 J Clin Aesthet Dermatol. 2021;14(4):38–40.
J Clin Aesthet Dermatol. 2021;14(4):38–40.
by Todd E. Schlesinger, MD, FAAD; Ashley E. Wilson, md; Lisa Trivedi, MD;
Phillip J. Latham, BA; and Robert Ball, MD, MPH, FACP
Dr. Schlesinger is with the Dermatology & Laser Center of Charleston and the Clinical Research Center of the Carolinas in Charleston, South Carolina. Dr. Wilson is with the Department of Dermatology at Tulane University School of Medicine in New Orleans, Louisiana. Mr. Latham is with the College of Medicine, Medical University of South Carolina in Charleston, South Carolina. Dr. Trivedi is with the Department of Dermatology and Dermatologic Surgery, Medical University of South Carolina in Charleston, South Carolina. Dr. Ball is with Division of Infectious Diseases, College of Medicine, Medical University of South Carolina in Charleston, South Carolina.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Mycobacterium fortuitum is a rapidly growing mycobacterium known to spread through many sources, including tap water. This organism can have variable presentation between patients which can lead to a delay in diagnosis. Here, we report a series of eight cases of tattoo-associated M. fortuitum infections that presented between December 2010 and January 2011, which were later linked to a single tattoo provider using gray tattoo ink made by diluting black ink with nonsterile tap water. In this case series, we emphasize the lack of pathognomonic features of these infections, the variability in culture and biopsy results, the importance of obtaining a culture in addition to a biopsy, and the importance of identifying the source of infection when determining management.
Keywords: Mycobacterium fortuitum, tattoo-associated infection, tap water, granulomatous dermatitis, acid-fast bacilli, culture, tattooing, granuloma, outbreak
Approximately 50 different mycobacterial species can cause disease in humans and several of them fall into the category of nontuberculous mycobacterium (NTM).1 Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium abscessus can be further categorized as rapidly growing mycobacteria (RGMs).3 This category of mycobacterium can be found in a variety of locations, such as surface water, tap water, soil, domestic and wild animals, milk, and food products.2–5 They have also been known to cause infections of surgical sites after exposure to nonsterile tap water.6 The distinct clinical symptoms include pulmonary disease, lymphadenitis, disseminated disease, and skin and soft tissue infections. However, they can also inhabit body surfaces or secretions without causing disease, making them difficult to diagnose in a clinical setting.7
Specifically, M. fortuitum infects by direct inoculation of skin and soft tissue and typically results in presentation as a single lesion.8 Mycobacterium share the common description of acid-fast bacilli, so further testing is required to differentiate the different organisms.7 Biopsy and culture followed by polymerase chain reaction (PCR) and nucleic acid-specific tests are used to determine the presence and type of RGM, but treatment can vary among the specific types.9 M. fortuitum can show resistance to certain macrolide antibiotics via the erm resistance gene, and the optimal drugs for treatment include amikacin, ciprofloxacin, levofloxacin, moxifloxacin, sulfonamides and imipenem.10
We report an eight-patient series of M. fortuitum infections in the Charleston, South Carolina area. The infections were associated with a single tattoo-administering establishment. Each patient was unique in terms of presentation and laboratory results, but the commonality of an M. fortuitum infection was concluded.
Case Presentations
Index case. On February 11, 2011, a 41-year-old Caucasian male received a tattoo on his right forearm containing red; yellow; and, applied last, gray inks. Three days after receiving the black ink, he experienced redness, swelling, painful bleeding, small pustules and scabs primarily in the areas tattooed with black-appearing ink (Figure 1A). After two months of persistent symptoms, he presented to the emergency department on March 7, 2011, and received anti-inflammatories, oral antibiotics, and topical therapy. These treatments provided temporary relief of symptoms; however, subsequent worsening occurred. On March 11, 2011, he returned to the emergency department due to lack of improvement and he was subsequently admitted and received intravenous clindamycin, topical clobetasol ointment, and a 12-day prednisone taper. He was discharged with instructions to continue oral antibiotics, prednisone, and topical clobetasol ointment. He followed up with his private-practice dermatologist, where he underwent a biopsy (Figure 1B) and tissue culture. An acid-fast stain was positive (Figure 1C), and the presence of a mycobacterium was confirmed by tissue culture (Figure 1D). Following the identification of M. fortuitum, he was treated with ciprofloxacin and linezolid for at least 3 to 4 months.
