J Clin Aesthet Dermatol. 2024;17(1):15–23.
J Clin Aesthet Dermatol. 2024;17(1):15–23.
by Nardin Awad, DO; John D. Hetzel, MD; Vishnu Bhupalam, BS; Mark S. Nestor, MD, PhD
All authors are with the Center for Clinical and Cosmetic Research in Aventura, Florida. Additionally, Dr. Nestor is with the Department of Dermatology and Cutaneous Surgery, as well as the Department of Surgery, Division of Plastic Surgery, at the University of Miami Miller School of Medicine in Miami, Florida.
FUNDING: No funding was provided for this study.
DISCLOSURES: Dr. Nestor is a consultant for Primus Pharmaceuticals.
ABSTRACT: Objective: We sought to examine the role of flavonoids, particularly diosmin, as a therapeutic agent for stasis dermatitis (SD) through discussion of pathophysiology, current treatment paradigms, potential mechanisms of action, and a systematic review of evidence on clinical efficacy. Methods: In addition to articles on pathophysiology and standard treatment, a search of PubMed was conducted using the following query: (“Diosmin” OR “MPFF” OR “Micronized Purified Flavonoid Fraction” OR “Flavonoid”) AND (“Stasis Dermatitis” OR “Venous Ulcer” OR “Lipodermatosclerosis”). Emphasis was placed on studies that were randomized controlled trials examining an oral flavonoid against a placebo or standard of care. Results: Diosmin is effective at improving stasis changes, increasing ulcer healing frequency, decreasing the time to ulcer healing, and reducing tissue edema. They also cause significant improvement in patient quality of life and reduction of venous symptoms. Diosmin has been shown to have a favorable safety profile with very few mild adverse events which did not differ significantly from placebo. Flavonoids also appear to be effective for other dermatologic conditions, including rosacea and senile purpura. Conclusion: There is a growing body of evidence indicating that diosmin has therapeutic efficacy in managing stasis dermatitis. Data from studies in diseases with pathogenic similarities suggests the potential for even broader dermatologic applications. Keywords: Flavonoid, diosmin, MPFF, stasis dermatitis, chronic venous disease, treatment
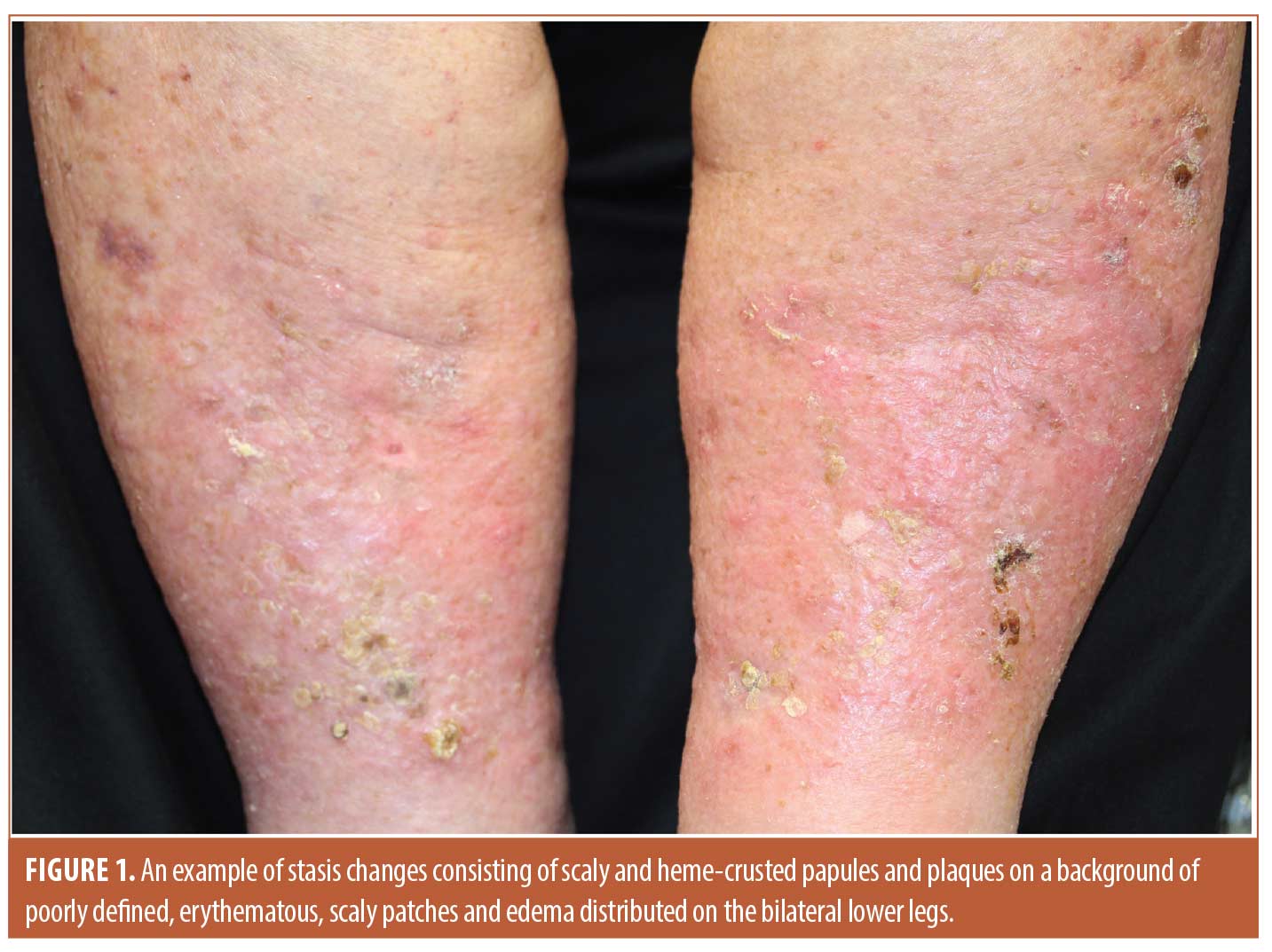
Cutaneous manifestations of chronic venous disease (CVD), such as stasis dermatitis (SD) and venous leg ulcers (VLU), are ubiquitous and frequently frustrating concerns often managed by dermatologists. Though few in-depth analyses of SD prevalence have been conducted, it is estimated that 6-7% of persons over the age of 50 have the disease, and prevalence is positively correlated with age.1 In the United States (U.S.), this constitutes a population of around 7-8 million, a number that is projected to increase in concert with current population aging trends. SD is a chronic, progressive condition clinically characterized by poorly demarcated erythematous, scaly patches and plaques with a variable presence of hyperpigmentation and tissue edema (Figure 1). Cutaneous findings are typically distributed on the lower leg with occasional extension to the foot or knee, and symptoms include localized pruritus, swelling, paresthesia, aching, and cramping, among which pruritus is often cited as being the most debilitating.1,2 SD may worsen acutely, presenting with increased erythema and edema alongside weeping bullae and vesicles. The spectrum of SD often culminates in the formation of VLUs, which can be challenging to treat and cause substantial patient morbidity.
