J Clin Aesthet Dermatol. 2024;17(7):24–36.
J Clin Aesthet Dermatol. 2024;17(7):24–36.
by Nika Kianfar, MD, MPH; Shayan Dasdar, MD, MPH; Amir Marashi, MD; Soheil Tavakolpour, PhD; Hamidreza Mahmoudi, MD; and Maryam Daneshpazhooh, MD
Drs. Kianfar, Dasdar, Marashi, Mahmoudi, and Daneshpazhooh are with the Autoimmune Bullous Diseases Research Center at Tehran University of Medical Sciences in Tehran, Iran. Dr. Tavakolpour is with the Dana-Farber Cancer Institute, Harvard Medical School in Boston, Massachusetts.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. Epidermolysis bullosa acquisita (EBA) is a rare dermatosis of the mucous membrane and/or skin. Employing biologic treatment modalities, specifically rituximab (RTX), have become pivotal measure in treating patients with blistering diseases. This study aims to summarize the current evidence on the safety and efficacy of RTX in EBA.
Methods. An extensive search was performed in MEDLINE/PubMed, Embase, Scopus, and Web of Science databases until the end of August 19th, 2023. Two independent reviewers screened the papers, and collected data. Two hundred thirty-three studies were screened using Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Results. Thirty-one studies were enrolled. The most common reason of RTX administration in patients with EBA was recalcitrant diseases. Clinical response and disease remission was recorded as 92.7 percent (63 patients) and 73.8 percent (45 patients) of the patients, respectively. A relapse rate of 39.5 percent (15 patients) in the mean follow-up of 23.0 months was reported in the studies. Of the patients, 28.2 percent (11 patients) experienced RTX-related side events, mostly mild and transient infusion reactions.
Conclusion. The results of this systematic review demonstrated that RTX is safe and effective in patients with EBA. This biological treatment modality can be routinely used in managing EBA.
Keywords. Autoimmune bullous disease, epidermolysis bullosa acquisita, rituximab, intravenous immunoglobulin, systematic review
Introduction
Epidermolysis bullosa acquisita is a rare bullous dermatoses triggered by IgG autoantibodies against collagen VII (COL7). The classical non-inflammatory mechanobullous subtype (MB) presents with trauma-induced tense blisters mainly limited to the skin. Contrariwise, in the inflammatory subtype, widespread vesiculobullous involvement is observed.1,2 Importantly, clinical presentations can switch from one subtype to the other over time, and also, the two subtypes may present simultaneously.3,4
Finding the optimal treatment for patients with EBA is still challenging. Rituximab, a chimeric anti-CD20 monoclonal antibody, has shown potential as an alternative treatment in the past few years.5–8 It is expressed on a wide range of B cells, from pre-B cell stage and on mature B cells in the bone marrow and in the periphery.9 Since B cells play a crucial role in the pathogenesis of EBA, this disease might benefit from this treatment.4
Herein, a systematic review of the current literature regarding RTX in EBA is performed. These data would be invaluable for choosing the best therapeutic regimen for patients suffering from EBA.
Methods
The systematic review of the literature was conducted based on Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guideline.10 The keywords related to “EBA” and “RTX” were determined using electronic databases and similar articles. The search syntheses were designed, and a systematic search was performed on MEDLINE/PubMed, Embase, Scopus, and Web of Science on August 19th, 2023 (Supplementary Table 1, see online version of this article). The references and citations of the relevant articles were also manually searched for the probable missing articles. Two independent reviewers screened the papers and collected data. All detected records were inserted into the Endnote X8 (Clarivate Analytics, Philadelphia), and duplicate reports were removed. Original studies that assessed the efficacy or safety of using RTX in patients with EBA were included. No limitation was set for language or study type. The EBA diagnosis had to be confirmed by histological, direct/indirect immunofluorescence, ELISA, and/or immunoblotting assessments. Studies with no sufficient data were excluded.
The screening process was done in two stages on titles/abstracts and full-texts. The following data were extracted: study characteristics, patients’ demographics, EBA subtype, disease duration until infusion, previous treatments, indication of RTX infusion, protocol of RTX infusion, adjuvant therapy, time to obtain clinical response, clinical remission, side effects of RTX, and treatment durability. All mentioned steps were done by two independent investigators (NK and SD).
