 J Clin Aesthet Dermatol. 2021;14(2):E69–E88.
J Clin Aesthet Dermatol. 2021;14(2):E69–E88.
by Riekie Smit, MD; Elena Gubanova, MD; Joely Kaufman, MD; Marina Landau, MD; Beatriz Molina, MD; Bill Andriopoulos, PhD; Pascal Maisonobe, MSc; Inna Prygova, MD; and Alessio Redaelli, MD
Dr. Smit is with the Dr. Riekie Smit Aesthetic Medical Practice in Pretoria, South Africa. Dr. Gubanova is with Vallex Med Clinic of Preventive Medicine, Moscow National University of Food Production in Moscow, Russian Federation. Dr. Kaufman is with the University of Miami, Miller School of Medicine and Skin Associates of South Florida in Coral Gables, Florida, United States of America. Dr. Landau is with Wolfson Medical Center in Holon, Israel. Dr. Molina is with Medikas Medispa in Street, Somerset, United Kingdom. Dr. Andriopoulos is with Galderma Aesthetics in Uppsala, Sweden. Mr. Maisonobe and Dr. Prygova are with Ipsen Pharma, Boulogne-Billancourt, France. Dr. Redaelli is with the Visconti di Modrone Medical Center in Milan, Italy.
FUNDING: This study was sponsored by Ipsen.
DISCLOSURES: Dr. Smit has received consultant fees from Ipsen, Fillmed, Hansbiomed, and Inova Pharmaceuticals. Dr. Gubanova has received consultant fees from Merz, Ipsen, Galderma, and SkinCeuticals. Dr. Kaufman has received research grants from Allergan, Galderma, Merz, Revance and Evolus and consultancy fees from Allergan, Galderma, Merz, Revance, and Evolus. Dr. Landau has received consultant fees from Ipsen. Dr. Molina has received consultant fees from IBSA International. Dr. Andriopoulos is an employee of Galderma. Mr. Maisonobe and Dr. Prygova are employees of Ipsen. Dr. Redaelli has received constultant fees from Ipsen and FillMed and is the editor of Officina Editoriale Oltrarno Firenze.
ABSTRACT: Background: AbobotulinumtoxinA (AboBoNT-A; Dysport®; Ipsen, Boulogne-Billancourt, France/Azzalure®; Galderma, Lausanne, Switzerland) is a botulinum neurotoxin type A approved for aesthetic use in the treatment of glabellar lines in adult patients under 65 years in Europe, the United States, and other countries.
Objective: We sought to analyze current literature on patient satisfaction with aboBoNT-A for upper facial aesthetic indications.
Methods: A systematic review of literature databases (PubMed/MEDLINE, Embase, the Cochrane Library, and Google Scholar) was performed to identify English-language publications reporting on patients with aesthetic indications (including glabellar lines and wrinkles) receiving aboBoNT-A, that assessed patient and/or physician satisfaction with treatment, with no restrictions on comparator studies. Structured data extraction was used to enable inter-study analysis. A post-hoc analysis was also performed to assess patient satisfaction by sex and age, using results from the noninterventional APPEAL study of patients’ satisfaction with aboBoNT-A for treating glabellar lines.
Results: Overall, 22 original research papers were identified. Patient satisfaction rates for aboBoNT-A treatment were significantly higher versus placebo from two weeks to between three and five months postinjection. At two to three weeks postinjection, patient satisfaction rates were 52% and 99% across studies. In studies with later time points, patient satisfaction rates were 85 to 87 percent at 5 months and between 25 and 100 percent at 6 months post-injection. Physician satisfaction was also high (97%–100%, across three treatments). No notable differences in patient satisfaction by sex or age were observed in the APPEAL study.
Conclusion: High rates of patient satisfaction have been achieved with aboBoNT-A treatment for upper facial aesthetic indications. Despite the current recommended interval of ?12 weeks, satisfaction with the aesthetic results of aboBoNT-A therapy is still evident up to 6 months post-injection in some patients.
KEYWORDS: AbobotulinumtoxinA, botulinum toxin, glabellar lines, lateral canthal lines, crow’s feet, wrinkles, patient satisfaction, Dysport
The short- and long-term efficacy and safety of abobotulinumtoxinA (aboBoNT-A; Dysport®; Boulogne-Billancourt, France/Azzalure®; Galderma, Lausanne, Switzerland) for treating glabellar lines have been well established in clinical studies.1–5 AboBoNT-A is a botulinum neurotoxin type A (BoNT-A) approved for aesthetic use in the treatment of glabellar lines in adult patients younger than 65 years in Europe, the United States and other countries.6,7 Approval of aboBoNT-A for facial aesthetic use extends to the treatment of lateral canthal lines (i.e., crow’s feet) in European and Asian countries as well as to hyperfunctional facial lines in Latin America.6,8,9 Current dosing guidelines recommend a minimum treatment interval of at least 12 weeks6,7 and studies have demonstrated that reduced wrinkle severity is commonly seen within 1 to 7 days after injection and persists for 3 to 6 months.1,10
Patients’ satisfaction with their appearance is of the utmost importance in the evaluation of cosmetic treatments and it is increasingly acknowledged that the evaluation of patients’ perspectives is a necessary aspect of providing patient-centered treatment and to inform decision-making.11 Furthermore, real-world data concerning patients’ beliefs and attitudes following long-term aboBoNT-A treatment and whether they vary according to age and sex are limited.
Here, we report results from a systematic review of patient and physician satisfaction with aboBoNT-A for the aesthetic treatment of glabellar lines and other areas of the upper face as reported in the literature up to September 2018. We also report the findings of a post-hoc analysis of results from a real-world observational study (APPEAL)12 of patient satisfaction with glabellar line treatment after three injection cycles of aboBoNT-A, stratifying results by sex and by age group.
Methods
Systematic literature search and study selection. This systematic literature review was performed in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.13 Searches were conducted in PubMed, EMBASE, the Cochrane Library, and Google Scholar on September 11 and 12, 2018. Search terms were developed by reviewing the background literature for terms related to the research question, in line with the objective outlined above. The full search strategy is presented in Supplementary Table 1. Searches were limited to English-language manuscripts, with no date restrictions. Abstracts from conferences and meetings were excluded. Review articles were also excluded, although their bibliographies were manually searched to identify further articles that met the study inclusion criteria. Citations were downloaded into the reference management software EndNote X7 (Clarivate Analytics, Philadelphia, Pennsylvania) to look for duplicates, which were rechecked manually. Citations and abstracts were screened for inclusion by two reviewers using the eligibility criteria defined using a population, intervention, comparator, outcomes, and setting (PICOS) strategy (Supplementary
Table 2). 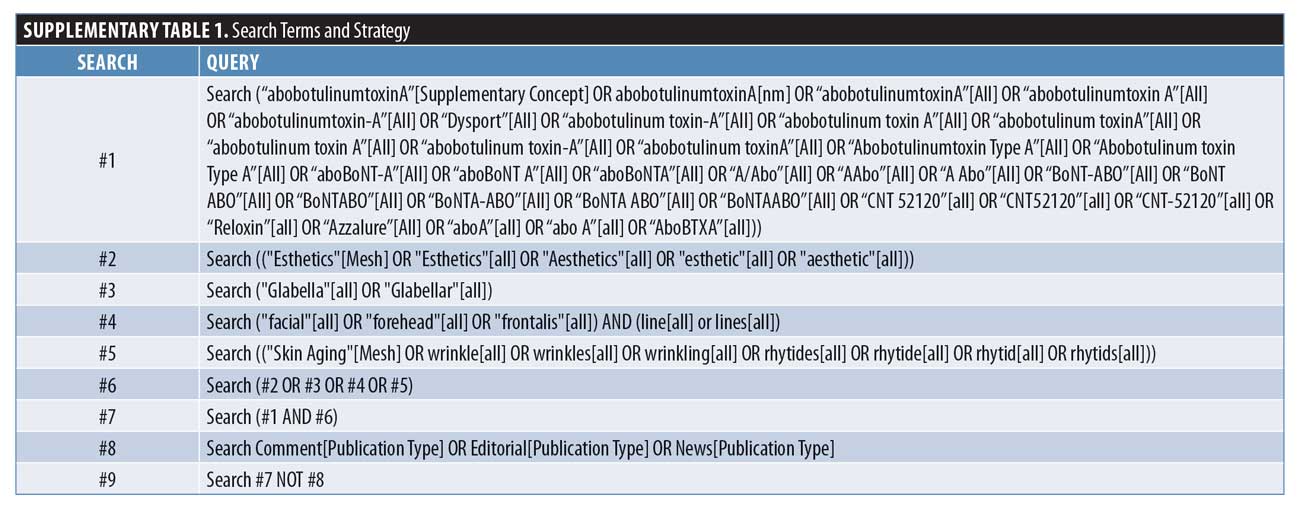
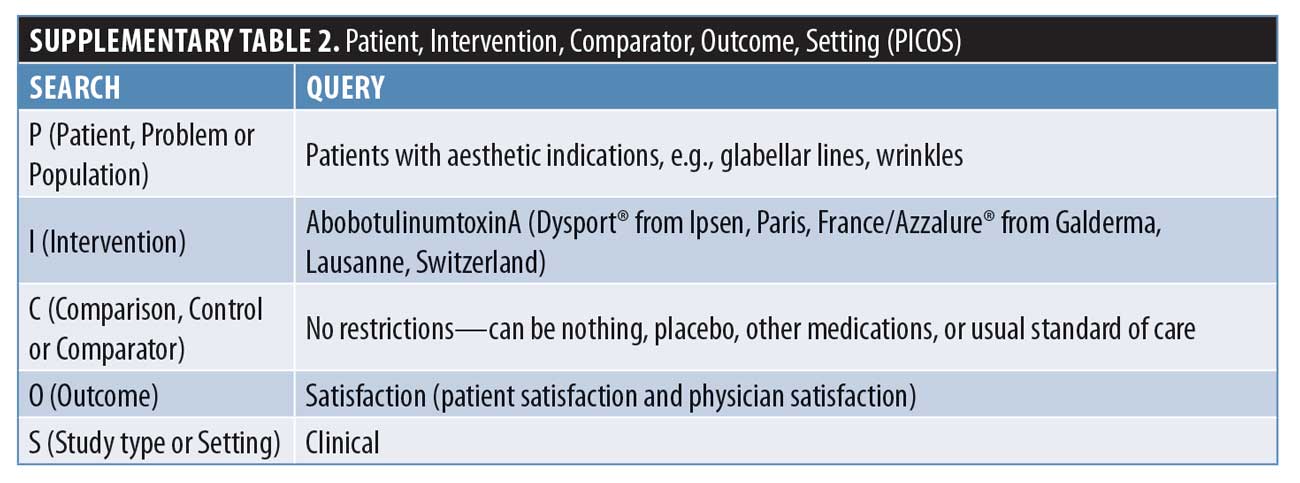
The patient population was defined as patients with aesthetic indications, such as glabellar lines, while the intervention was aboBoNT-A. No restrictions were placed on comparators. Outcomes were defined as either patient or physician satisfaction with treatment. Study type was any clinical setting, excluding case reports.
Full manuscripts were obtained for abstracts considered eligible from the initial screening. For each manuscript, the relevance was assessed by two reviewers; one reviewer checked all manuscripts for inclusion and consistency of data-extraction forms.
Data extraction and assessment. Study design, population, and outcome data were extracted by each reviewer using a standardized data-extraction form (Supplementary Appendix 1). An assessment of bias was completed using the Cochrane Collaboration’s (London, England) tool for assessing the risk of bias in randomized controlled trials.14 A similar approach was used to assess the risk of bias in nonrandomized studies using the Methodological Index for Nonrandomized Studies (MINORS) checklist.15 As a statistical meta-analysis was not conducted, an overall assessment of quality across studies (e.g., heterogeneity and publication bias) was not performed.
The primary objective was to assess patient and physician satisfaction with aboBoNT-A for aesthetic use in the upper face. Results are presented here, in tables and figures and narratively described.
