, Barozzino T, Mirghani HM, et al. Pregnancy outcome post renal transplantation. Teratology. 2002;65(1):5–9.
- Bangsgaard N, Rorbye C, Skov L. Treating psoriasis during pregnancy: safety and efficacy of treatments. Am J Clin Dermatol. 2015;16(5):389–398.
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6): 524–530.e1; quiz e60.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatology. 2013;149(10):1173–1179.
- Malatjalian DA, Ross JB, Williams CN, et al. Methotrexate hepatotoxicity in psoriatics: report of 104 patients from Nova Scotia, with analysis of risks from obesity, diabetes and alcohol consumption during long term follow-up. Can J Gastroenterol. 1996;10(6):369–375.
- Shetty A, Cho W, Alazawi W, Syn WK. Methotrexate hepatotoxicity and the impact of nonalcoholic fatty liver disease. Am J Med Sci. 2017;354(2):172–181.
- Sauder MB, Cheung L, Beecker J. Acitretin-induced hepatitis: when to monitor cholestatic enzymes. J Cutan Med Surg. 2015;19(2):115–120.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27 Suppl 3:36–46.
- Gelfand JM, Shin DB, Neimann AL, et al. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126(10):2194–2201.
- Brauchli YB, Jick SS, Miret M, Meier CR. Psoriasis and risk of incident cancer: an inception cohort study with a nested case-control analysis. J Invest Dermatol. 2009;129(11):2604–2612.
- Mazaud C, Fardet L. Relative risk of and determinants for adverse events of methotrexate prescribed at a low dose: a systematic review and meta-analysis of randomized placebo-controlled trials. Br J Dermatol. 2017;177(4):978–986.
- Stern RS. Lymphoma risk in psoriasis: results of the PUVA follow-up study. Arch Dermatol. 2006;142(9):1132–1135.
- Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort crossover study. Lancet. 2001;358(9287):1042–1045.
- Paul CF, Ho VC, McGeown C, et al. Risk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J Invest Dermatol. 2003;120(2):211–216.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49(4): 644–650.
- de Sevaux RG, Smit JV, de Jong EM, et al. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. J Am Acad Dermatol. 2003;49(3):407–412.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. 2010;163(3):586–592.
- Svedbom A, Dalen J, Mamolo C, et al. Increased cause-specific mortality in patients with mild and severe psoriasis: a population-based Swedish register study. Acta Derm Venereol. 2015;95(7): 809–815.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961.
- Kremer JM, Petrillo GF, Hamilton RA. Pharmacokinetics and renal function in patients with rheumatoid arthritis receiving a standard dose of oral weekly methotrexate: association with significant decreases in creatinine clearance and renal clearance of the drug after 6 months of therapy. J Rheumatol. 1995;22(1):38–40.
- Kim BR, Yang S, Doh EJ, et al. Risk factors affecting adverse effects of cyclosporine A in a real-world psoriasis treatment. Ann Dermatol. 2018;30(2): 143–149.
- Liu Y, Zhou S, Assaf M, et al. Impact of Renal impairment on the pharmacokinetics of apremilast and metabolite M12. Clin Pharmacol Drug Dev. 2016;5(6):469–479.
- Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149(10):1180–1185.
- Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US Perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17(1):87–97.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80(1):43–53.