Management options for SD historically have addressed compromised venous flow and fluid accumulation (e.g., leg compression, leg elevation, and procedural interventions, such as sclerotherapy). Chronic venous and cutaneous inflammation have an equally significant role in the development and progression of SD, but there are few options for targeting these aspects of the disease. Topical therapies, such as corticosteroids (TCS), are one of the few tools that can be used to treat cutaneous inflammation and reduce pain/pruritus, but they do not address the underlying venous inflammatory cascade.3 This limitation highlights the need to search for therapeutic agents that ideally address both chronic inflammation and vascular compromise in SD. Orally administered flavonoids and particularly diosmin are associated with a host of health-promoting benefits and minimal side effects, and they are an emerging option for the medical management of a number of diseases, including CVD.4 Diosmin is the major constituent of both micronized purified flavonoid fraction (MPFF), prescribed as a drug in Europe for over 40 years, and Vasculera® (Primus Pharmaceuticals; Scottsdale, Arizona), prescribed as a medical food in the United States. Each formulation contains diosmin at a concentration of at least 90%. The Society for Vascular Surgery (United States) and the European Society for Vascular Surgery include diosmin in their societal guidelines for the medical management of CVD.5,6 The significant advantage of the approval as a medical food by prescription is the adherence to pharmaceutical standards of production and quality assurance similar to FDA-approved medications. Given the effectiveness of diosmin in managing CVD, there is reason to believe it is a viable therapy for SD. This review provides an overview of relevant pathophysiology and current treatments for the spectrum of SD, highlights key examples of diosmin’s therapeutic mechanisms, and systematically reviews studies investigating clinical efficacy in the management of SD as well as other dermatologic conditions.
Methods
A literature search was conducted using PubMed to obtain key articles on CVD and SD pathophysiology, current SD treatment paradigms, and diosmin pharmacology and therapeutic mechanisms. This search was supplemented with articles obtained through review of citations in the initially returned studies, and these collected works were used to synthesize narrative reviews on the aforementioned subjects. In order to assess the efficacy of diosmin and other flavonoids in the treatment of SD, a systematic literature search with the following search terms was conducted using PubMed: (“Diosmin” OR “MPFF” OR “Micronized Purified Flavonoid Fraction” OR “Flavonoid”) AND (“Stasis Dermatitis” OR “Venous Ulcer” OR “Lipodermatosclerosis”). Further exploration of the potential of diosmin and other flavonoids in the management of dermatologic disease and aesthetic concerns was conducted using a combination of citation harvesting, PubMed searches, and consultation with experts.
Understanding the Dermatologic Manifestations of CVD
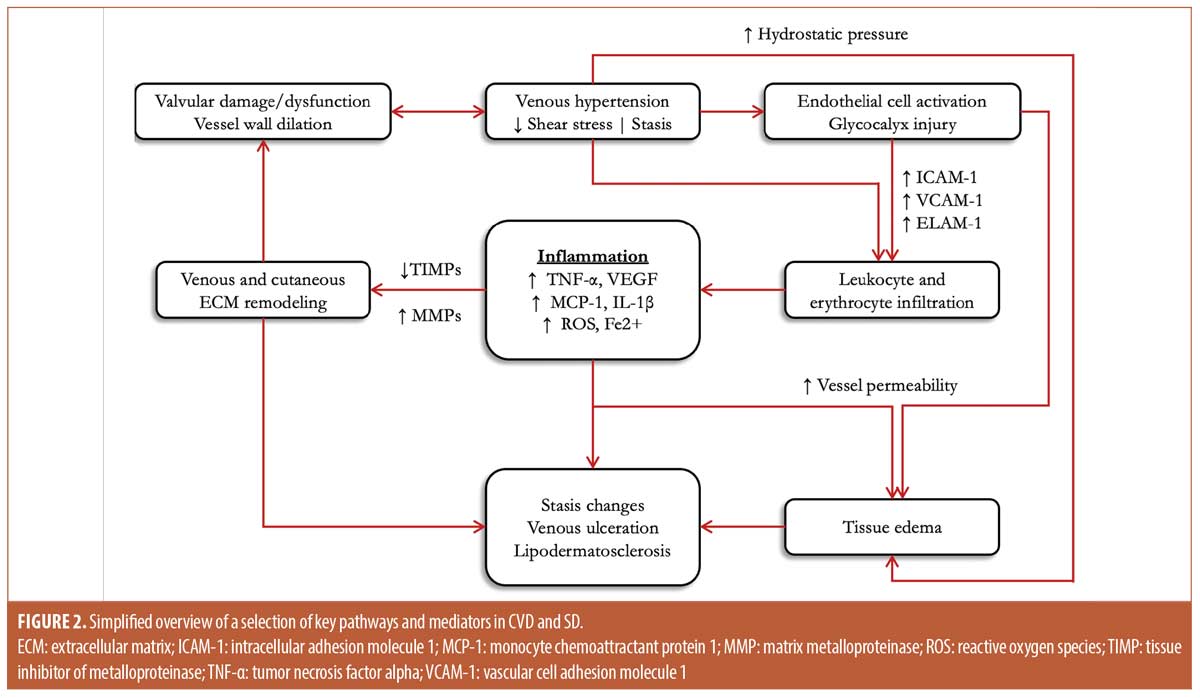
CVD is a common circulatory disorder that generally affects the lower extremities and manifests in the skin as SD, which is a disease spectrum that includes entities like lipodermatosclerosis (LDS) and VLU. Though the pathogenesis of CVD is not fully understood, it seems to arise from a cycle of interactions between two key determinants: venous dysfunction and chronic inflammation. In healthy individuals, the venous system of the lower extremity is pumped by the calf muscles, and the directionality of flow is maintained by a system of one-way venous valves.7 As a result of environmental and/or genetic factors, these valves and vessels may lose functionality, and this leads to venous reflux and hypertension. Increased venous pressure and decreased fluid shear stress promote the activation of numerous pro-inflammatory signaling pathways that cause pathologic changes in vein structure, biomechanics, and cellular behaviors.8 As various alterations caused by inflammation accumulate, vein function is further compromised, and the disease progresses via positive feedback (Figure 2).3,9,10
SD and other cutaneous complications are the byproducts of pathological changes stemming from the venous microenvironment.11 Lower extremity tissue edema is the result of increased vascular fluid leakage caused by high venous pressure and VEGF-mediated permeability of vein endothelium.12 As tissue edema accumulates, it can interfere with nutrient/waste management, reduce oxygenation, and increase tissue tension.7 Upregulation of adhesion molecules (ICAM-1, VCAM-1, ELAM-1), damage to the endothelial glycocalyx, the release of pro-inflammatory cytokines, decreased forward flow, and low fluid shear stress all promote the attachment and infiltration of leukocytes from veins into surrounding dermal tissues.10,13,14 These infiltrating leukocytes, which include a high proportion of T-lymphocytes and macrophages, are inappropriately activated by elevated levels of pro-inflammatory mediators such as TNF-α, IL-1β, and MCP-1.13,15 Together, these are thought to be responsible for much of the chronic cutaneous inflammation that typifies SD. Similarly, the permeability of vein endothelium allows for the increased extravasation of erythrocytes, which are subsequently consumed by macrophages that degrade hemoglobin into the pro-inflammatory hemosiderin pigment observed in LDS.16 Chronically elevated inflammatory signaling also induces the expression of matrix metalloproteinases (MMPs) and suppresses their tissue-specific inhibitors, the combination of which promotes pathologic venous and cutaneous tissue remodeling (e.g., fibrosis).17,18