APPEAL study satisfaction by sex and age. The APPEAL study (NCT02353897) was a multicenter, noninterventional, prospective, longitudinal study in patients with moderate-to-severe glabellar lines conducted across six countries between October 2014 and December 2016. Full details of the APPEAL study methodology and results have been previously published.12 In brief, patients received three injection cycles of aboBoNT-A, which were administered in accordance to the investigators’ routine clinical practice. Meanwhile, the minimal interval between injections was in accordance to the local summary of product characteristics, while the maximum recommended treatment interval was less than six months. Patients attended three injection visits and a follow-up visit at three weeks ± seven days postinjection. At each follow-up visit, patients completed a satisfaction questionnaire and, at follow-up visits 1 and 3, investigator satisfaction was recorded. The questions asked at each follow-up visit are available in the supplementary material of the primary publication.12
In this post-hoc analysis of the APPEAL study, we aimed to compare patient satisfaction, stratified by age and sex, after three injection cycles of aboBoNT-A for the treatment of glabellar lines. Patients were adults (aged >18 or >21 years, depending on local legislation) with moderate-to-severe glabellar lines at maximum frown as assessed on the four-point glabellar lines severity scale (0=none, 1=mild, 2=moderate, 3=severe) who were naïve to aesthetic treatments or procedures for glabellar lines. Further exclusion criteria are defined in the primary manuscript.12 All patients provided written informed consent to participate and the decision to receive long-term (?3 cycles) treatment with aboBoNT-A for glabellar lines was made prior to and independently of the decision to participate in the study.
The primary effectiveness endpoint was patient satisfaction with the treatment of glabellar lines after three injection cycles, assessed on a five-point Likert scale (very satisfied, satisfied, neutral, dissatisfied, very dissatisfied) and dichotomized variables (satisfied vs. not satisfied [inclusive of neutral]). Secondary endpoints included patient satisfaction after one and two injection cycles and patient satisfaction with individual factors related to satisfaction, including aesthetic outcomes, self-perceived age, natural look, expectations being met, self-feeling improvement, recommendations to family or friends, and the desire to receive another injection.
Statistical analyses. Data are summarized as frequency counts and percentages for all patient satisfaction and questionnaire data, with 95% confidence intervals (CIs) calculated using the Agresti–Coull method for approximate binomial CIs. Analyses were performed on the full study population, including all patients who provided informed consent and who received at least one injection. All statistical analyses were performed using the SAS version 9.4 software program (SAS Institute Inc., Cary, North Carolina).
Results
Literature search and selected studies. In total, 320 studies were identified from the surveyed medical literature databases and the supplementary manual selection of manuscripts from the bibliographies of review articles. Of these, 22 publications met the criteria for primary analysis. Figure 1 shows the PRISMA diagram of all publications evaluated for inclusion and the reasons for exclusion. In Tables 1 to 3, we present the study design, outcome measures, and a summary of satisfaction results from the identified publications. 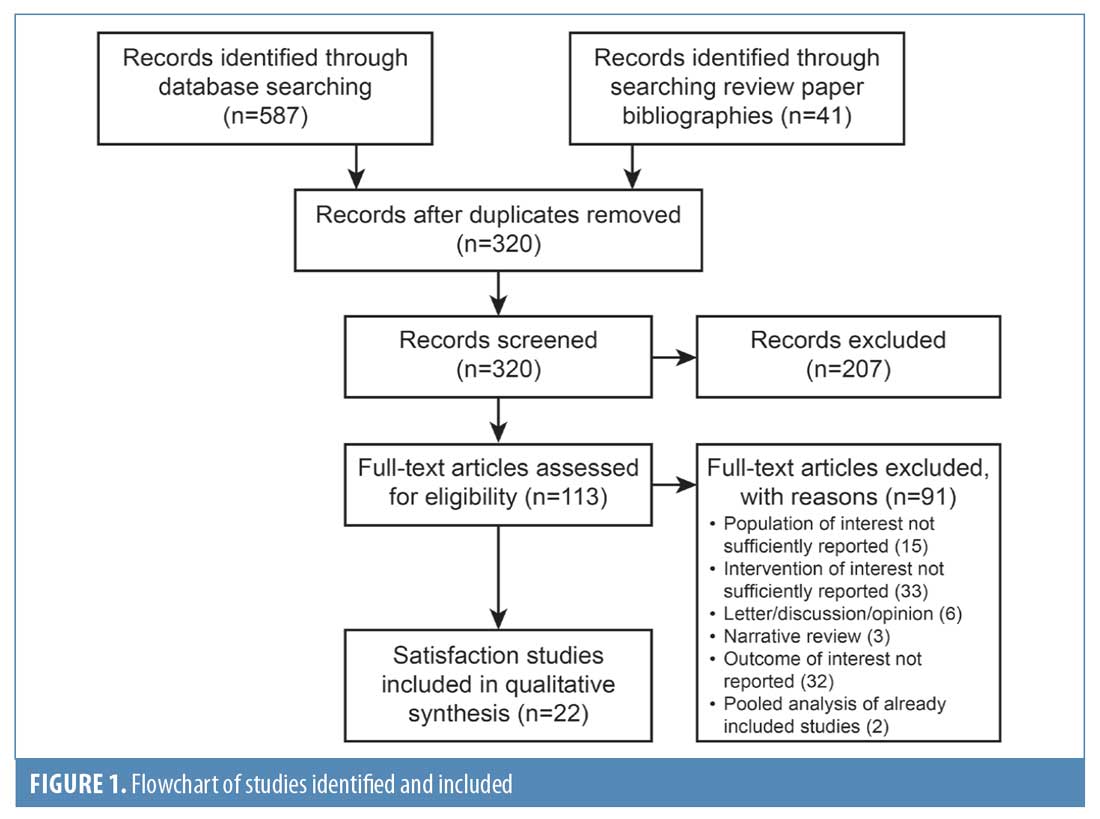
Studies of similar design (i.e., placebo-controlled, comparator-controlled, or uncontrolled) are discussed together and by indication within these sections. Furthermore, a summary of patient satisfaction reported as the percentage of patients satisfied with aboBoNT-A treatment at or after 12 weeks (i.e., three months) postinjection is presented in Figure 2. 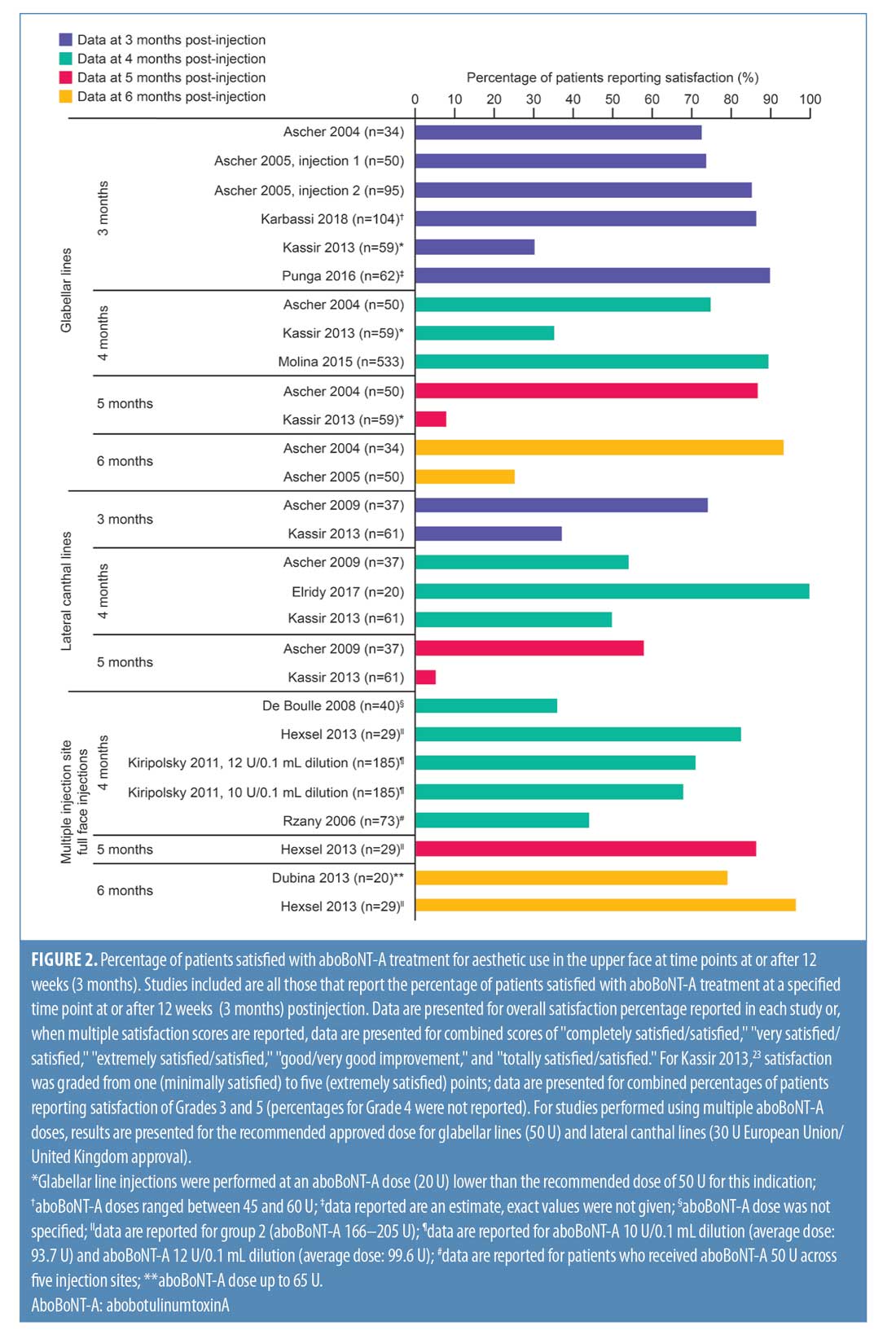
Placebo-controlled studies. Overall, four double-blind, randomized, placebo-controlled studies were identified that assessed patient satisfaction following treatment with aboBoNT-A (Table 1).2,3,16,17 Three of these studies assessed the statistical significance of aboBoNT-A versus placebo, demonstrating the superiority of aboBoNT-A at various doses for achieving patient satisfaction following treatment of glabellar lines, lateral canthal lines, or multiple sites. The earliest time point at which satisfaction was reported was Week 2, with 66 to 85 percent of patients satisfied with treatment.3,16 At later time points across these studies, satisfaction rates of 45 to 75 percent were reported at four months postinjection,2,16,17 that of 87 percent was reported at five months postinjection,17 and those from 25 to 93 percent were reported at six months following treatment.3,16,17 Results from each of these studies are presented in Figure 2.