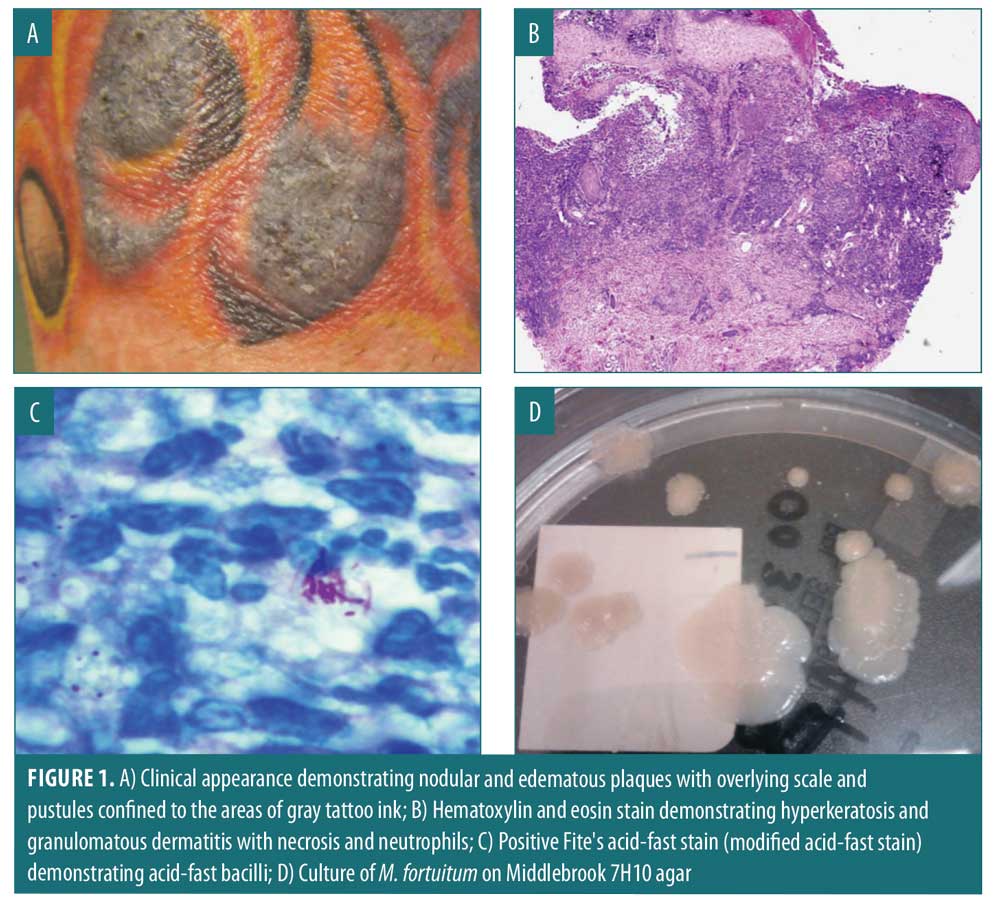
Additional cases. We subsequently conducted a review of the medical records of eight patients who had been a part of an isolated outbreak of M. fortuitum infections associated with tattoos in the Charleston, South Carolina area between December 15, 2010, and January 28, 2011. Clinical course, biopsy results, and culture results were reviewed. Once public health officials confirmed an isolated outbreak of tattoo-associated MF infections, these eight cases were linked back to a single tattoo parlor and a single tattoo artist.