In the case of VLU formation, each of the aforementioned pathological changes may play a role. Skin that is structurally compromised and possesses low tensile strength as a result of MMP-mediated tissue remodeling will be more strained by increases in skin tension from tissue edema burden. This, in conjunction with the direct damage caused by sequestered leukocytes, increases tissue fragility and may lead to the initial formation of VLU. After VLUs form, upregulated MMPs and other inflammatory mediators may interfere with many aspects of the wound-healing process, from angiogenesis to re-epithelialization. This dynamic contributes to the challenge inherent in treating VLUs and may result in them becoming chronic.19
Current Treatment Paradigms
Most mainstays of therapy for SD focus on improving venous return and limiting edema, such as with extrinsic compression using stockings or bandages; topical agents are also frequently used for the management of specific cutaneous manifestations. Though compression does not affect disease progression, it may reduce symptoms and improve the quality of life in patients with a high degree of compliance. However, studies indicate compliance is often as low as 50-60%.2,6 The presence of comorbidities such as obesity or skin lesions that frequently co-occur with CVD may limit the practicality or desirability of compression therapy because of difficulty donning and doffing tightly fitting gear.6,20 Leg elevation, another common practice intended to improve fluid return, is effective at reducing edema and venous symptoms, though low compliance remains an issue.6 More invasive interventions for improving fluid return, such as sclerotherapy, ablation, or phlebectomy, work by redirecting flow to competent vessels and can significantly improve symptoms. However, these methods are still associated with a high recurrence rate and have an increased risk of complications.8,9,25,29
Available topical therapy options focus on maintaining the skin barrier or limiting the level of cutaneous inflammation.6 SD may present with significant xerosis that can be improved by moisturizing with bland emollients. Mid- to high-potency TCS formulations are routinely used for the management of cutaneous inflammation and can be effective at improving pain and pruritus. Acute exacerbations of SD seem to benefit the most from topical corticosteroids, with mid-potency options being favored for this purpose.21 Topical calcineurin inhibitors have also been shown to improve symptoms in SD, though they have seen much more limited use.22,21 While topical anti-inflammatory therapies are a useful tool and can help manage symptoms, they are limited by an inability to address the underlying venous inflammatory cascade. Additionally, frequent exposure to common vehicle ingredients, such as propylene glycol, may lead to sensitization and further skin barrier disruption.
Active venous ulceration, the most advanced stage of CVD, is managed primarily by facilitating healing and mitigating infection risk through diligent wound care, which may include the use of zinc oxide, iodine, or hydrocolloid dressings.6,7 The Unna boot has been a popular and anecdotally favored inelastic bandage system impregnated with moist zinc oxide, and it has been used in combination with other topical therapies.11 However, a meta-analysis of eight studies published between 2000 and 2015 including patients with a clinical diagnosis of venous leg ulcers showed that elastic, multilayer bandages were more effective at reducing ulcer healing times than their inelastic, single-layer counterparts.23 Furthermore, though excellent wound care can reduce ulcer healing times, the degree of success is highly dependent on patient compliance. Simple bandages applied at home may become tedious to replace, and more complex dressings regularly applied in an office setting may pose significant burdens, not just in time or financial cost, but also in travel and ambulation requirements for patients whose mobility is often restricted.
Current treatment paradigms therefore are more heavily focused on increasing fluid return as compared to decreasing inflammation, and treatments are generally more palliative than curative. Though somewhat effective, conservative management with compression or lifestyle changes is hampered by low compliance. More invasive interventions are still associated with high rates of recurrence. Topical therapies may actively target inflammation, but they do not address it at a venous level.6,7,20,24 Given the limitations of current practices, it is important that additional treatment options be explored.