In a dose-ranging study by Ascher et al,3 the percentage of patients who were satisfied or completely satisfied by their aboBoNT-A dose (25U, 50U, or 75U) varied across time points, ranging from 66 to 70 percent at two weeks, 66 to 86 percent at one month, 72 to 82 percent at three months, and 62 to 93 percent at six months following treatment, with the highest satisfaction rates recorded in the aboBoNT-A50 U group. In the placebo group, the percentage of patients satisfied or completely satisfied with treatment ranged from 7 to 13 percent at each time point.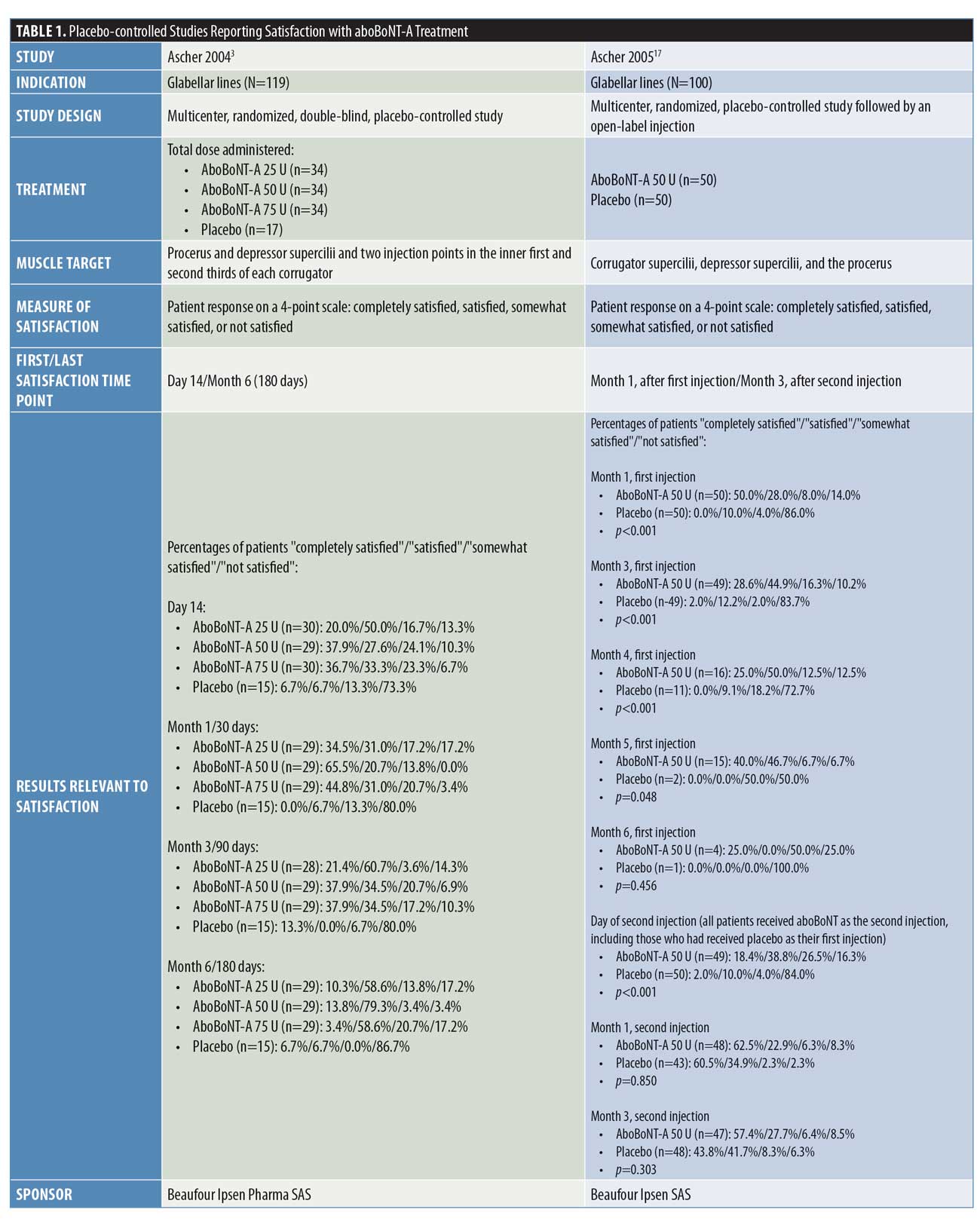
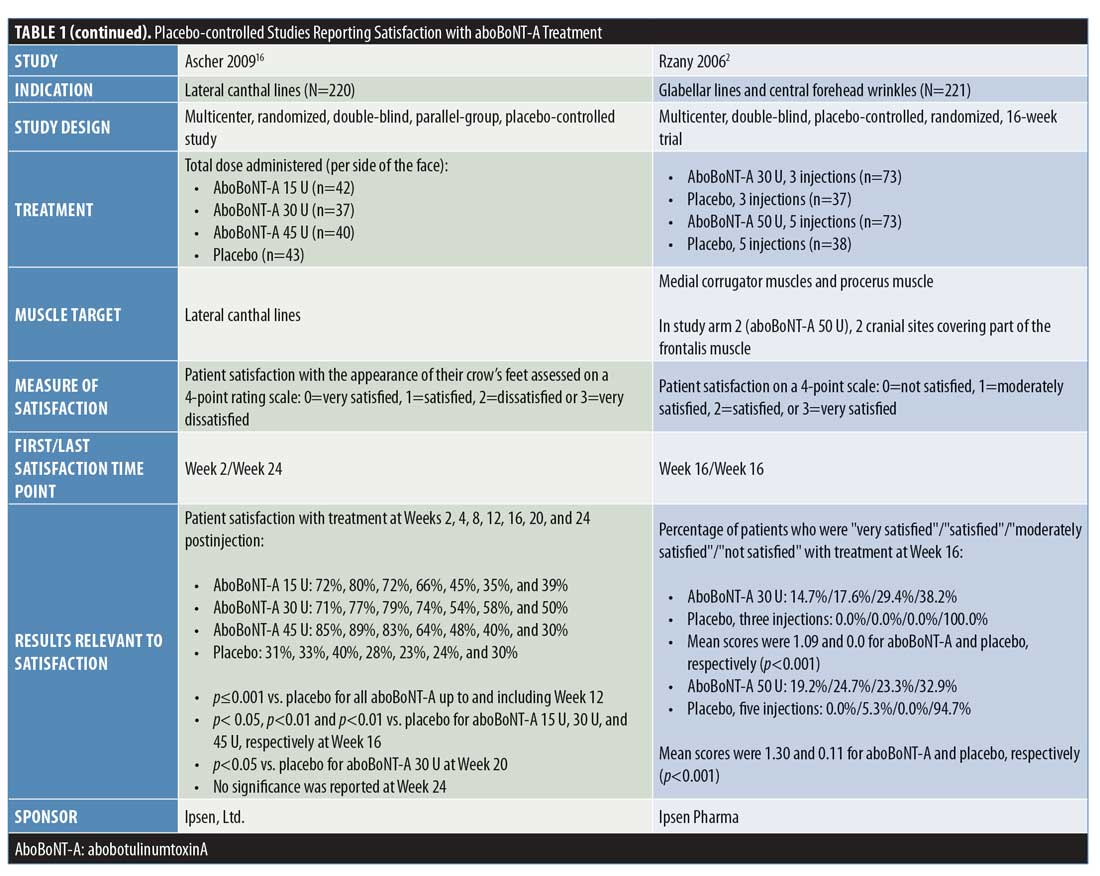
In another study by Ascher et al,17 patients received an injection cycle of aboBoNT-A50 U or placebo for the treatment of glabellar lines, which was followed by a second injection cycle in which all patients received aboBoNT-A 50 U. The proportion of patients reporting satisfaction with treatment was significantly higher in the aboBoNT-A group than in the placebo group at each time point assessed from 1 month to 5 months after the first injection (p<0.01 to p=0.048), with 75 percent and 87 percent of patients reporting that they were satisfied/completely satisfied with treatment at four and five months postinjection (vs. 9% and 0% in the placebo group), respectively. At six months, the satisfaction rates were higher with aboBoNT-A 50 U but not statistically significant relative to that in the placebo group (25% vs. 0%, respectively). After the second injection cycle, the proportions of patients satisfied/completely satisfied with treatment at one and three months postinjection with aboBoNT-A were 85 percent and 85 percent, respectively, for those who received aboBoNT-A during both injection cycles, and 95 percent and 86 percent, respectively, for those who had received the placebo during the first injection cycle.
In a dose-ranging study by Ascher et al16 of aboBoNT-A versus placebo for the treatment of lateral canthal lines, satisfaction rates were reported from two weeks up to 24 weeks (6 months) postinjection. With all aboBoNT-A doses tested (15 U, 30 U, or 45 U per side), the proportion of patients reporting treatment satisfaction was significantly higher than with the placebo up to 16 weeks (4 months) postinjection (p?0.001 up to and including Week 12; p<0.05, p<0.01, and p<0.01 for aboBoNT-A 15 U, 30 U, and 45 U, respectively, at Week 16). However, at 20 weeks postinjection, satisfaction rates were significantly higher than with the placebo in the aboBoNT-A 30 U group only.
These high rates of satisfaction (very satisfied or satisfied) across doses were 71 to 85 percent at the first time point recorded (Week 2), peaking at 77 to 89 percent at Week 4, with the highest satisfaction rates associated with the aboBoNT-A dose of
45 U at these time points. At later time points, satisfaction was maintained in 45 to 54 percent of patients at Week 16, 35 to 58 percent at Week 20, and 30 to 50 percent at Week 24, with the highest rates achieved in the aboBoNT-A30 U group from Week 12 onwards.
Rzany et al2 reported patient satisfaction with treatment at 16 weeks following injection with either aboBoNT-A 30 U or placebo across three injection sites or with aboBoNT-A50 U or placebo across five injection sites for glabellar lines and central forehead wrinkles. At Week 16 after injections at three sites, 62 percent of patients receiving aboBoNT-A were at least “moderately satisfied” with treatment compared to zero percent in the placebo group (p<0.001). Similarly, after injections were performed at five sites, 67 percent of patients receiving aboBoNT-A were at least “moderately satisfied” with treatment relative to just five percent of patients in the placebo group (p<0.001) at 16 weeks. No statistical comparisons were performed for results according to the number of aboBoNT-A injection sites.
