J Clin Aesthet Dermatol. 2025;18(8):13–15.
by Ibukunoluwa Omole, BA; Brindley Brooks, BA; Tyler Pham, BA; Raj Chovatiya, MD, PhD, MSCI; and Steven Daveluy, MD
Ms. Omole and Mr. Pham are with the Michigan State University College of Human Medicine in East Lansing, Michigan. Ms. Brooks is with HS Connect in Puyallup, Washington. Dr. Daveluy is with the Wayne State University Department of Dermatology in Detroit, Michigan. Dr. Chovatiya is with the Rosalind Franklin University of Medicine and Science Chicago Medical School in North Chicago, Illinois and the Center for Medical Dermatology and Immunology Research in Chicago, Illinois.
FUNDING: No funding was provided for this article.
DISCLOSURES: Ms. Brooks is the Founder and Executive Director of HS Connect. Dr. Daveluy has served as an advisor, consultant, speaker, and/or investigator for AbbVie, Incyte, Insmed, Moonlake, Novartis, Pfizer, Regeneron, Sanofi, UCB. Dr. Chovatiya has served as an advisor, consultant, speaker, and/or investigator for AbbVie, Acelyrin, Alumis, Amgen, AnaptysBio, Apogee Therapeutics, Arcutis Biotherapeutics Inc., Argenx, Astria Therapeutics Inc., Avalere Health, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Castle Biosciences, Celldex CLn Skin Care, Dermavant, Eli Lilly and Company, EMD Serono, Formation Bio, Galderma, Genentech, GSK, Incyte, Johnson & Johnson, Kenvue, LEO Pharma, L’Oréal, Nektar Therapeutics, Novartis, Opsidio, Pfizer Inc., RAPT, Regeneron, Sanofi, Sitryx, Takeda, TRex Bio, UCB, Zai Lab. The remaining authors have no conflicts of interest to declare.
Abstract. Hidradenitis suppurativa (HS) is a chronic skin condition characterized by nodules, abscesses, and tunnels that may develop in various parts of the body, particularly in the axillary, gluteal, and inguinal regions.1 Treatment for HS varies based on clinical presentation and disease progression and encompasses antibiotics, hormonal therapy, biologics, topical treatments, and surgical procedures. Despite the array of available treatment options, patients typically require multiple treatment modalities to alleviate symptoms, which can include antimicrobial cleansers and washes, though there is limited evidence regarding their effectiveness in managing HS. Here, we evaluated real-world patient-assessed efficacy of sodium hypochlorite body wash in the management of hidradenitis suppurativa. Of the 165 participants enrolled, 145 completed a four-week study evaluating daily use of sodium hypochlorite body wash for hidradenitis suppurativa (HS), representing all Hurley stages and a wide range of disease durations. Significant improvements were observed across key symptoms, with the greatest reduction in pain (3.52 to 1.62, p<0.001), and over 60 percent of participants reported fewer and shorter flares. Most participants found the wash beneficial, with 88.8 percent recommending it, and there was strong support for the dab method among those who used it. These data support clinical utility of hypochlorite washes in the chronic management of HS. Keywords: Hidradenitis suppurativa, wash, cleanser, sodium hypochlorite
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by nodules, abscesses, and tunnels that can develop on any areas of hair-bearing skin, with a predilection for the axillae, gluteal region, and inguinal areas.1 Presently, there are multiple treatment modalities and approaches aimed at alleviating symptoms and preventing disease progression. These include systemic antibiotic therapy, hormonal treatments, biologics, topical therapies, and surgical interventions, often utilized in combination to manage this debilitating disease.1
Despite limited evidence to support their effectiveness in HS, antimicrobial cleansers and washes are commonly employed for HS in routine clinical practice and recommended as a component of the treatment regimen across all stages of disease in treatment guidelines.1–11 Antimicrobial washes, such as chlorhexidine, zinc pyrithione, bleach baths, and benzoyl peroxide aim to reduce inflammation, restore normal skin and hair follicle flora, and prevent resistance to concomitant topical antibiotics.¹ While sodium hypochlorite containing solutions (commonly in the form of “bleach baths”) have shown efficacy in treating other dermatological conditions like atopic dermatitis and acne, their utility in managing HS remains unexplored.2 We sought to evaluate the efficacy of a commercially available body wash containing sodium hypochlorite in alleviating symptoms experienced by individuals with HS.