Therapeutic Mechanisms of Diosmin in Venous Disease
Found abundantly in certain fruits, vegetables, and leaves, flavonoids are a diverse family of bioactive compounds that have been the subject of contemporary therapeutics research for a number of diseases, including CVD.25 Diosmin is a flavone glycoside derived from the citrus-sourced flavanone hesperidin, and it is a well-studied flavonoid with venotonic, anti-inflammatory, anti-diabetic, anti-cancer, and antioxidant properties.4 Semisynthetic preparations of diosmin have been investigated extensively as a supplemental therapy for CVD, and they have excellent safety parameters. In pre-clinical studies of chronic and acute toxicity in both single doses of 3,000mg/kg and repeated doses of 583mg/kg, diosmin was shown to have no toxic effect, and acute dosing of 5,000mg/day in humans has been performed without observed toxicity. Diosmin is available as micronized purified flavonoid fraction (MPFF) in Europe and as Vasculera® (Primus Pharmaceuticals, Scottsdale, AZ) in the United States, with each formulation containing diosmin at a minimum concentration of 90%.26,27 Clinical evidence supports the use of diosmin in patients with SD and CVD, and studies using various models suggest that diosmin may influence the pathophysiology of CVD and its dermatologic manifestations in several ways.6
An investigation in rats with experimentally induced saphenous vein hypertension demonstrated that oral administration of 100mg/kg/day MPFF significantly attenuated several features of venous disease progression over a 21-day period of observation.28 MPFF was initiated in the treatment group 4 days prior to surgical induction of hypertension by saphenofemoral arteriovenous fistula, and the treatment group was compared to the control at one-week intervals. The MPFF group had a significantly lower increase in limb circumference and valvular diameter, as well as a lesser decrease in venous flow velocity. The MPFF group also had significantly less infiltration of neutrophils and macrophages into the vein wall. Another study investigating the effects of 20mg/kg/day oral MPFF on bradykinin and histamine-induced vascular leakage in hamster cheek pouches indicated a similarly attenuating effect of MPFF on venular permeability and edema.29 Experiments assessing levels of key pathophysiologic mediators of CVD following diosmin administration indicate that observed effects on venous hemodynamics and endothelial permeability may result from several mechanisms. Diosmin increased eNOS expression and limited GCX shedding (measured by plasma hyaluronan) in mice following carotid artery ligation.30 Additionally, plasma levels of the permeability-promoting ligand VEGF were observed to decrease following diosmin administration in multiple animal and human studies.31–33 Decreases in leukocyte extravasation may be due in part to decreased levels of ICAM-1 and VCAM, which have been observed in CVD patients taking diosmin.34
In addition to positively influencing venous function, diosmin may more directly mitigate the progressive pathologic tissue remodeling of CVD and SD by promoting ECM homeostasis. An in vitro study using human skin fibroblast cultures treated with lipopolysaccharide (a powerful inflammatory agent) indicated that diosmin inhibits MMP-1 (interstitial collagenase) and elastase 2 in a dose-dependent manner.35 This inhibitory effect is congruent with in silico structure-activity relationship analysis examining interactions between other flavonoids and elastases, and it could be allosteric and/or competitive.36 A semi-quantitative real-time PCR analysis of cultured human articular chondrocytes treated with peroxide demonstrated decreased expression of MMPs 3 & 9 and increased expression of TIMP-1 in cells treated with diosmin.37 Similarly, suppressive effects on serum levels of MMPs 1, 3, and 9 have also been observed in an arthritic rat model treated with 20 mg/kg/day of oral diosmin.38 These results suggest that diosmin may affect major regulators of the ECM through direct inhibition, indirect inhibition (through stimulation of TIMP-1), and transcription-level effects.39
Diosmin also appears to effectuate a multimodal suppression of inflammation beyond attenuation of leukocyte adherence and endothelial transmigration. A 3-month study of 35 CEAP C2-4 patients taking 600 mg diosmin twice daily showed a significant reduction in serum levels of pleiotropic inflammatory signaling molecules TNF-α and IL-6, as well as a reduction in pro-angiogenic FGF-2, which may have a role in potentiating dermal inflammatory responses.33,40 Likewise, levels of TNF-α were noted to decrease following MPFF therapy in a placebo-controlled study of women with varicose veins which also demonstrated significant improvement in metrics of oxidation activity.41 A number of other inflammatory mediators have also been shown to be reduced by diosmin in vitro, including IL-1β, IL-10, COX-2, and PGE-2.35 Furthermore, a study investigating the effect of 600 mg twice daily diosmin on oxidative stress in 47 CEAP 2-5 patients showed a significant reduction in F2 isoprostane, which is a product of non-enzymatic lipid peroxidation and a proxy for free radical activity.42
Taken together, these investigations indicate that diosmin including the MPFF preparation, positively influence many aspects of both the vascular and inflammatory processes of CVD and SD. Because of these diverse mechanisms, diosmin seems well-suited to comprehensively address venous and cutaneous inflammation as well as venous hypertension and dysfunction in ways that current therapies cannot achieve.
Efficacy of Diosmin in Treating Cutaneous Manifestations of Venous Disease
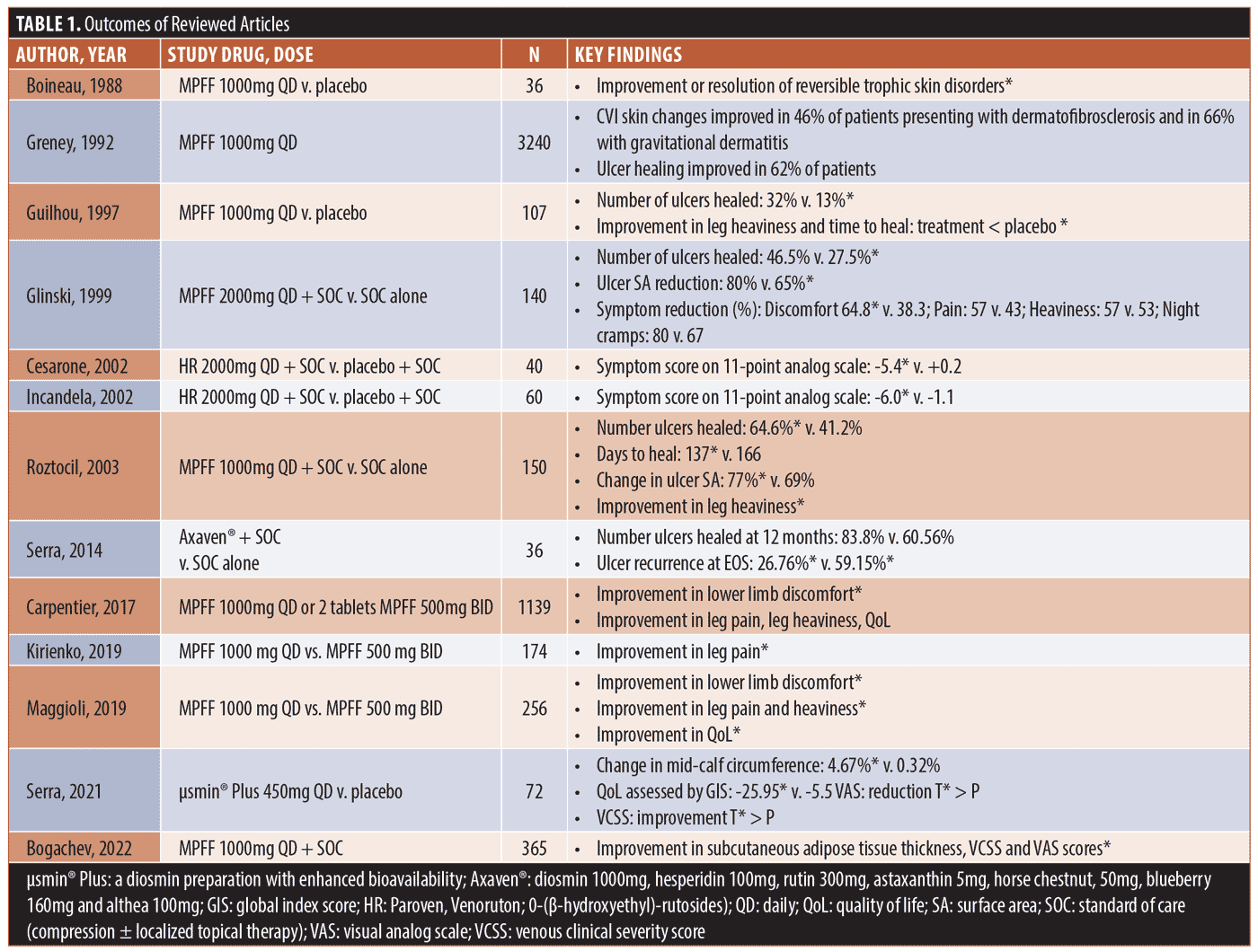
Per the literature review, the initial controlled studies included a total of 605 patients across the seven separate published studies. This was supplemented with other published literature and case studies (Table 1). MPFF was the most commonly studied drug, and the most commonly administered dosage was 1,000 mg per day. Other studied interventions included flavonoid combination drugs, such as Axaven®, which contains diosmin and hesperidin among other ingredients, and Paroven, which contains rutosides. The studies varied greatly in duration of follow-up, which ranged from four weeks to 78 weeks. The primary study outcomes were largely objective and included the number of ulcers healed by the end of the study, the time (in days) to ulcer healing, change in ulcer surface area (SA), rate of ulcer recurrence, change in local and systemic free radicals, and change in venous flux. Secondary outcomes were largely subjective and focused on venous symptoms, though some studies used the change in ulcer SA as a secondary outcome.