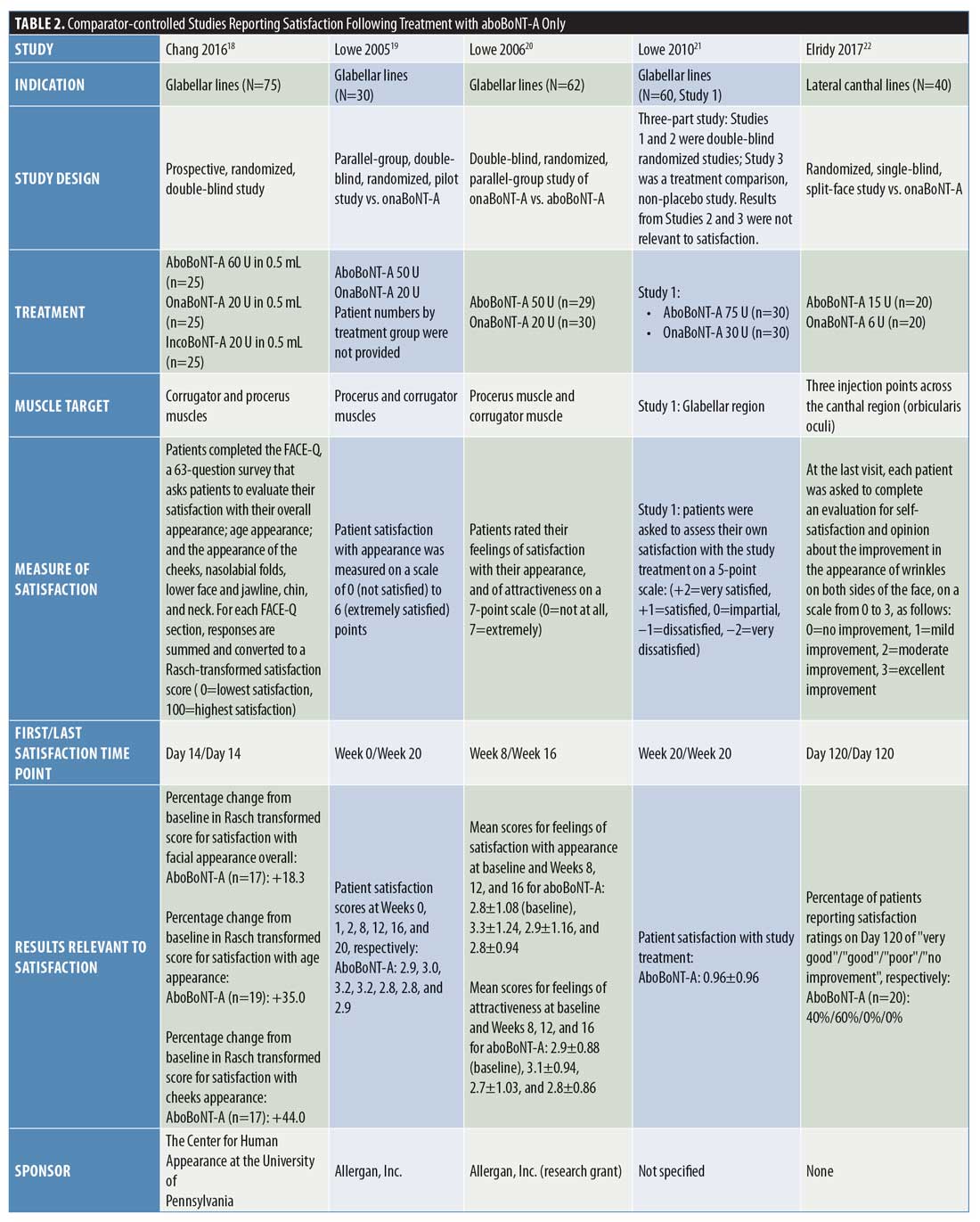
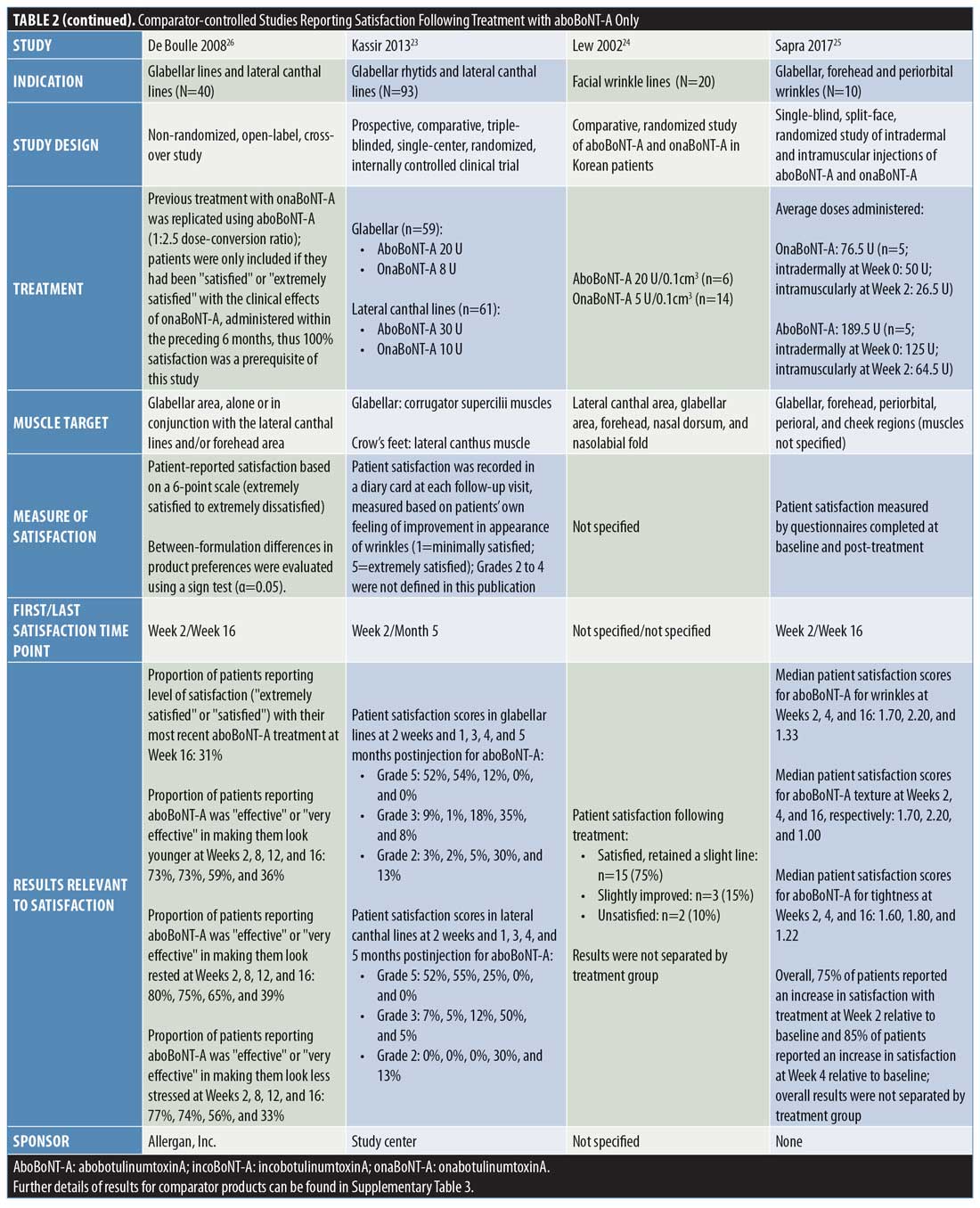
Comparator-controlled studies. Overall, nine comparator-controlled studies were identified that assessed patient satisfaction following treatment with aboBoNT-A (Table 2),18–26 with all except one26 being randomized. Comparator product results for these studies can be found in Supplementary Table 3.
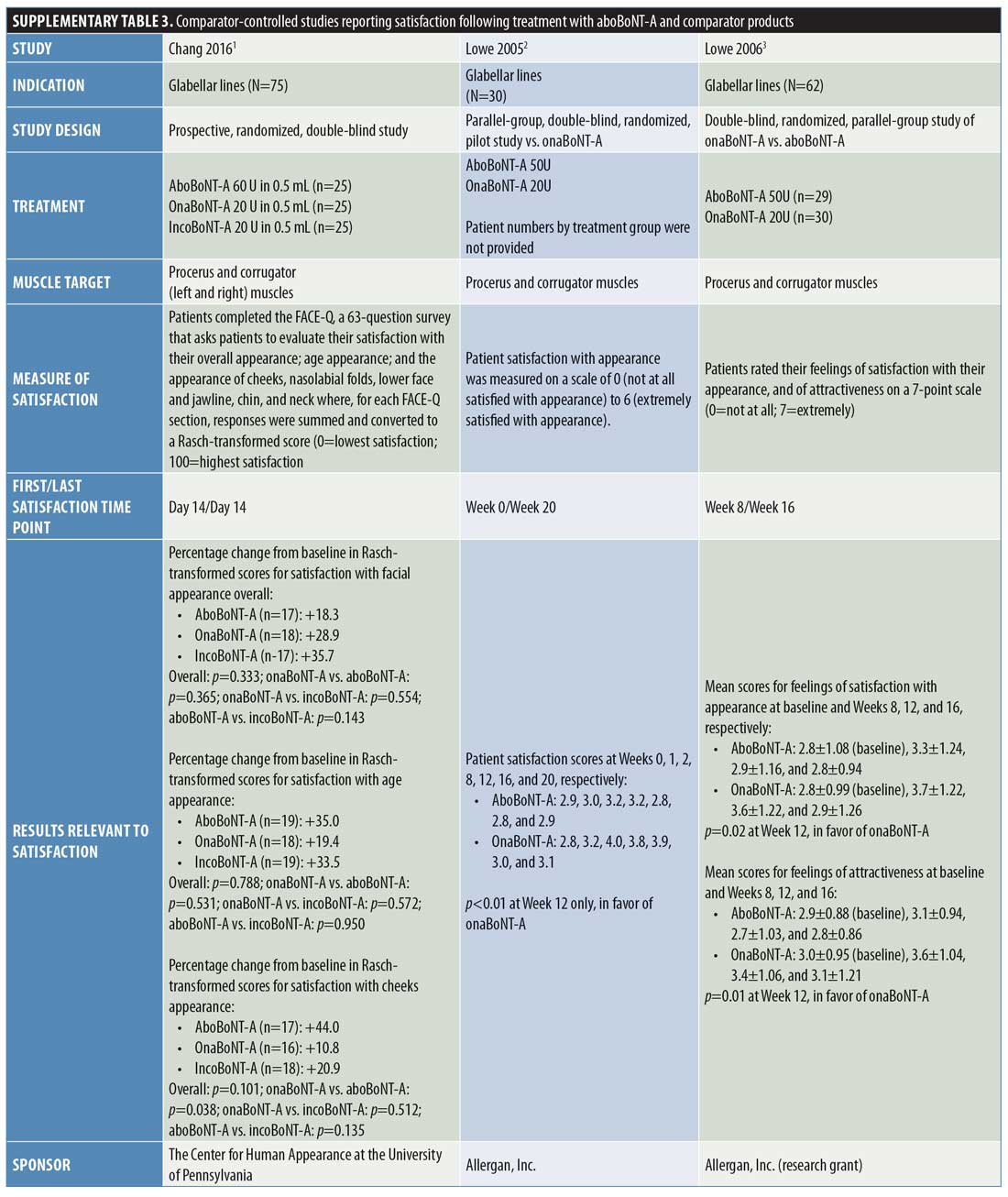
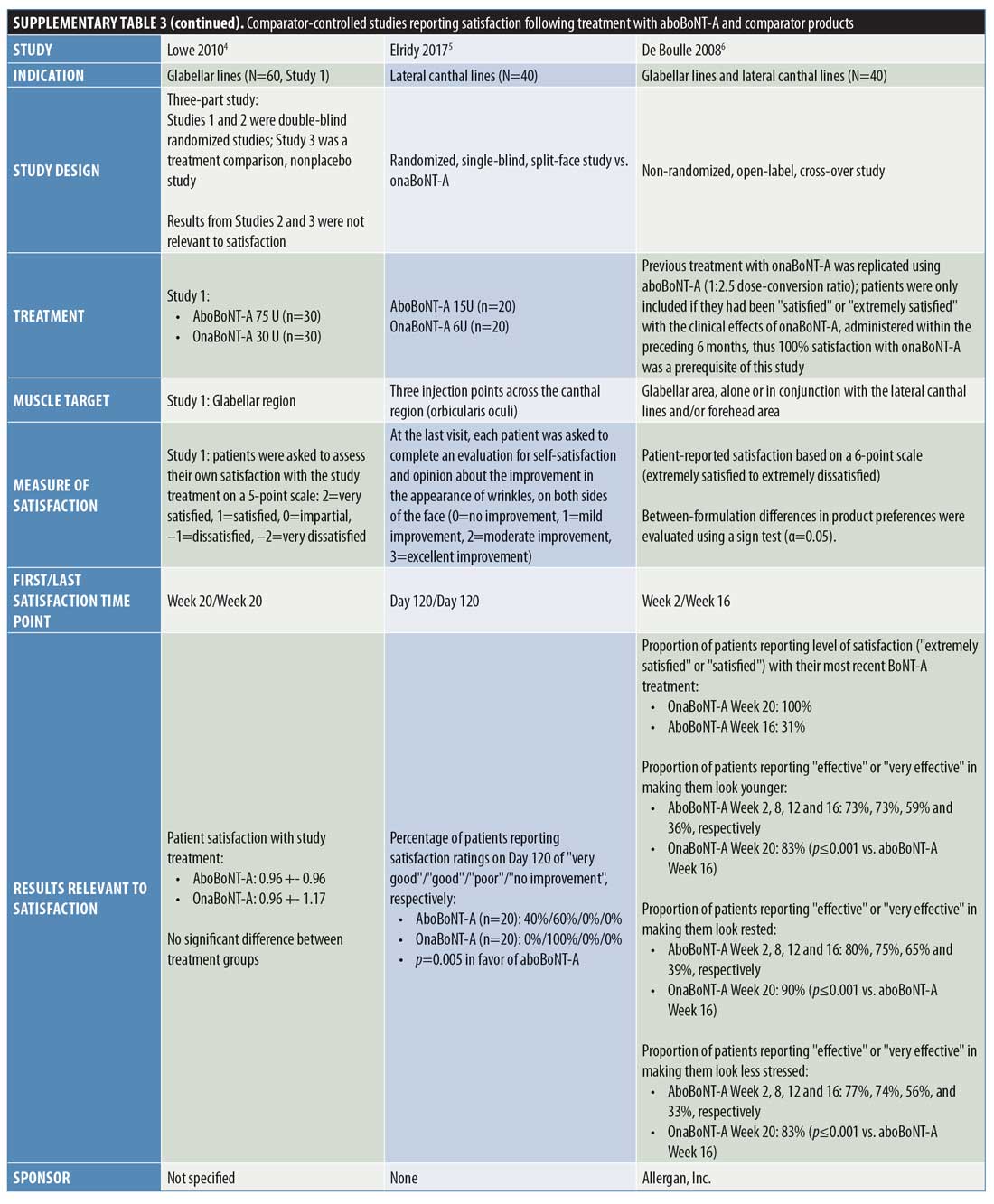
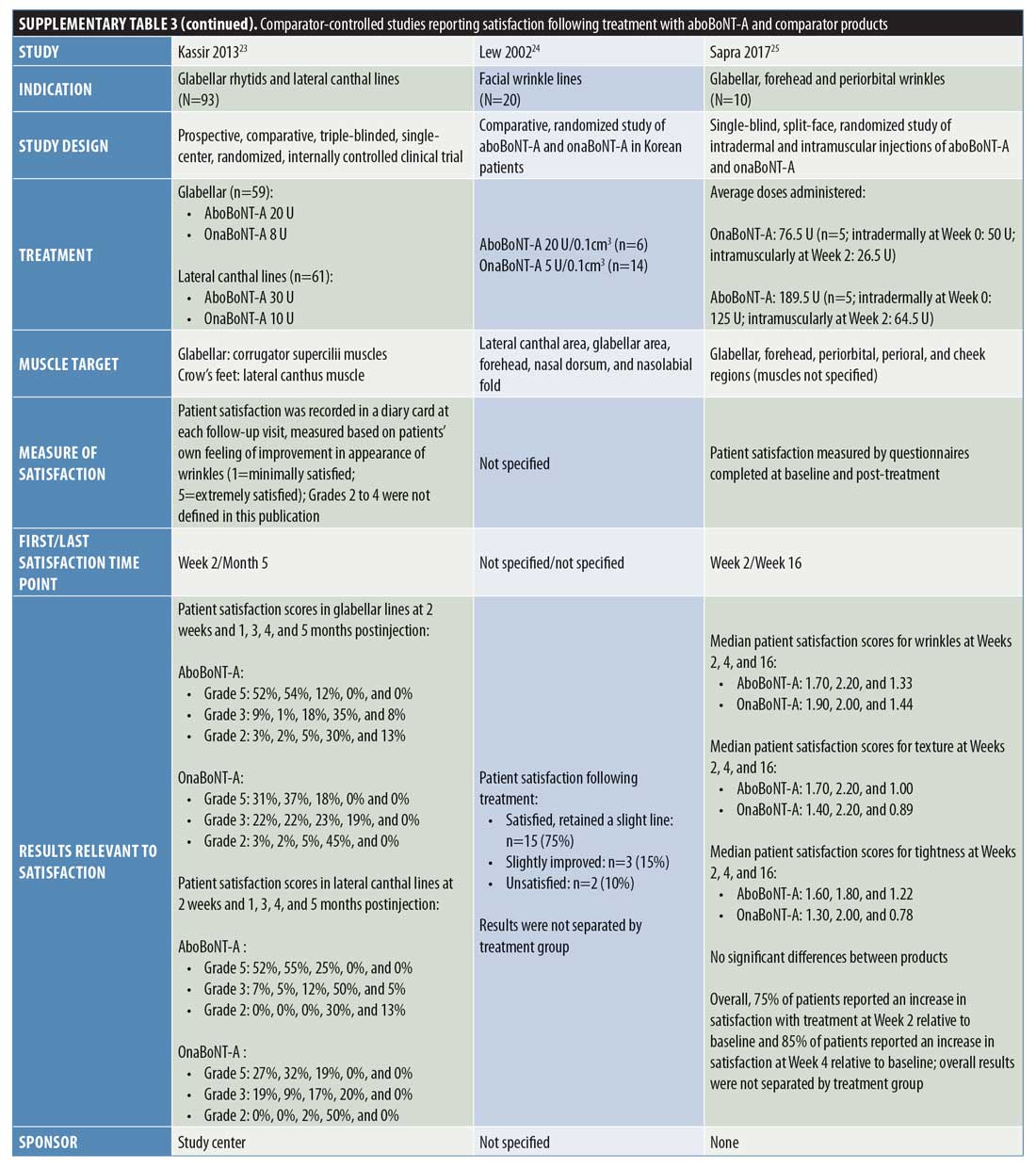
At two weeks postinjection, a change in satisfaction from baseline of +18 percent was reported,18 and between 52 percent and 75 percent of patients reported satisfaction with treatment23,25 after receiving aboBoNT-A injections for glabellar lines or lateral canthal lines or at multiple treatment sites. Additionally, a satisfaction score of 3.2 out of six points was reported at two weeks postinjection (vs. 2.9 points at Week 0).19 At later time points, studies reported between 31-percent and 100-percent rates for patient satisfaction with aboBoNT-A at four months postinjection,22,23,26 as well as satisfaction scores of 2.9 out of six points at five months postinjection (vs. 2.9 at Week 0)19, and 0.96 (on a score range of -2 [dissatisfied] to +2 [very satisfied] points) at five months postinjection.21 In another study, no improvement was observed at four months following aboBoNT-A injection compared to Week 0.20 Three of these comparator-controlled studies had results for the proportion of patients satisfied with treatment after 12 weeks or later and are included in Figure 2.22,23,26
In a study by Chang et al,18 the percentage increase from baseline in satisfaction with overall facial appearance following aboBoNT-A treatment was 18 percent and the percentage increase from baseline in satisfaction with perceived age was 35 percent for aboBoNT-A, both assessed at two weeks postinjection.
Lowe et al19 reported scores of 2.8 to 3.2 points (0=not at all satisfied; 6=extremely satisfied) for aboBoNT-A between Weeks 1 and 20 postinjection (vs. 2.9 points at Week 0), with the highest satisfaction score of 3.2 points first achieved at two weeks postinjection. Similarly, Lowe et al20 evaluated patient satisfaction with appearance, reporting mean scores of between 2.8 and 3.3 points (0=not at all satisfied; 7=extremely satisfied) after eight, 12, and 16 weeks postinjection with aboBoNT-A (2.8 points at Week 0). Mean scores for feelings of attractiveness, assessed on the same scale, ranged between 2.7 and 3.1 points following injection with aboBoNT-A across these time points (vs. 2.9 points at Week 0). For both assessments, the highest scores were achieved at Week 8 postinjection with aboBoNT-A. In another study by Lowe et al,21 a mean value of 0.96 points on a satisfaction scale ranging from -2 (very dissatisfied) to +2 (very satisfied) points was achieved at 20 weeks postinjection with aboBoNT-A.