Methods
We conducted a prospective study spanning four weeks in participants diagnosed with HS recruited online from the patient group HS Connect (www.hsconnect.org). Consent was obtained from all participants. Participants were instructed to use the sodium hypochlorite body wash (0.006% NaOCl; CLn Bodywash®) daily for four weeks. Patient-reported evaluations were performed at baseline, two weeks, and four weeks via survey. The treatment regimen consisted of 0.006% NaOCl body wash during bathing as part of the regular skincare routine, with the option to additionally utilize the “dab” method. The dab method entails applying the solution directly to a flared lesion and leaving it on the skin for up to 24 hours. Participants were asked to assess disease activity based on parameters such as redness, swelling, drainage, skin pain, itching, and odor, using a scale of 0 to 5, where 5 indicated the most severe symptoms. Additionally, participants who opted for the dab method were given an extra survey with the same set of questions specifically addressing their experience with this technique.
Descriptive statistics were utilized in this study to summarize patient demographics, disease characteristics, and treatment responses. The use of paired t-tests was used to compare mean symptom severity scores (on a scale of 0–5) at baseline and at Week 4 for both the overall body wash regimen and the dab method subgroup. Statistical significance was set to p-value less than 0.05, with attention paid to severity of changes in swelling, skin pain, drainage, itching, and odor across the treatment period.
Results
One hundred and sixty-five participants were enrolled, with 145 completing all surveys across the four-week period. Within this cohort, 18 (11%) participants reported Hurley Stage 1, 77 (47%) Hurley Stage 2, and 70 (42%) Hurley Stage 3. Participants represented a range of HS duration, consisting of 1 to 5 years (10.4%), 5 to 10 years (18.9%), 11 to 15 years (25.0%), 16 to 20 years (16.5%), 21 to 30 years (20.7%), and over 31 years (8.5%).
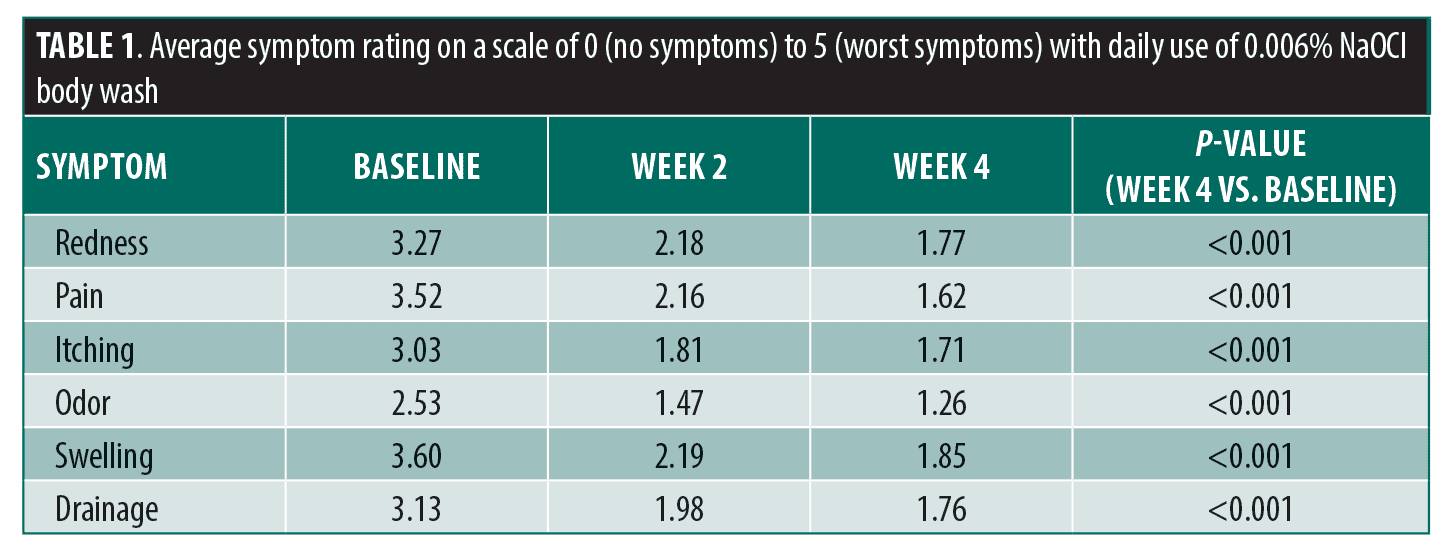
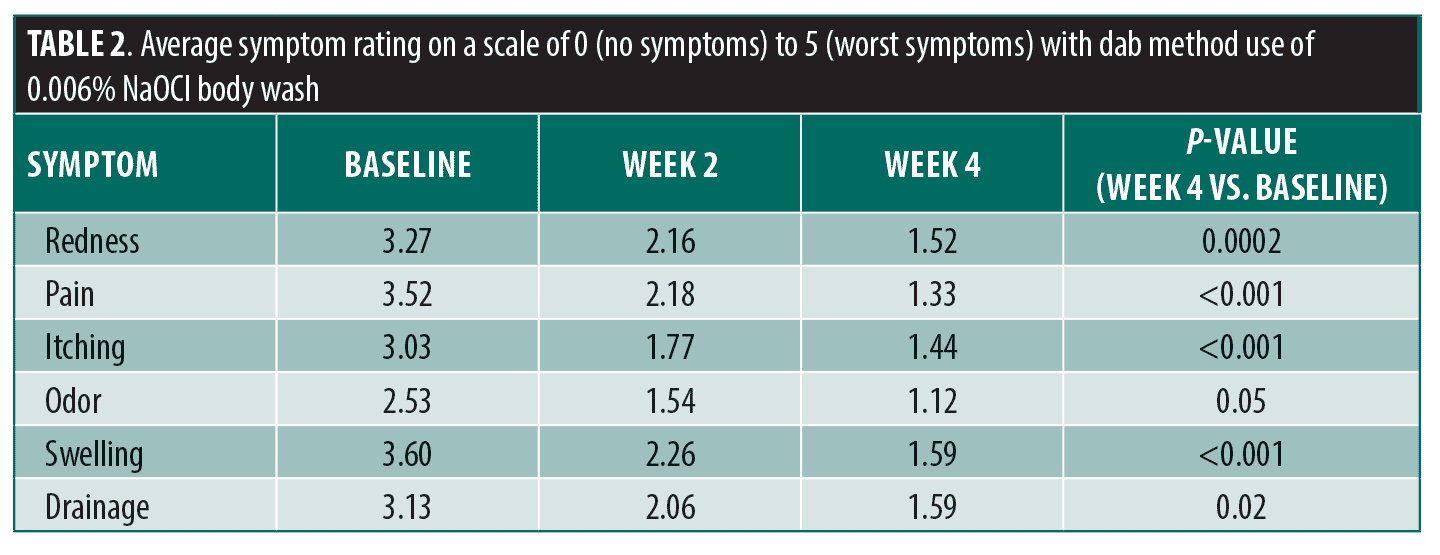