The results indicated that diosmin is effective at improving ulcer healing frequency, with rates of 32% to 83.8% in treatment groups versus 13% to 60.56% in placebo groups. Additionally, they were effective at reducing the time to ulcer healing. Outcomes assessing overall ulcer SA reduction favored the use of diosmin, with 2 studies reporting a significantly greater reduction in SA for treatment versus placebo and one study reporting no significant difference. It is worth noting that the studies showing a significant difference in ulcer SA reduction had a more homogenous selection of ulcers since they did not include patients with ulcers >10cm in diameter, while the paper that did not show significance did include such patients. It is possible that a less variable patient population in the non-significant study could have yielded a significant difference in ulcer SA reduction. In one study, diosmin was highly efficacious for preventing ulcer recurrence with a significant difference in ulcer recurrence with rates of 26.76% and 59.15% at 12 months in treatment and placebo groups.
These results indicate that diosmin (as MPFF or a similar compound) in particular, is effective in managing venous ulcers, and this echoes the results of a previous meta-analysis of published and unpublished data from five randomized clinical trials (published trials are included here). Subgroup analysis of the 616 patients in six-month trials in those studies yielded a relative risk ratio of 32% (95% CI 3-70%) for ulcer healing, and time to ulcer healing was shortened by about five weeks. MPFF appeared to be most effective at improving results for ulcers of 5-10 cm2 in area and >6 months in duration.43 Similarly positive results were noted in an uncontrolled study examining the effects of 2 months of 1000 mg MPFF on trophic skin disorders (including SD, VLU, and LDS) with clinical improvement in 46-66% of patients.44 Likewise, a smaller placebo-controlled French study of MPFF in patients with gravitational eczema (SD) showed improvement or resolution of clinical signs in 88% of treated patients vs. only 21% of placebo patients.44
Diosmin was also found to be effective at reducing tissue edema. One study showed a 4.67% reduction in mid-calf circumference with 8 weeks of low-dose micronized diosmin therapy compared to a reduction of 0.32% in those using a placebo comparator. Venous clinical severity scores, which are a subjective assessment of several factors such as cutaneous induration, inflammation, and trophic changes, were noted to progressively improve over the same 8-week period. Improvements in edema are congruent with a 6-month uncontrolled clinical trial of MPFF in 365 patients, which showed significant decreases in both subjective clinical severity scores for edema and ultrasound measurements of subcutaneous tissue thickness.45
Outcomes of subjective assessments indicate that diosmin and related flavonoids are more effective than placebo at reducing patient reports of various venous symptoms, including heaviness and discomfort. Two studies that used a 10-point visual analog scale (VAS) encompassing edema, pain, subjective swelling, leg restlessness, and skin changes observed VAS reductions ranging from -6.0 to -5.4 points in patients treated with flavonoids, compared to -1.1 to +0.2 points in placebo. Another study demonstrated a significant reduction in pain VAS score compared to baseline after 4 weeks of therapy, and that reduction became significantly different from placebo at 8 weeks. The same study also showed treatment groups had a significant and approximately 5-fold greater improvement in Global Index Scores obtained from CIVIQ-20 quality-of-life (QoL) questionnaires. Similar progressive improvements in symptoms, particularly pain, discomfort, and heaviness, as well as QoL are well reported in numerous uncontrolled trials in the literature.45–48
All studies displayed a favorable safety profile, and rates of adverse event reports in treatment groups did not differ significantly from those taking a placebo. Aside from one report of headache and one report of gastrointestinal upset, no adverse events were reported that were deemed related to the study treatment.