At 120 days (approximately 4 months) following treatment with aboBoNT-A in a split-face study by Elridy et al,22 100 percent of patients were satisfied with treatment, with 40 percent reporting their treatment as “very good” and the remaining 60 percent reporting theirs as “good.”
In a study by Kassir et al,23 patients reported satisfaction with the improvement in their appearance following treatment for glabellar lines and lateral canthal lines. At two weeks and one month following aboBoNT-A treatment for glabellar lines, 52 percent and 54 percent of patients, respectively, were “extremely satisfied” (Grade 5 on a scale of Grades 1 to 5). At four months following treatment, a moderate level of satisfaction (Grade 3; range of grades not clearly defined) was reported by 35 percent of patients receiving aboBoNT-A. Similar results were observed at these time points following aboBoNT-A treatment for lateral canthal lines, with 52 percent and 55 percent of patients being “extremely satisfied” at two weeks and one month postinjection, respectively, and a moderate level of satisfaction observed in 50 percent of patients at four months following treatment.
In a study by Lew et al,24 patients reported satisfaction following full-face treatment with BoNT-A (either aboBoNT-A or onaBoNT-A [2.5:1 U], results not provided by treatment group). Overall, 15 patients (75%) were satisfied with the results of treatment and retained only a slight line, three patients (15%) felt that the treatment had slightly improved their condition, and two patients (10%) were unsatisfied with their treatment (time point for evaluation was not specified).
Following aboBoNT-A injections in the glabellar, forehead, and periorbital regions, Sapra et al25 reported a high level of patient satisfaction. Patients scored their satisfaction with treatment for several factors (details of the scale used were not specified), including wrinkles (following intramuscular injections), skin texture, and skin tightness, with scores of 1.60 to 1.70 points across factors reported at Week 2, 1.80 to 2.20 points reported at Week 4, and 1.00 to 1.33 points reported at Week 16 postinjection with aboBoNT-A. Overall, regardless of BoNT-A product, 75 percent of patients reported an increase from baseline in satisfaction at Week 2 and 85 percent reported the same at Week 4.
De Boulle26 assessed patients’ satisfaction with aboBoNT-A following treatment of glabellar lines and lateral canthal lines in patients previously treated with another BoNT-A product (dosing was kept consistent with previous treatment at a ratio of 2.5:1 U). Assessments occurred from two weeks until 16 weeks (4 months) postinjection for aboBoNT-A. At 16 weeks postinjection with aboBoNT-A, patients reported a satisfaction rate of 31 percent. The proportions of patients reporting that aboBoNT-A treatment was “effective” or “very effective” in making them look younger, rested, and less stressed were 73 to 80 percent at Week 2 postinjection, 73 to 75 percent at Week 8, 56 to 65 percent at Week 12, and 33 to 39 percent at Week 16 across these parameters.