With daily use of sodium hypochlorite body wash, participants experienced significant improvement across all assessed signs and symptoms (redness, swelling, drainage, pain, itching, odor) when comparing baseline and Week 4 scores (Table 1). The greatest decrease was observed in pain, with an average score of 3.52 at baseline and 1.62 at Week 4 (p<0.001), representing a 1.90-point reduction. A decrease in the number of flares was reported by 63.5 percent of participants and a decrease in duration of flares by 62.7 percent. When utilizing the dab method, participants experienced significant improvements in redness, swelling, drainage, pain, and itching comparing Week 4 to baseline (Table 2). The scores for odor showed a trend toward improvement with the dab method, but did not reach statistical significance (baseline: 1.54, Week 4: 1.12; p=0.05). The majority (88.8%) of participants stated that they would recommend the body wash to patients with HS. Seventy-five percent of participants tried the dab method. Of those, 85.0 percent indicated they will continue using it and 90.0 percent would recommend it to others.
Discussion
HS remains a challenging disease to treat and is associated with an enormous impact on quality of life, including depression and low self-esteem.4 Most patients require a combination treatment regimen to achieve disease control. Antimicrobial washes, including benzoyl peroxide, chlorhexidine, zinc pyrithione, and bleach baths, are commonly used across all stages and severity of disease, but their use is only supported by anecdotal evidence.1 Choice of antimicrobial wash is guided by expert opinion, patient preference, accessibility, and affordability. Adherence to daily use of antimicrobial washes can be low (with 30 percent reported in one study), with difficulty finding the wash in the store and affordability reported as the most significant barriers.10 With the frequent use of antibiotics in HS, bacterial resistance is a common concern. In a cross-sectional analysis, concomitant use of antimicrobial washes, including chlorhexidine, bleach baths, benzoyl peroxide, or a combination, showed decreased bacterial resistance to several classes of antibiotics, including penicillins and cephalosporins.8 While clinical experience supports a role for antimicrobial washes in HS, rigorous study of their potential benefits may provide better evidence-guided treatment decisions.