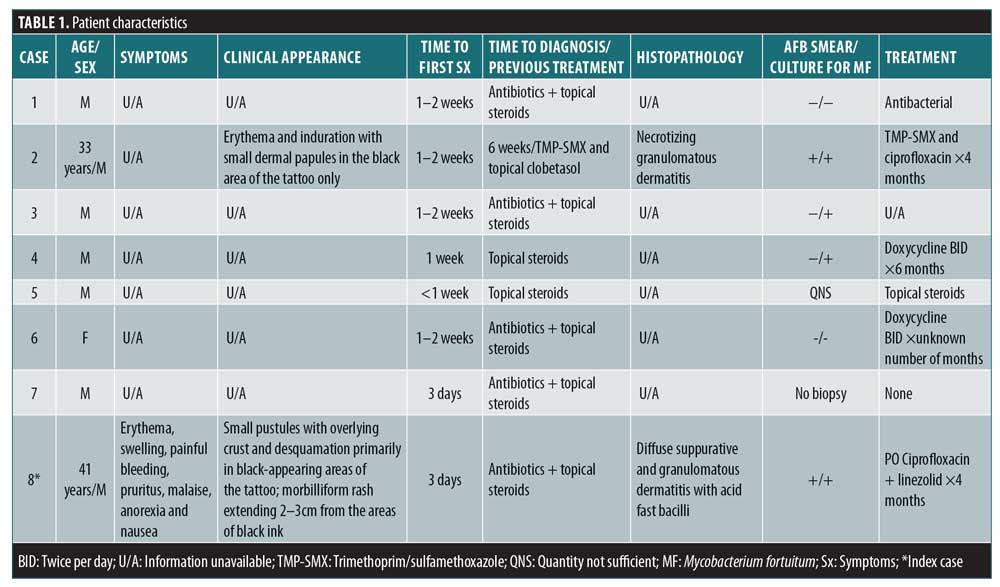
This series consisting of eight patients included seven male patients and one female patient. All patients received tattoos from a single tattoo artist in a single tattoo parlor between December 2010 and January 2011. The clinical appearance of their lesions ranged from small dermal papules to edematous and indurated plaques, all of which were confined to black and gray areas of tattoo ink. All cases initially presented between January and February 2011. Six patients were initially treated with topical steroids and antibiotics, while two were initially treated with topical steroids only. Two patients required hospitalization, while the remaining six were managed in an outpatient setting.
Two of six acid-fast bacilli smears were positive for M. fortuitum. Four of six cultures were positive for M. fortuitum. One patient did not undergo a biopsy, and another patient’s sample quantity was not sufficient. All bacterial cultures were negative, except one that was positive for methicillin-resistant Staphylococcus aureus. It is unclear whether this was a concomitant infection or secondary superinfection. Patient characteristics for each case in this series is summarized in Table 1.
Discussion
Nontuberculous mycobacteria are known culprits in tattoo-associated infections. Outbreaks have been reported in which the etiology was gray ink that has been diluted with contaminated water. This contamination has been known to occur at both the level of the manufacturer and the tattoo artist themselves.11,12 Nontuberculous mycobacteria consist of M. chelonae, M. fortuitum, and M. abscessus. In this report, we focus on NTM infections related to M. fortuitum, which has predominantly been implicated in skin and soft tissue, surgical wound, and catheter-related infections by direct inoculation.13 However, it has been reported more rarely in pulmonary, lymphadenitis, disseminated, and prosthetic device infections.14 Although none of the patients in this series were immunosuppressed, the rate of infections associated with NTM is higher in the immunosuppressed population. The nonspecific clinical manifestations are what make M. fortuitum infections a diagnostic and therapeutic challenge. This case series demonstrates how the nonspecific presentation of this infection can contribute to a delay in diagnosis, which unfortunately led to hospitalization of two of the patients discussed here. Because the diagnosis may or may not be confirmed by culture, it must be emphasized that a biopsy is needed in addition to a culture to aid in diagnosis. One should consider M. fortuitum infection when a patient presents with inflammatory signs and symptoms in a tattoo, particularly when the lesions are confined to an area of tattoo with gray ink.