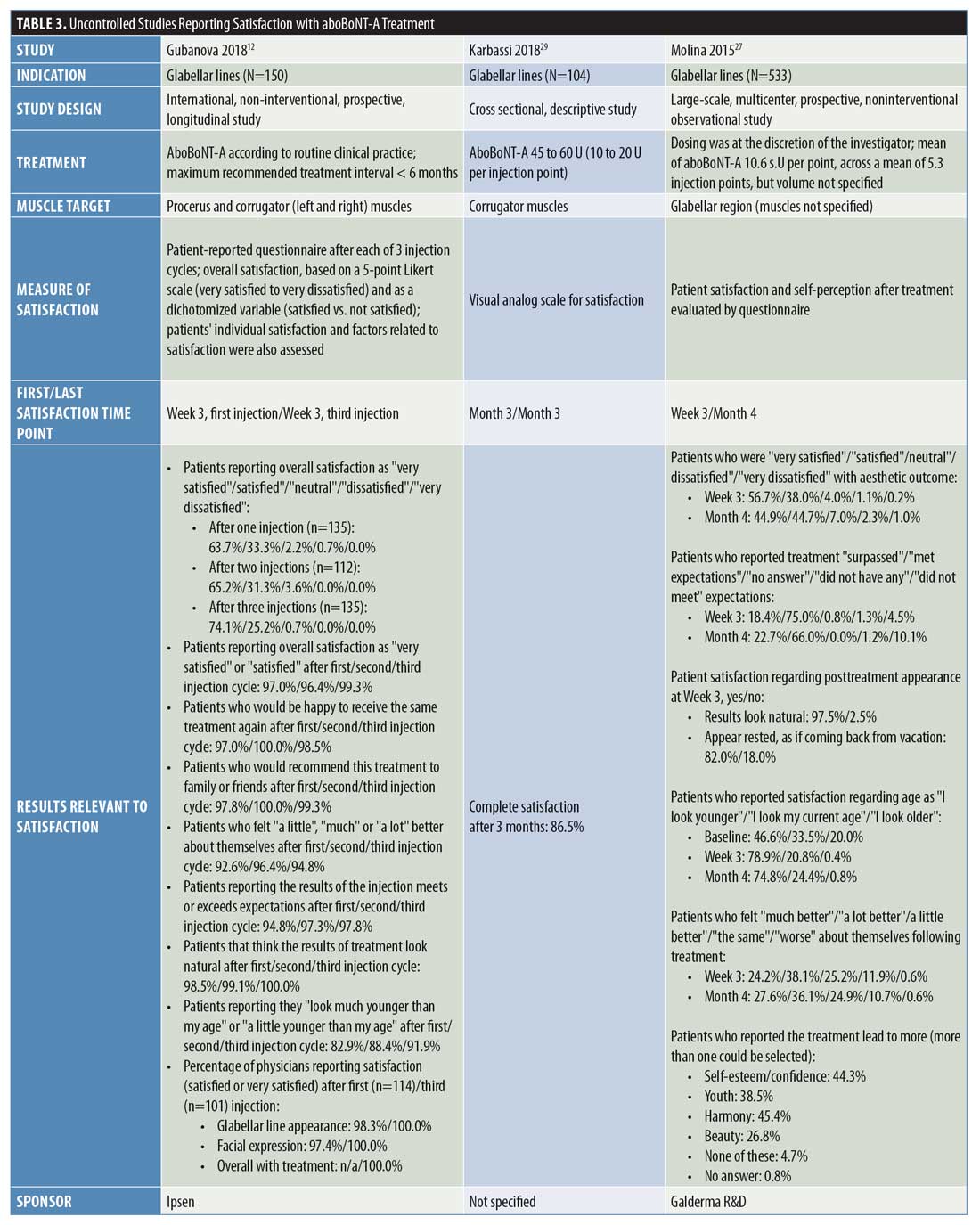
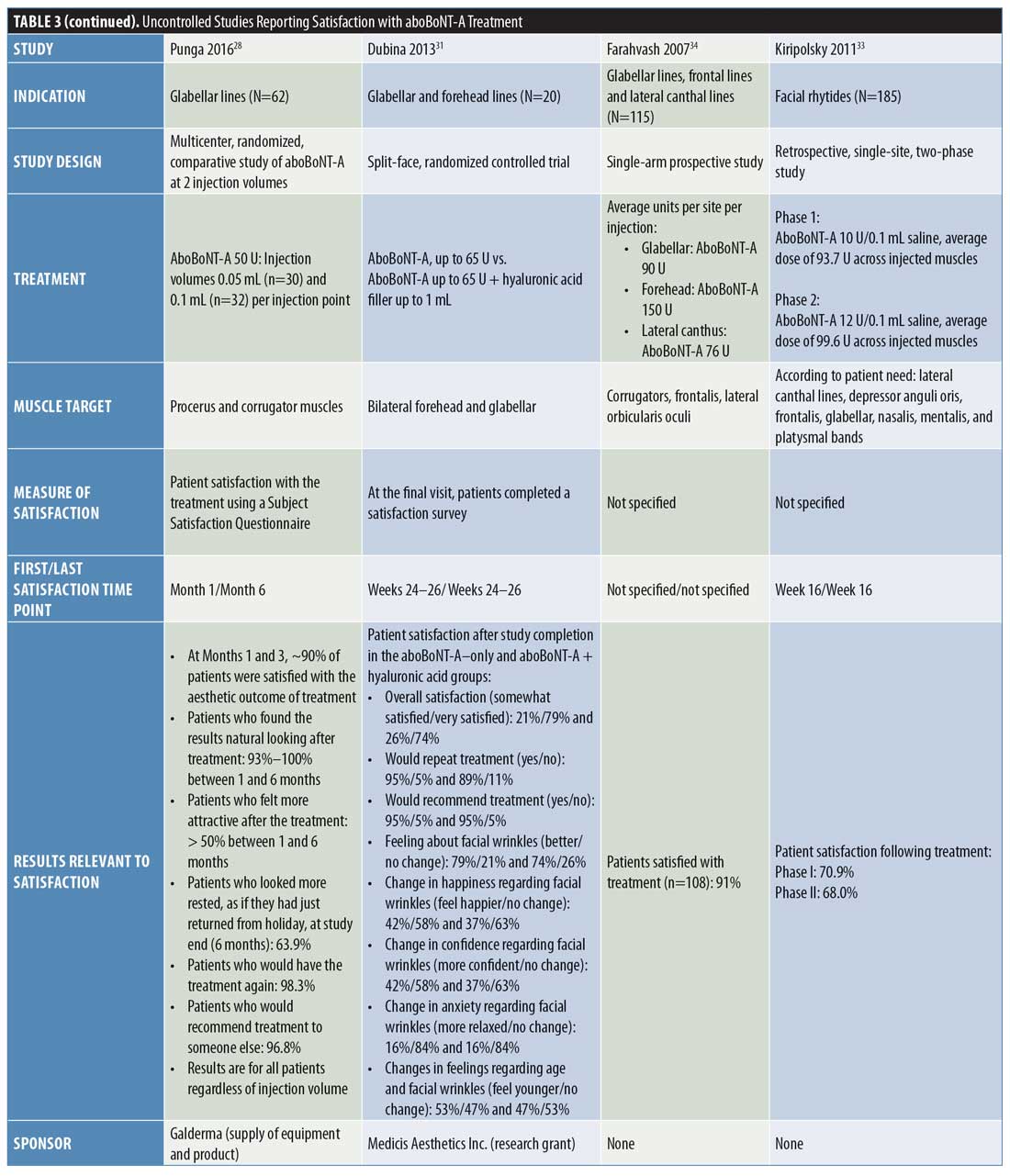
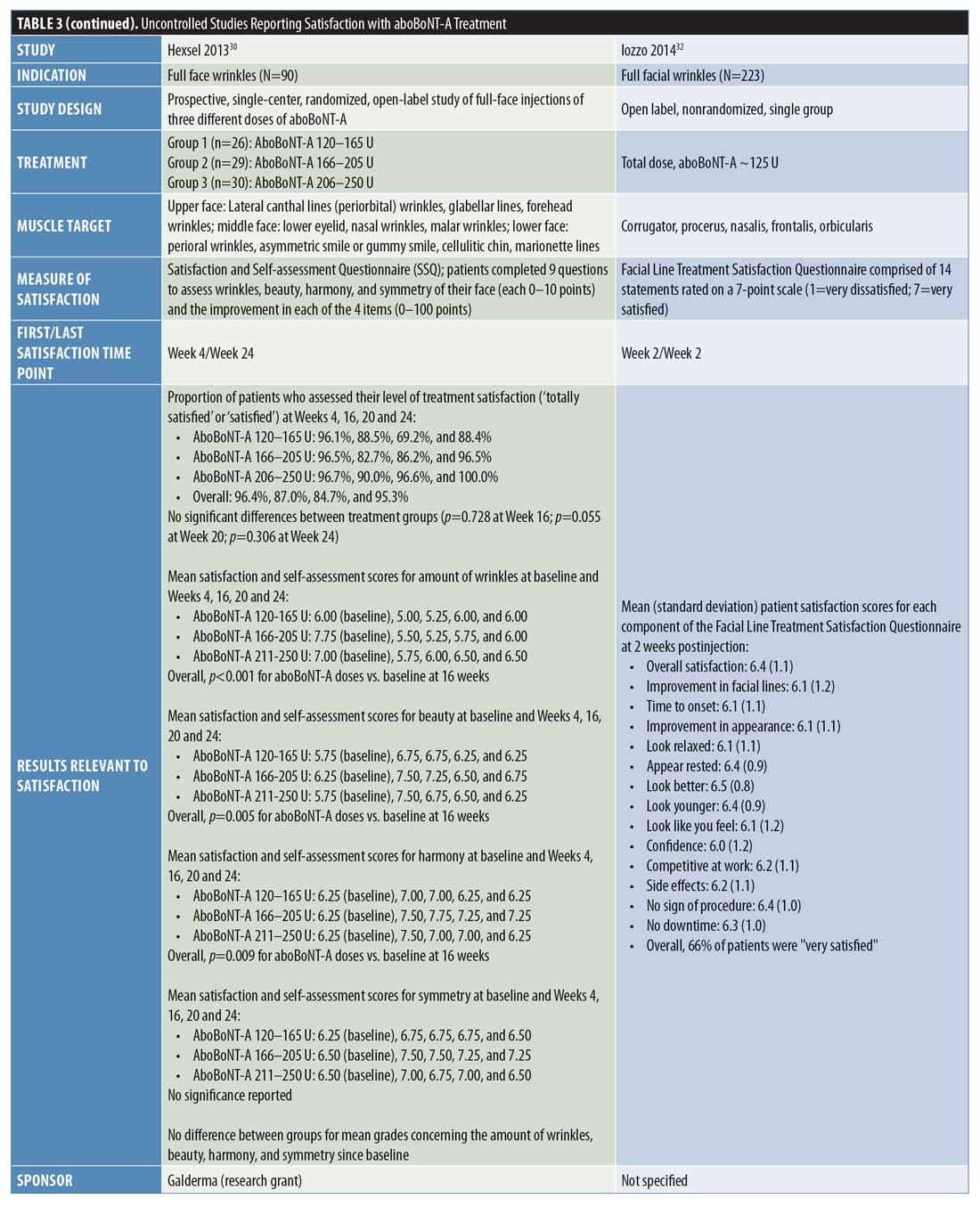
Uncontrolled studies. Overall, nine uncontrolled studies were identified that assessed patient satisfaction following treatment with aboBoNT-A (Table 3).12,27–34 These uncontrolled studies had varying study designs, including noninterventional, cross-sectional, open-label, single-arm, or retrospective and some compared different treatment regimens of aboBoNT-A. All studies where patients were grouped according to varying aboBoNT-A treatment plans were randomized.28,30,31 Overall, high satisfaction rates were reported across these studies. At the earliest recorded time points, a satisfaction score of 6.4 out of seven points was reported at two weeks postinjection32 and the proportion of patients satisfied or very satisfied with treatment was 95 to 99 percent at Week 3 postinjection.12,27 At later time points, 68 to 90 percent of patients reported satisfaction with treatment at four months postinjection,27,30,33 85 percent reported the same at five months postinjection,30 and 95 to 100 percent reported the same at six months postinjection.30,31 The results from six of these studies are included in Figure 2.27–31,33
In the noninterventional APPEAL study by Gubanova et al,12 patients received three injection cycles of aboBoNT-A for glabellar lines and patients completed a satisfaction questionnaire three weeks after each injection. Regarding overall satisfaction, 97 percent of patients were either “satisfied” or “very satisfied” after one injection cycle, 97 percent were the same after two injection cycles, and 99 percent were the same after three injection cycles. In response to questions regarding factors associated with patient satisfaction (e.g., would receive treatment again or recommend to others, feelings about themselves, expectations being met, natural look, self-perceived age), patient satisfaction rates ranged from 83 to 100 percent, depending on the question after the first and second aboBoNT-A injection cycles and from 92 to 100 percent after the third injection cycle.
Similarly, in a large-scale, single-cycle, noninterventional study of aboBoNT-A in glabellar lines, Molina et al27 reported that 95 and 90 percent of patients reported satisfaction (i.e., either being “satisfied” or “very satisfied”) with treatment at three weeks and four months postinjection, respectively. In response to specific factors affecting satisfaction, at three weeks and four months, respectively, 93 percent and 89 percent of patients said treatment met or surpassed their expectations, 79 percent and 75 percent felt they looked younger, and 88 percent and 89 percent felt better (either “much,” “a lot,” or “a little”) about themselves. At Week 3, 98 percent of patients also reported that their posttreatment appearance looked natural; results at four months were not recorded.
In a study by Punga et al,28 patients reported satisfaction after receiving aboBoNT-A 50 U at two injection volumes (0.05 mL and 0.1 mL) in the glabellar lines. Overall, patient satisfaction results were similar between the two injection volumes and, at one and three months postinjection, approximately 90 percent of patients were satisfied with their aesthetic outcomes. Regarding factors affecting satisfaction, 93 to 100 percent of patients across the one-to six-month time period (results not presented by time point) reported that the results were natural looking and at least 50 percent of patients felt more attractive after treatment. At six months following treatment, 64 percent of patients said they looked more rested, 98 percent would receive treatment again, and 97 percent would recommend treatment to someone else. A further study by Karbassi et al29 reported that 87 percent of patients were completely satisfied with treatment at three months after receiving aboBoNT-A 45 to 60 U for glabellar lines.
In a study by Hexsel et al,30 patients received a range of aboBoNT-A doses (120–165 U, 166–205 U, and 206–250 U) injected in wrinkles or other aesthetic indications across the full face. Overall (and across all doses injected), 96 percent (96%–97%) of patients reported they were “satisfied” or “totally satisfied” at four weeks postinjection, 87 percent (83%–90%) of patients reported the same at 16 weeks, 85 percent (69%–97%) of patients reported the same at 20 weeks, and 95 percent (88%–100%) of patients reported the same at 24 weeks. The highest satisfaction rates were consistently reported in the aboBoNT-A 206 to 250 U group, although no statistically significant differences were observed between the dose groups. In response to specific factors associated with patient satisfaction, there were no differences between treatment groups concerning wrinkles, beauty, harmony, or symmetry. Compared with at baseline, there was a significant reduction in wrinkles (p<0.001) and improvements in beauty (p=0.005) and harmony (p=0.009) at 16 weeks postinjection.