Sodium hypochlorite containing washes, which includes bleach baths, have demonstrated benefit in multiple skin conditions such as atopic dermatitis (AD), acne, and herpes simplex.5-7,12 They have been utilized and studied most extensively in AD, potentially conferring benefits through anti-inflammatory properties as well as decreasing Staphylococcus aureus colonization.5 A recent meta-analysis of bleach baths in AD that included 10 randomized, controlled trials with 307 participants revealed overall improvement in clinician-reported severity by approximately 22.0 percent, with 10.0 percent of patients achieving a 50.0 percent or greater improvement measured by the Eczema Area and Severity Index (EASI).3 The risk of S. aureus colonization was slightly decreased (RR: 0.89, 95% CI: 0.73–1.09) across seven studies that performed culture.3 This specific formulation of sodium hypochlorite body wash has been studied in two open-label trials in 68 total pediatric participants with AD, demonstrating a significant reduction in EASI (46.0%) after six weeks of daily use with bathing.3,9
We investigated the efficacy of 0.006% NaOCl body wash in the treatment of HS in a real-world setting. Participants experienced significant improvement across all measured signs and symptoms (redness, swelling, drainage, pain, itching, odor) with four weeks of daily use with bathing. The greatest improvement was seen for pain, the most significant symptom in HS, with a reduction of 1.9 points on the 0–5 scale. A decrease in both the number and duration of self-reported flares was also reported by most participants. When utilizing the dab method, wherein the wash is applied to a flared lesion and left in place for up to 24 hours, participants also experienced significant improvements in redness, swelling, drainage, pain, and itch. Most importantly, most participants agreed that they would recommend the body wash daily (88.8%) and the dab method (90.0%) to other patients with HS. Limitations of the current study include the small sample size, lack of control arm, and self-reporting of outcomes. The absence of a lack of control arm prevents direct comparison with standard care or placebo, making it harder to isolate the true effects of the hypochlorite wash. In addition, relying on self-reported results may introduce the possibility of recall bias, though the goal of this study was to assess symptoms purely from the patient perspective. An additional limitation is the use of a non-validated questionnaire to assess symptom changes. While this tool allowed for the collection of relevant patient-reported outcomes, the lack of validation may impact the reliability and reproducibility of the findings. However, it is important to note that no validated questionnaires currently exist specifically for evaluating short-term patient-assessed treatment response in HS, highlighting the need for the development of standardized, validated instruments tailored to this population.
This study demonstrates the real-world efficacy of a sodium hypochlorite wash in improving the symptoms of hidradenitis suppurativa, with improvements across all studied symptoms: redness, swelling, drainage, itching, odor, and even pain. An over-the-counter wash that is available to patients of all HS severities, well-tolerated, and may counter antibiotic resistance is an important addition to the current treatment armamentarium. Furthermore, a significant issue faced by HS patients is delayed diagnosis, often due to difficulties in accessing healthcare or a lack of awareness among providers, which can lead to progression of HS and irreversible tissue destruction.11 A readily available sodium hypochlorite body wash presents an option for patients to initiate a treatment when they suspect HS but have not yet received a diagnosis or have been unable to seek specialized clinical care.
References
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1): 91–101.
- An JH, Moon SJ, Shin JU, et al. Clindamycin Mono-Therapy of Hidradenitis Suppurativa Patients: A Single-Center Retrospective Study. Ann Dermatol. 2021;33(6):515–521.
- Bakaa L, Pernica JM, Couban RJ, et al. Bleach baths for atopic dermatitis: A systematic review and meta-analysis including unpublished data, Bayesian interpretation, and GRADE. Ann Allergy Asthma Immunol. 2022;128(6):660–668.
- Chernyshov PV, Finlay AY, Tomas-Aragones L, et al. Quality of Life in Hidradenitis Suppurativa: An Update. Int J Environ Res Public Health. 2021;18(11):6131.
- Chopra R, Vakharia PP, Sacotte R, Silverberg JI (2017). Efficacy of bleach baths in reducing severity of atopic dermatitis: A systematic review and meta-analysis. Ann Allergy Asthma Immunol. 2017;119(5):435–440.
- Dorostkar A, Ghahartars M, Namazi M, et al. Sodium Hypochlorite 0.005% Versus Placebo in the Treatment of Mild to Moderate Acne: A Double-Blind Randomized Controlled Trial. Dermatol Pract Concept. 2021;11(3):e2021046.
- Hunter DT. Sodium hypochlorite in the treatment of herpes simplex virus infections. Cutis. 1983;31(3):328–332.
- Leiphart P, Ma H, Naik HB, Kirby JS. The effect of antimicrobial washes on antibacterial resistance in hidradenitis suppurativa lesions. J Am Acad Dermatol. 2019;80(3):821–822.
- Majewski S, Bhattacharya T, Asztalos M, et al. Sodium hypochlorite body wash in the management of Staphylococcus aureus-colonized moderate-to-severe atopic dermatitis in infants, children, and adolescents. Pediatr Dermatol. 2019;36(4):442–447.
- Leiphart P, Kitts S, Kirby JS. Adherence to Over-the-Counter Antimicrobial Washes in Hidradenitis Suppurativa Patients. Dermatology. 2019; 235(5):440–441.
- Hunger RE, Laffitte E, Läuchli S, et al. Swiss Practice Recommendations for the Management of Hidradenitis Suppurativa/Acne Inversa. Dermatology. 2017;233(2–3): 113–119.
- Ryan C, Shaw RE, Cockerell CJ, et al. Novel sodium hypochlorite cleanser shows clinical response and excellent acceptability in the treatment of atopic dermatitis. Pediatr Dermatol. 2013;30(3):308–315.