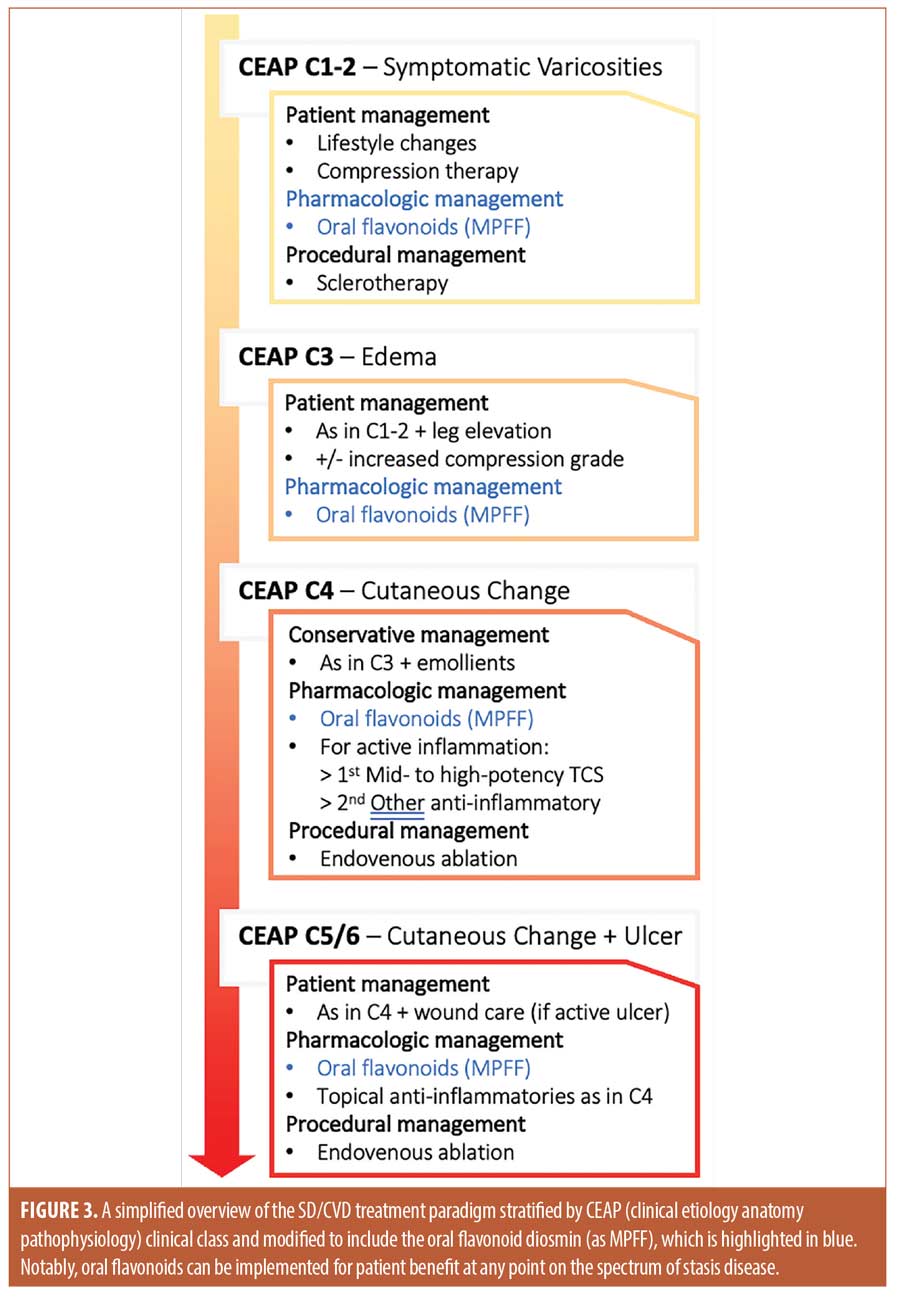
Based on the data, the current treatment paradigm should be modified by initiating oral diosmin alongside patient-centered management as soon as patients develop symptomatic venous disease. Patients in later disease stages, such as those with edema or ulcers, will also see stage-specific benefits (e.g., decreased edema and improved ulcer healing) from adding oral diosmin to the current standard of care (Figure 3).
The Future of Diosmin and other Flavonoids in Dermatology
Therapeutics in dermatology are increasingly focusing on and demonstrating the value of agents targeting key mediators of pathogenic processes. Animal and human pharmacology studies indicate that flavonoids interact with a broad range of these key mediators, particularly those with roles in inflammation and microvascular function. Therefore, it is worthwhile to consider the role of diosmin and perhaps other flavonoids in treating dermatologic illnesses involving similar processes and mediators.
Early animal studies of the flavonoid kaempferol in systemic sclerosis indicate significant reductions in dermal thickness, reactive oxygen species, mRNA markers of oxidative stress, and inflammatory and pro-fibrotic cytokines IL-6, TNF-α, and TGF- β.49 A randomized placebo-controlled trial of 1% Chrysanthellum indicum extract for rosacea demonstrated an improvement in erythema and overall rosacea severity with only mild side effects that were similar to placebo; the reductions in erythema scores were similar to those observed in trials using metronidazole.50 Another trial using a 12-week course of a rutosides complex to treat rosacea demonstrated effectiveness at clearance, and recurrence during the 12-month follow-up period was only observed in the control group.51
Flavonoids have also seen success in treating purpuric and pigmentary diseases. A 1% citrus flavonoid blend was found effective in treating post-inflammatory hyperpigmentation and age spots, with better results seen with longer use.52 A randomized placebo-controlled trial using a novel citrus bioflavonoid blend to treat senile purpura found a significant 50% reduction in the number of purpuric lesions from baseline compared to a 9% increase in lesions in the placebo group with no reports of adverse events.53 Similarly, a case report of a 45-year-old male demonstrated complete resolution of his Schamberg disease after treatment with MPFF, Euphorbia prostata extract, and calcium dobesilate.54 In another case, a 19-year-old woman with Schamberg disease had a recurrence of her lesions after a prednisone taper but achieved complete clearance after four months of treatment with rutosides and ascorbic acid.55,56,57
The studies and diseases referenced here constitute only a small subset of a growing body of literature investigating flavonoids in the management of dermatologic illness. It is worth noting that since many flavonoids share pharmacologic mechanisms, the agents investigated above may be interchangeable with the other flavonoids, like diosmin and MPFF. This evidence suggests a promising future for flavonoids as therapeutic agents in dermatology, and further investigation into their potential applications is warranted.
Conclusion
The literature highlights the complex interaction of various mechanical and biomolecular pathophysiologic determinants and their contributions to CVD and SD. A growing body of knowledge suggests that venous mechanical dysfunction and hemodynamic changes reflect the combined results of myriad alterations in physiologic processes including microvascular structural homeostasis and inflammation. Given that current treatment paradigms for CVD are relatively limited in scope and efficacy, it is unsurprising that efforts to improve patient quality of life are so often stymied; this is likewise true of the typical management for SD. Investigations into the pharmacology of diosmin demonstrate a diverse set of mechanisms by which flavonoids may provide therapeutic benefits in CVD and SD, including multimodal anti-inflammatory action, modulation of vascular permeability and tone, and inhibition of pathologic structural remodeling. Available data on the efficacy of diosmin (as MPFF) suggests significant benefits in ulcer healing rates and reduced ulcer duration. Diosmin has also been shown to reduce leg edema and improve subjective assessments of cutaneous inflammation. Accompanying subjective outcome measures also suggest a host of symptomatic benefits, such as reduction in pruritus, pain, and pigmentation—concerns seen commonly in SD patients. Considering that the adverse effects of diosmin are infrequent and mild, this evidence makes a case for using it as a therapy for SD. Based on their mechanisms of action and early clinical data, flavonoids, particularly diosmin, may have a significant number of applications in both treating various dermatologic diseases and addressing aesthetic concerns.