In a study by Dubina et al,31 patients received split-face injections of aboBoNT-A and aboBoNT-A with hyaluronic acid filler for the treatment of glabellar lines and forehead lines. Overall, at 24 to 26 weeks following injection with aboBoNT-A and aboBoNT-A with hyaluronic acid filler (respectively) all patients were either somewhat satisfied (21% and 26%) or very satisfied (79% and 74%) and most patients would receive treatment again (95% and 89%) and recommend treatment to others (95% and 95%). In patients who received aboBoNT-A and aboBoNT-A with hyaluronic acid filler, respectively, 79 and 74 percent reported feeling better about their facial wrinkles, 53 and 47 percent felt younger, 42 and 37 percent felt happier about their facial wrinkles, 42 and 37 percent also felt more confidence regarding facial wrinkles, and 16 and 16 percent were more relaxed in terms of their anxiety regarding facial wrinkles.
In a study of aboBoNT-A for full facial wrinkles, Iozzo et al32 reported a mean score of 6.4 points on a seven-point scale (1=very dissatisfied; 7=very satisfied) at two weeks postinjection and 66 percent of patients reported being “very satisfied” with their treatment results. Across specific factors affecting patient satisfaction rated on the same scale, mean scores ranged from six (for “confidence”) to 6.5 (for “look better”) points. Following injection with aboBoNT-A at two dilutions (10 U/0.1 mL, average dose: 93.7 U; 12 U/0.1 mL, average dose: 99.6 U) for full facial wrinkles, Kiripolsky et al33 reported that 71 and 68 percent of patients were satisfied with aboBoNT-A treatment at Week 16 using the doses of 10 U/0.1 mL and 12 U/0.1 mL, respectively. Furthermore, Farahvash et al34 reported that 91 percent of 108 patients were satisfied with treatment following injection with aboBoNT-A across the glabellar lines, frontal lines, and lateral canthal lines, although the time point for this assessment was not specified.
Physician satisfaction. Only one uncontrolled study of glabellar lines was identified that assessed satisfaction of the treating physician. The APPEAL study, by Gubanova et al,12 reported high rates of physician satisfaction (either “satisfied” or “very satisfied”) at three weeks after one injection cycle with aboBoNT-A, whereby 98 and 97 percent of physicians were satisfied with the appearance of patients’ glabellar lines and facial expressions, respectively. Furthermore, after a third injection cycle with aboBoNT-A, all participating physicians (100%) reported satisfaction with the appearance of patients’ glabellar lines, facial expressions, and overall satisfaction with treatment.
APPEAL study satisfaction by sex and age. Of 150 patients enrolled into the APPEAL study,12 137 (91%) were women and 13 (9%) were men. Across age groups, there were 13 patients (9%) aged 18 to 30 years, 32 (21%) aged 31 to 40 years, 58 (39%) aged 41 to 50 years, 34 (23%) aged 51 to 60 years, and 13 (9%) aged older than 60 years. Of the 150 patients enrolled, 137 (91%) completed all three aboBoNT-A treatment cycles. Of the 13 withdrawn patients, 10 were women. By age, seven were aged 31 to 40 years; three were aged 18 to 30 years; and one each was aged 41 to 50, 51 to 60, and older than 60 years.
As shown in Figure 3, all female patients and all except one male patient reported overall satisfaction with treatment, while no patients were “dissatisfied” or “very dissatisfied.” Overall, a higher proportion of female patients were “very satisfied” with treatment compared to male patients (75% vs. 67%, respectively), although the number of male patients enrolled in the study was low (Table 4). 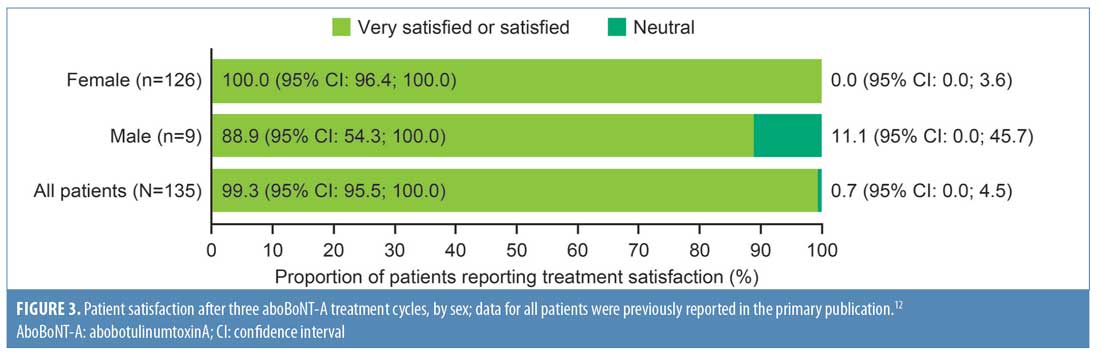
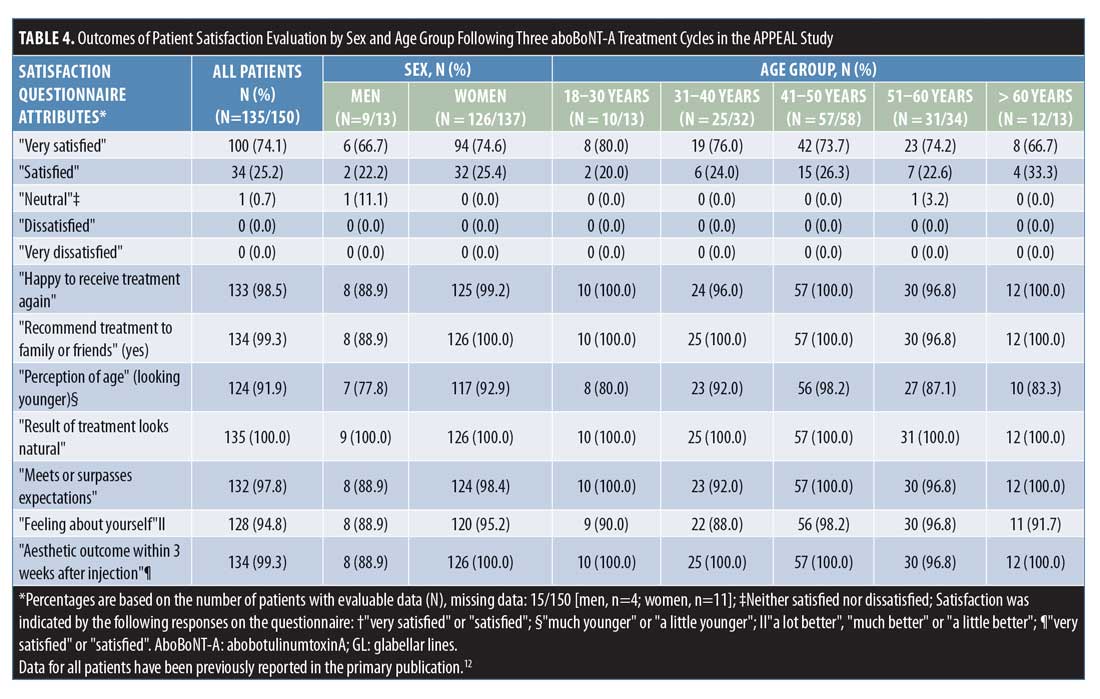 In response to questions regarding factors affecting patients’ satisfaction, the percentage of female patients reporting satisfaction with each factor, as listed in Table 4, was consistently higher, although the small number of male patients enrolled should be taken into account. In particular, a greater proportion of female participants than male participants felt they looked younger (either “a little” or “much”) than their actual age (93% vs. 78%, respectively).
In response to questions regarding factors affecting patients’ satisfaction, the percentage of female patients reporting satisfaction with each factor, as listed in Table 4, was consistently higher, although the small number of male patients enrolled should be taken into account. In particular, a greater proportion of female participants than male participants felt they looked younger (either “a little” or “much”) than their actual age (93% vs. 78%, respectively).
As shown in Figure 4, all patients except one in the age group of 51 to 60 years reported overall satisfaction with treatment. The proportion of “very satisfied” patients was similar across age groups from 31 to 60 years (74%–76%), but slightly higher among patients aged 18 to 30 years and slightly lower among those aged older than 60 years (80% and 67%, respectively) (Table 4). 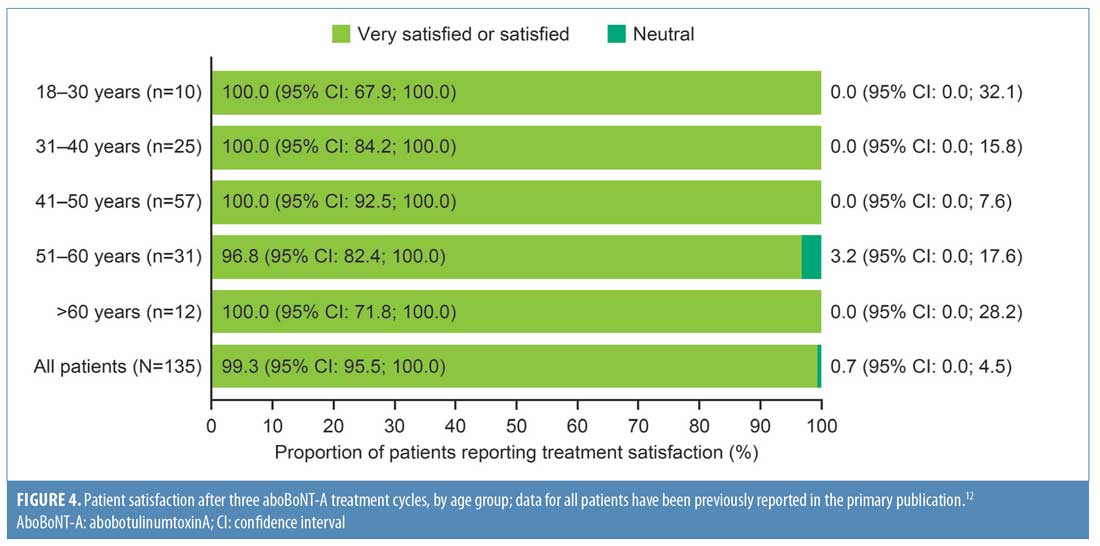
In response to questions regarding factors affecting patients’ satisfaction, the proportion of patients satisfied with each factor, as listed in Table 4, was similar across age groups, with only responses to the “perception of age” and “feelings about yourself” falling below 90 percent in some age groups (Table 4). Patients aged 18 to 30 years and older than 60 years reported the lowest proportion of positive responses for “perceptions of age” (80% and 83%, respectively), while the lowest proportion of positive responses to “feelings about yourself” was in the 31 to 40 years age group. For these two factors, the highest proportion of positive responses were found in the 41 to 50 years age group (98% for both factors). Additionally, across all age groups, 100 percent of patients felt that the “results of treatment look natural.”