With respect to treatment, there is a variety of antimicrobial susceptibility described in the literature.15–17 For infections limited to the skin and soft tissue, current recommendations state that two of the following antimicrobial drugs should be orally administered for a minimum of four months: trimethoprim-sulfamethoxazole, doxycycline, levofloxacin, and clarithromycin or azithromycin. For more extensive skin and soft tissue disease, recommendations consist of parenteral therapy with at least two of the following drugs for two to six weeks: an aminoglycoside (amikacin or tobramycin), cefoxitin, imipenem, and levofloxacin. This should be followed by 6 to 12 months of oral therapy with any of the agents previously mentioned.18
Three notable limitations of our case series include the inability to confirm the gray ink as source of infection due to a failure to obtain a sample; because one case went without an associated biopsy; and that, once cultures were obtained, most patients had already been treated with antimicrobials.
Conclusion
We report a series of eight tattoo-associated M. fortuitum infections confined to the areas of gray ink made by a dilution of black ink with tap water, which is the presumed source of infections. Here, we emphasize the lack of pathognomonic features of these infections, the variability in culture and biopsy results, and the importance of obtaining a culture in addition to a biopsy.
References
- Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection. 2004;32(5):257–270.
- Chapman JS. The ecology of the atypical mycobacteria. Arch Environ Health. 1971;22(1): 41–46.
- Goslee S, Wolinsky E. Water as a source of potentially pathogenic mycobacteria. Am Rev Respir Dis. 1976;113(3):287–292.
- Gruft H, Falkinham JO 3rd, Parker BC. Recent experience in the epidemiology of disease caused by atypical mycobacteria. Rev Infect Dis. 1981;3(5): 990–996.
- Wolinsky E, Rynearson TK. Mycobacteria in soil and their relation to disease-associated strains. Am Rev Respir Dis. 1968;97(6):1032–1037.
- Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria. Clin Infect Dis. 2001;33(8):1363–1374.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4): 367–416.
- Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142(10):1287–1292.
- Huebner RE, Good RC, Tokars JI. Current practices in mycobacteriology: results of a survey of state public health laboratories. J Clin Microbiol. 1993;31(4): 771–775.
- Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53(4):1367–76.
- Drage LA, Ecker PM, Orenstein R, et al. An outbreak of Mycobacterium chelonae infections in tattoos. J Am Acad Dermatol. 2010;62(3):501–506.
- Kluger N, Muller C, Gral N. Atypical mycobacteria infection following tattooing: review of an outbreak in 8 patients in a French tattoo parlor. Arch Dermatol. 2008;144(7):941–942.
- Wallace RJ Jr., Swenson JM, Silcox VA, et al. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983;5(4):657–659.
- Hoffman PC, Fraser DW, Robicsek F, et al. Two outbreaks of sternal wound infection due to organisms of the Mycobacterium fortuitum complex. J Infect Dis. 1981;143(4):533–542.
- Swenson JM, Wallace RJ Jr, Silcox VA, Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985;28(6):807–811.
- Brown BA, Wallace RJ Jr, Onyi GO, et al. Activities of four macrolides, including clarithromycin, against Mycobacterium fortuitum, Mycobacterium chelonae, and M. chelonae-like organisms. Antimicrob Agents Chemother. 1992;36(1):180–184.
- Wallace RJ Jr, Brown-Elliott BA, Ward SC, et al. Activities of linezolid against rapidly growing mycobacteria. Antimicrob Agents Chemother. 2001;45(3):764–767.
- Wallace RJ Jr, Swenson JM, Silcox VA, Bullen MG. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonae on the basis of in vitro susceptibilities. J Infect Dis. 1985;152(3):500–514.