References
- Sundaresan S, Migden MR, Silapunt S. Stasis Dermatitis: Pathophysiology, Evaluation, and Management. Am J Clin Dermatol. 2017;18(3):383-390. doi:10.1007/s40257-016-0250-0
- Yosipovitch G, Nedorost ST, Silverberg JI, Friedman AJ, Canosa JM, Cha A. Stasis Dermatitis: An Overview of Its Clinical Presentation, Pathogenesis, and Management. Am J Clin Dermatol. 2023;24(2):275-286. doi:10.1007/s40257-022-00753-5
- Pocock ES, Alsaigh T, Mazor R, Schmid-Schönbein GW. Cellular and molecular basis of Venous insufficiency. Vasc Cell. 2014;6(1):24. doi:10.1186/s13221-014-0024-5
- Huwait E, Mobashir M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines. 2022;10(5). doi:10.3390/biomedicines10051076
- Gloviczki P, Lawrence PF, Wasan SM, et al. The 2023 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society Clinical Practice Guidelines for the Management of Varicose Veins of the Lower Extremities. Part II. J Vasc Surg Venous Lymphat Disord. Published online August 2023. doi:10.1016/j.jvsv.2023.08.011
- Wittens C, Davies AH, Bækgaard N, et al. Editor’s Choice – Management of Chronic Venous Disease: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery. 2015;49(6):678-737. doi:10.1016/j.ejvs.2015.02.007
- Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130(4):333-346. doi:10.1161/circulationaha.113.006898
- Castro-Ferreira R, Cardoso R, Leite-Moreira A, Mansilha A. The Role of Endothelial Dysfunction and Inflammation in Chronic Venous Disease. Ann Vasc Surg. 2018;46:380-393. doi:10.1016/j.avsg.2017.06.131
- Smith PDC. The Microcirculation in Venous Hypertension. Vascular Medicine. 1997;2(3):203-213. doi:10.1177/1358863X9700200306
- Mansilha A, Sousa J. Pathophysiological Mechanisms of Chronic Venous Disease and Implications for Venoactive Drug Therapy. Int J Mol Sci. 2018;19(6). doi:10.3390/ijms19061669
- Yosipovitch G, Nedorost ST, Silverberg JI, Friedman AJ, Canosa JM, Cha A. Stasis Dermatitis: An Overview of Its Clinical Presentation, Pathogenesis, and Management. Am J Clin Dermatol. 2023;24(2):275-286. doi:10.1007/s40257-022-00753-5
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77(7):527-543. doi:10.1007/s001099900019
- Costa D, Andreucci M, Ielapi N, et al. Molecular Determinants of Chronic Venous Disease: A Comprehensive Review. Int J Mol Sci. 2023;24(3). doi:10.3390/ijms24031928
- Peschen TLBHM. Expression of the Adhesion Molecules ICAM-1, VCAM-1, LFA-1 and VLA-4 in the Skin is Modulated in Progressing Stages of Chronic Venous Insufficiency. Acta Derm Venereol. 1999;79(1):27-32. doi:10.1080/000155599750011651
- Wilkinson LS, Bunker C, Edwards JC, Scurr JH, Smith PD. Leukocytes: their role in the etiopathogenesis of skin damage in venous disease. J Vasc Surg. 1993;17(4):669-675.
- Ackerman Z, Seidenbaum M, Loewenthal E, Rubinow A. Overload of iron in the skin of patients with varicose ulcers. Possible contributing role of iron accumulation in progression of the disease. Arch Dermatol. 1988;124(9):1376-1378.
- Serra R, Buffone G, Falcone D, et al. Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair Regen. 2013;21(3):395-401. doi:10.1111/wrr.12035
- Serra R, Gallelli L, Butrico L, et al. From varices to venous ulceration: the story of chronic venous disease described by metalloproteinases. Int Wound J. 2017;14(1):233-240. doi:10.1111/iwj.12594
- Raffetto JD, Ligi D, Maniscalco R, Khalil RA, Mannello F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J Clin Med. 2020;10(1):29. doi:10.3390/jcm10010029
- Hamdan A. Management of varicose veins and venous insufficiency. JAMA. 2012;308(24):2612-2621. doi:10.1001/jama.2012.111352
- Rzepecki AK, Blasiak R. Stasis Dermatitis: Differentiation from Other Common Causes of Lower Leg Inflammation and Management Strategies. Curr Geriatr Rep. 2018;7(4):222-227. doi:10.1007/s13670-018-0257-x
- Dissemond, Knab, Lehnen, Franckson, Goos. Successful treatment of stasis dermatitis with topical tacrolimus. Vasa. 2004;33(4):260-262. doi:10.1024/0301-1526.33.4.260
- Paranhos T, Paiva CSB, Cardoso FCI, et al. Systematic review and meta-analysis of the efficacy of Unna boot in the treatment of venous leg ulcers. Wound Repair and Regeneration. 2021;29(3):443-451. doi:10.1111/wrr.12903
- Sundaresan S, Migden MR, Silapunt S. Stasis Dermatitis: Pathophysiology, Evaluation, and Management. Am J Clin Dermatol. 2017;18(3):383-390. doi:10.1007/s40257-016-0250-0
- Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi:10.1017/jns.2016.41
- Amato C. Advantage of a micronized flavonoidic fraction (Daflon 500 mg) in comparison with a nonmicronized diosmin. Angiology. 1994;45(6 Pt 2):531-536.