Discussion
Based on a systematic review of data from studies conducted using various methodologies, most studies report a high rate of patient satisfaction with aboBoNT-A. In three placebo-controlled studies that performed statistical comparisons, patient satisfaction with aboBoNT-A given at varying doses was significant versus with the placebo at time points ranging from two weeks to between three and five months postinjection, depending on the study.2,16,17
In a previous review, Nestor et al35 identified the onset of effect and duration of action as factors of BoNT-A efficacy that are key in determining patient satisfaction. Many of the studies identified in the present review had time points for assessing satisfaction at early and late stages postinjection. Initial satisfaction results were commonly reported at 2 to 3 weeks postinjection, with a patient satisfaction rate of 66 to 85 percent in placebo-controlled studies,3,16 52 to 75 percent in comparator-controlled studies,23,25 and 95 to 99 percent in uncontrolled studies.12,27 However, there were limited results for patient satisfaction in the first two weeks postinjection; thus, the reported studies cannot provide an indication of when patients first start to feel satisfied with the results of treatment. Furthermore, a number of studies demonstrated satisfaction with aboBoNT-A treatment at time points after the recommended retreatment injection of three months (?12 weeks). At four months postinjection, the proportion of patients satisfied with treatment were 45 to 75 percent in placebo-controlled studies,2,16,17 31 and 100 percent in comparator-controlled studies,22,23,26 and 68 to 90 percent in uncontrolled studies.6,16,23 At five months postinjection, patient satisfaction rates were 87 and 85 percent in placebo-controlled and uncontrolled studies,17,30 respectively, while, at six months postinjection, the reported patient satisfaction rates were 25 to 93 percent in placebo-controlled studies3,16,17 and 95 to 100 percent in uncontrolled studies.30,31
Despite the current recommended interval of at least 12 weeks, this systematic review showed that satisfaction with the aesthetic results of aboBoNT-A therapy were still evident up to six months postinjection in some studies. Similarly, a recent real-word study of satisfaction with twice-yearly aboBoNT-A injections reported that 86 percent of patients were “satisfied” or “very satisfied” with treatment at three months after their first injection and 75 percent of patients remained so at six months after first injection.36 The long-lasting effects of aboBoNT-A were not only reported in terms of patient satisfaction but also have been established in terms of treatment efficacy, as discussed in a recent review by Nestor et al10 This prolonged duration of effect with aboBoNT-A is thought to be due to the higher quantity of active neurotoxins present in doses of aboBoNT-A approved by the United States Food and Drug Administration relative to doses of other approved toxins, as determined in a preclinical study by Field et al.37
It is widely accepted that the assessment of patient satisfaction is the optimal way to determine patients’ opinions about aesthetic procedures and should be considered as the most important factor for defining treatment success.38 In some cases, patients remain satisfied with the treatment effect even after wrinkle severity has returned to baseline condition, as assessed by the investigator.3,36
The post-hoc analyses of data from the APPEAL study showed that, regardless of patient sex and age at baseline, high levels of patient satisfaction could be achieved with aboBoNT-A injections for the treatment of glabellar lines. Interestingly, some small differences were identified in factors affecting satisfaction between women and men, as a higher proportion of women than men reported positive changes after treatment, although the number of male patients included in the APPEAL study was low. Additionally, the lowest proportion of positive responses to “perceptions of age” and “feelings about yourself” were in the younger age group (18–30 and 31–40 years, respectively), yet overall satisfaction in these age groups was high, with 80 and 76 percent of patients, respectively, reporting they were “very satisfied” with treatment. It is possible that, for younger age groups, perception of age is a less important factor in determining their satisfaction with treatment compared to patients who are older. Overall, outcomes of this analysis support an individualized treatment approach that should respect patients’ expectations and preferences.
Limitations. In terms of limitations, it is evident from the results of the systematic review, as summarized in Figure 2, that the proportion of patients satisfied with treatment does vary between studies in all treatment indications, possibly due to differing study designs and patient expectations for treatment. However, in large-scale studies, such as Gubanova et al12 and Molina et al,27 for which the assessment of patient satisfaction with aboBoNT-A was the primary objective, particularly high levels of satisfaction were achieved, with rates of between 95 and 99 percent for satisfaction at Week 3 and 90 percent for satisfaction at four months postinjection reported. Additionally, both the APPEAL study by Gubanova et al12 and the study by Molina et al27 were noninterventional studies performed in a real-life setting, which may explain the high levels of satisfaction, as the physician-patient relationship and overall experience during the consultation can also influence patient satisfaction with treatment.39 In a more formal trial setting, treatment is performed according to a protocol and patients may not feel the consultation is as personalized compared with a real-life setting. Furthermore, the APPEAL post-hoc analysis of satisfaction by sex is limited by the small number of male patients involved and should be interpreted with caution.
Conclusion
The results of this systematic literature review demonstrated high rates of patient satisfaction exist following treatment with aboBoNT-A. Satisfaction was observed at the first time points recorded, usually 2 to 3 weeks postinjection, and was maintained in many patients beyond the current recommended retreatment interval (?12 weeks), with satisfaction evident for up to five or six months in some patients. Furthermore, post-hoc analysis of the APPEAL study data showed that high levels of satisfaction with aboBoNT-A treatment can be achieved regardless of sex or age.
Acknowledgments
The authors thank all patients involved in the APPEAL study as well as all investigators and research staff at the participating institutions. The authors thank Watermeadow Medical, an Ashfield Company, for their support in developing this systematic literature review.
The authors also thank Jacqueline Harte, BSc (Hons), of Watermeadow Medical, an Ashfield Company, for providing medical writing support, which was sponsored by Ipsen in accordance with Good Publication Practice guidelines.
References
- Rzany B, Ascher B, Monheit G. Treatment of glabellar lines with botulinum toxin type A (Speywood Unit): a clinical overview. J Eur Acad Dermatol Venereol. 2010;24 Suppl 1:1–14.
- Rzany B, Ascher B, Fratila A, et al. Efficacy and safety of 3-and 5-injection patterns (30 and 50 U) of botulinum toxin A (Dysport) for the treatment of wrinkles in the glabella and the central forehead region. Arch Dermatol. 2006;142(3):320–326.
- Ascher B, Zakine B, Kestemont P, et al. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. J Am Acad Dermatol. 2004;51(2):223–233.
- Rubin MG, Dover J, Glogau RG, et al. The efficacy and safety of a new U.S. botulinum toxin type A in the retreatment of glabellar lines following open-label treatment. J Drugs Dermatol. 2009;8(5):439–444.
- Rubin M, Dover J, Maas C, Nestor M. An analysis of safety data from five phase III clinical trials on the use of botulinum neurotoxin type A-ABO for the treatment of glabellar lines. Aesthet Surg J. 2009;29(6_Supplement):S50-S56.
- Electronic Medicines Compendium. Azzalure Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/6584/smpc. Accessed October 21, 2019.
- Ipsen Biopharm Ltd. Dysport Full Prescribing Information. Available at: https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/9/2020/01/09195739/S115_2019_09_25_sBLA_Approval_PMR_Fulfilled_PI_MG_Sept-2019.pdf. Accessed February 26, 2020.
- Sundaram H, Huang PH, Hsu NJ, et al. Aesthetic applications of botulinum toxin A in Asians: an International, multidisciplinary, pan-Asian consensus. Plast Reconstr Surg Glob Open. 2016;4(12):e872.
- Hexsel D, Cartier H, Hedén P, et al. Efficacy, safety, and subject satisfaction after abobotulinumtoxinA treatment of upper facial lines. Dermatol Surg. 2018;44(12):1555–1564.
- Nestor M, Cohen JL, Landau M, et al. Onset and duration of abobotulinumtoxinA for aesthetic use in the upper face: a systematic literature review. J Clin Aesthet Dermatol. 2020. 13(12):E56–E83.
- Mori S, Lee EH. Beyond the physician’s perspective: A review of patient-reported outcomes in dermatologic surgery and cosmetic dermatology. Int J Womens Dermatol. 2019;5(1):21–26.
- Gubanova E, Haddad Tabet M, Bergerova Y, et al. Assessment of subject and physician satisfaction after long-term treatment of glabellar lines with abobotulinumtoxinA (Dysport®/Azzalure®: primary results of the APPEAL noninterventional study. Aesthetic Plast Surg. 2018;42(6):1672–1680.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716.
- Ascher B, Rzany BJ, Grover R. Efficacy and safety of botulinum toxin type A in the treatment of lateral crow’s feet: double-blind, placebo-controlled, dose-ranging study. Dermatol Surg. 2009;35(10):1478–1486.
- Ascher B, Zakine B, Kestemont P, et al. Botulinum toxin A in the treatment of glabellar lines: scheduling the next injection. Aesthet Surg J. 2005;25(4):365–375.
- Chang BL, Wilson AJ, Taglienti AJ, et al. Patient perceived benefit in facial aesthetic procedures: FACE-Q as a tool to study botulinum toxin injection outcomes. Aesthet Surg J. 2016;36(7):810–820.
- Lowe PL, Patnaik R, Lowe NJ. A comparison of two botulinum type a toxin preparations for the treatment of glabellar lines: double-blind, randomized, pilot study. Dermatol Surg. 2005;31(12):1651–1654.
- Lowe P, Patnaik R, Lowe N. Comparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: a double-blind, randomized study. J Am Acad Dermatol. 2006;55(6):975–980.
- Lowe NJ, Shah A, Lowe PL, Patnaik R. Dosing, efficacy and safety plus the use of computerized photography for botulinum toxins type A for upper facial lines. J Cosmet Laser Ther. 2010;12(2):106–111.
- Elridy AS, Zaki RGE, Elshinawy RF. Comparison of the clinical efficacy of abobotulinumtoxin A (ABO) and onabotulinumtoxin A (ONA) in the treatment of crow’s feet wrinkles: a split-face study. Semin Ophthalmol. 2017;33(6): 739–747.
- Kassir R, Kolluru A, Kassir M. Triple-blind, prospective, internally controlled comparative study between abobotulinumtoxinA and onabotulinumtoxinA for the treatment of facial rhytids. Dermatol Ther (Heidelb). 2013;3(2):179–189.
- Lew H, Yun YS, Lee SY, Kim SJ. Effect of botulinum toxin A on facial wrinkle lines in Koreans. Ophthalmologica. 2002;216(1):50–54.
- Sapra P, Demay S, Sapra S, et al. A Single-blind, split-face, randomized, pilot study comparing the effects of intradermal and intramuscular injection of two commercially available botulinum toxin A formulas to reduce signs of facial aging. J Clin Aesthet Dermatol. 2017;10(2):34–44.
- De Boulle K. Patient satisfaction with different botulinum toxin type A formulations in the treatment of moderate to severe upper facial rhytids. J Cosmet Laser Ther. 2008;10(2):87–92.
- Molina B, Grangier Y, Mole B, et al. Patient satisfaction after the treatment of glabellar lines with Botulinum toxin type A (Speywood Unit): a multi-centre European observational study. J Eur Acad Dermatol Venereol. 2015;29(7):1382–1388.
- Punga AR, Alimohammadi M, Fagrell D, et al. A randomized, comparative study to evaluate efficacy and safety of two injection volumes of abobotulinumtoxinA in treatment of glabellar lines. Dermatol Surg. 2016;42(8):967–976.
- Karbassi E, Nakhaee N, Zamanian M. The efficacy and complications of a new technique of Abobotulinum-toxin A (Dysport) injection in patients with glabellar lines. J Cosmet Dermatol. 2019;18(1):55–58.
- Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363–1367.
- Dubina M, Tung R, Bolotin D, et al. Treatment of forehead/glabellar rhytide complex with combination botulinum toxin a and hyaluronic acid versus botulinum toxin A injection alone: a split-face, rater-blinded, randomized control trial. J Cosmet Dermatol. 2013;12(4):261–266.
- Iozzo I, Tengattini V, Antonucci VA. Multipoint and multilevel injection technique of botulinum toxin A in facial aesthetics. J Cosmet Dermatol. 2014;13(2):135–142.
- Kiripolsky MG, Peterson JD, Guiha I, Goldman MP. A two-phase, retrospective analysis evaluating efficacy of and patient satisfaction with abobotulinumtoxina used to treat dynamic facial rhytides. Dermatol Surg. 2011;37(10):1443–1447.
- Farahvash MR, Arad S. Clostridium botulinum type A toxin for the treatment of upper face animation lines: an Iranian experience. J Cosmet Dermatol. 2007;6(3):152–158.
- Nestor M, Ablon G, Pickett A. Key parameters for the use of abobotulinumtoxinA in aesthetics: Onset and duration. Aesthet Surg J. 2017;37(Suppl_1):S20–S31.
- Shamban A, Cohen J, Schlessinger J, Jacob C, Karimi K, Maas C. Subject satisfaction with a twice-yearly retreatment schedule for abobotulinumtoxinA—interim results from a multi-center study. Abstract 99884. Presented at the 22nd International Master Course on Aging Science World Congress; January 30–February 1, 2020; Paris, France.
- Field M, Splevins A, Picaut P, et al. AbobotulinumtoxinA (Dysport®, onabotulinumtoxinA (Botox®, and incobotulinumtoxinA (Xeomin® Neurotoxin content and potential implications for duration of response in patients. Toxins (Basel). 2018;10(12):535.
- Ching S, Thoma A, McCabe RE, Antony MM. Measuring outcomes in aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg. 2003;111(1):469–480; discussion 481–462.
- Redaelli A, Siddiqui Syed S, Liu X, et al. Two multinational, observational surveys investigating perceptions of beauty and attitudes and experiences relating to aesthetic medical procedures. J Cosmet Dermatol. 2020;19(11):3020–3031