- das Graças C de Souza M, Cyrino FZ, de Carvalho JJ, Blanc-Guillemaud V, Bouskela E. Protective Effects of Micronized Purified Flavonoid Fraction (MPFF) on a Novel Experimental Model of Chronic Venous Hypertension. Eur J Vasc Endovasc Surg. 2018;55(5):694-702. doi:10.1016/j.ejvs.2018.02.009
- Pascarella L, Lulic D, Penn AH, et al. Mechanisms in experimental venous valve failure and their modification by Daflon 500 mg. Eur J Vasc Endovasc Surg. 2008;35(1):102-110. doi:10.1016/j.ejvs.2007.08.011
- Bouskela E, Donyo KA, Verbeuren TJ. Effects of Daflon 500 mg on increased microvascular permeability in normal hamsters. Int J Microcirc Clin Exp. 1995;15 Suppl 1:22-26. doi:10.1159/000179091
- Mitra R, Nersesyan A, Pentland K, Melin MM, Levy RM, Ebong EE. Diosmin and its glycocalyx restorative and anti-inflammatory effects on injured blood vessels. FASEB J. 2022;36(12):e22630. doi:10.1096/fj.202200053RR
- Shoab SS, Scurr JH, Coleridge-Smith PD. Plasma VEGF as a Marker of Therapy in Patients with Chronic Venous Disease Treated with Oral Micronised Flavonoid Fraction – a Pilot Study. European Journal of Vascular and Endovascular Surgery. 1999;18(4):334-338. doi:10.1053/ejvs.1999.0890
- Tong N, Zhang Z, Zhang W, et al. Diosmin Alleviates Retinal Edema by Protecting the Blood-Retinal Barrier and Reducing Retinal Vascular Permeability during Ischemia/Reperfusion Injury. PLoS One. 2013;8(4):e61794. doi:10.1371/journal.pone.0061794
- Feldo M, Wójciak-Kosior M, Sowa I, et al. Effect of Diosmin Administration in Patients with Chronic Venous Disorders on Selected Factors Affecting Angiogenesis. Molecules. 2019;24(18). doi:10.3390/molecules24183316
- Shoab SS, Porter J, Scurr JH, Coleridge-Smith PD. Endothelial Activation Response to Oral Micronised Flavonoid Therapy in Patients with Chronic Venous Disease – a Prospective Study. European Journal of Vascular and Endovascular Surgery. 1999;17(4):313-318. doi:10.1053/ejvs.1998.0751
- Feldo M, Wójciak M, Ziemlewska A, Dresler S, Sowa I. Modulatory Effect of Diosmin and Diosmetin on Metalloproteinase Activity and Inflammatory Mediators in Human Skin Fibroblasts Treated with Lipopolysaccharide. Molecules. 2022;27(13). doi:10.3390/molecules27134264
- Jakimiuk K, Gesek J, Atanasov AG, Tomczyk M. Flavonoids as inhibitors of human neutrophil elastase. J Enzyme Inhib Med Chem. 2021;36(1):1016-1028. doi:10.1080/14756366.2021.1927006
- Chen YR, Yang KC, Lu DH, Wu WT, Wang CC, Tsai MH. The chondroprotective effect of diosmin on human articular chondrocytes under oxidative stress. Phytother Res. 2019;33(9):2378-2386. doi:10.1002/ptr.6425
- Shaaban HH, Hozayen WG, Khaliefa AK, El-Kenawy AE, Ali TM, Ahmed OM. Diosmin and Trolox Have Anti-Arthritic, Anti-Inflammatory and Antioxidant Potencies in Complete Freund’s Adjuvant-Induced Arthritic Male Wistar Rats: Roles of NF-κB, iNOS, Nrf2 and MMPs. Antioxidants (Basel). 2022;11(9). doi:10.3390/antiox11091721
- Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803(1):55-71. doi:10.1016/j.bbamcr.2010.01.003
- Zittermann SI, Issekutz AC. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am J Pathol. 2006;168(3):835-846. doi:10.2353/ajpath.2006.050479
- Pietrzycka A, Kózka M, Urbanek T, Stpniewski M, Kucharzewski M. Effect of Micronized Purified Flavonoid Fraction Therapy on Endothelin-1 and TNF-α Levels in Relation to Antioxidant Enzyme Balance in the Peripheral Blood of Women with Varicose Veins. Curr Vasc Pharmacol. 2015;13(6):801-808. doi:10.2174/1570161113666150827124714
- Feldo M, Woźniak M, Wójciak-Kosior M, et al. Influence of Diosmin Treatment on the Level of Oxidative Stress Markers in Patients with Chronic Venous Insufficiency. Oxid Med Cell Longev. 2018;2018:2561705. doi:10.1155/2018/2561705
- Coleridge-Smith P, Lok C, Ramelet AA. Venous Leg Ulcer: A Meta-analysis of Adjunctive Therapy with Micronized Purified Flavonoid Fraction. European Journal of Vascular and Endovascular Surgery. 2005;30(2):198-208. doi:10.1016/j.ejvs.2005.04.017
- Ramelet AA. Clinical Benefits of Daflon 500 mg in the Most Severe Stages of Chronic Venous Insufficiency. Angiology. 2001;52(1_suppl):S49-S56. doi:10.1177/0003319701052001S07
- Bogachev V, Boldin B, Turkin P, Samenkov A, Dzhenina O. Micronized purified flavonoid fraction-based conservative treatment of chronic venous disease in a real-world setting. Future Cardiol. 2022;18(10):777-785. doi:10.2217/fca-2022-0026
- Kirienko A, Radak D, Maggioli A. Clinical efficacy of once-daily micronized purified flavonoid fraction 1000 mg tablet in patients with symptomatic chronic venous disease. Curr Med Res Opin. 2019;35(3):553-557. doi:10.1080/03007995.2018.1499508
- Maggioli A, Carpentier P. Efficacy of MPFF 1000 mg oral suspension on CVD C0s-C1-related symptoms and quality of life. Int Angiol. 2019;38(2):83-89. doi:10.23736/S0392-9590.18.04054-3
- Carpentier P, van Bellen B, Karetova D, et al. Clinical efficacy and safety of a new 1000-mg suspension versus twice-daily 500-mg tablets of MPFF in patients with symptomatic chronic venous disorders: a randomized controlled trial. International Angiology. 2017;36(5). doi:10.23736/S0392-9590.17.03801-9
- Sekiguchi A, Motegi SI, Fujiwara C, et al. Inhibitory effect of kaempferol on skin fibrosis in systemic sclerosis by the suppression of oxidative stress. J Dermatol Sci. 2019;96(1):8-17. doi:10.1016/j.jdermsci.2019.08.004
- Rigopoulos D, Kalogeromitros D, Gregoriou S, et al. Randomized placebo-controlled trial of a flavonoid-rich plant extract-based cream in the treatment of rosacea. Journal of the European Academy of Dermatology and Venereology. 2005;19(5):564-568. doi:10.1111/j.1468-3083.2005.01248.x
- Tsiskarishvili N V, Katsitadze A, Tsiskarishvili T, Tchitanava L, Tsiskarishvili NI. [Angioprotectors in the treatment of rosacea]. Georgian Med News. 2014;(228):51-54.
- Kiefer S, Weibel M, Smits J, Juch M, Tiedke J, Herbst N. Citrus Flavonoids with Skin Lightening Effects – Safety and Efficacy Studies. International Journal of Applied Sciences SOFW. Published online December 1, 2010.
- Berlin JM, Eisenberg DP, Berlin MB, Sarro RA, Leeman DR, Fein H. A randomized, placebo-controlled, double-blind study to evaluate the efficacy of a citrus bioflavanoid blend in the treatment of senile purpura. J Drugs Dermatol. 2011;10(7):718-722.
- Gupta A, Sardana K, Kishan Gautam R. Venoprotective drugs in pigmented purpuric dermatoses: A case report. J Cosmet Dermatol. 2019;18(5):1580-1583. doi:10.1111/jocd.12850
- Morquette AJ, Lee JB, Grossman SK, Hsu S. Rutoside and Ascorbic Acid in the Treatment of Schamberg Pigmented Purpuric Dermatosis. Cureus. 2021;13(4):e14592. doi:10.7759/cureus.14592
- Peng B, Hu Q, He R, et al. Baicalein alleviates fibrosis and inflammation in systemic sclerosis by regulating B-cell abnormalities. BMC Complement Med Ther. 2023;23(1):62. doi:10.1186/s12906-023-03885-1
- Xian D, Guo M, Xu J, Yang Y, Zhao Y, Zhong J. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26(1):134-146. doi:10.1080/13510002.2021.1962094