Results
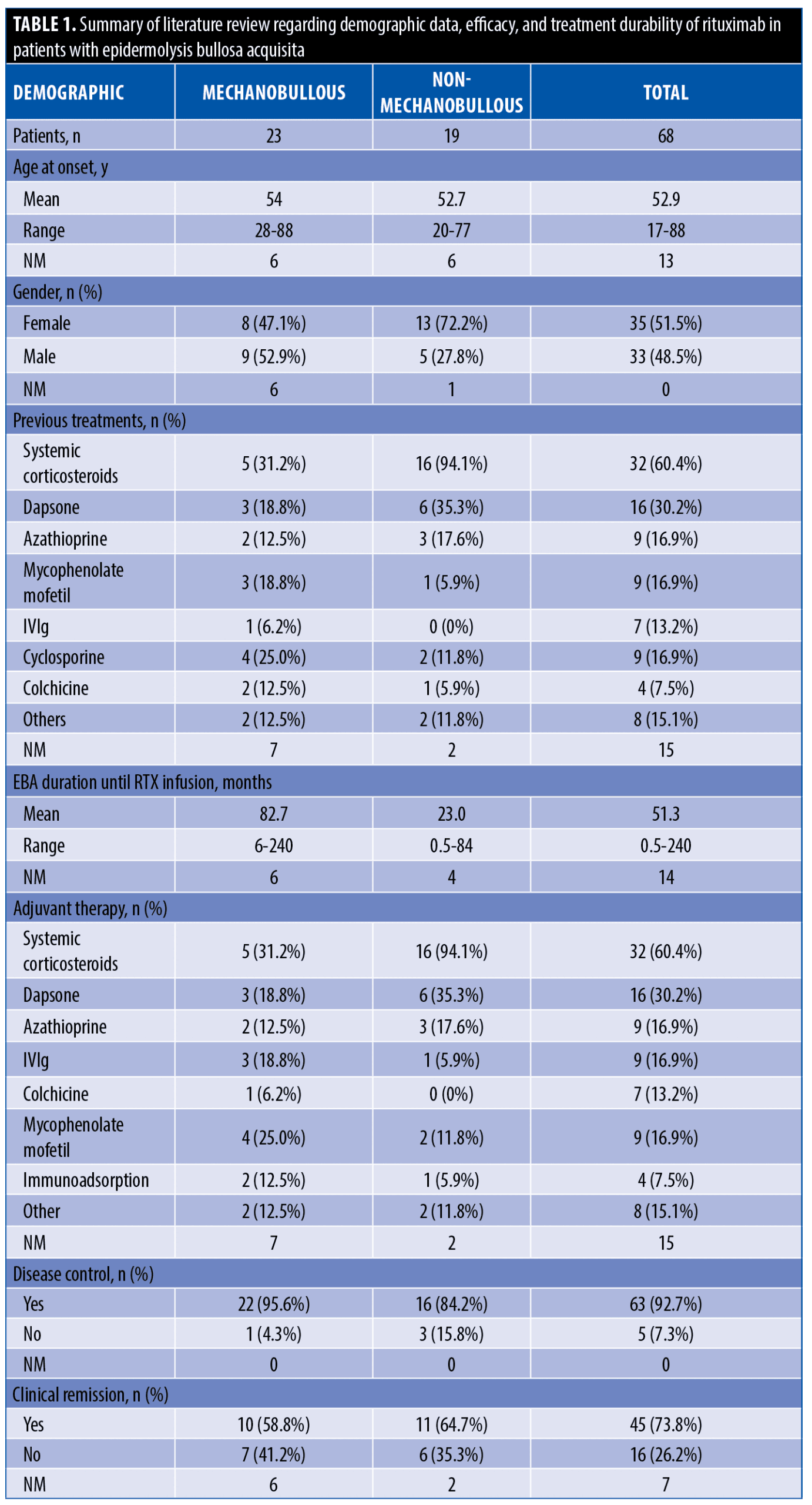
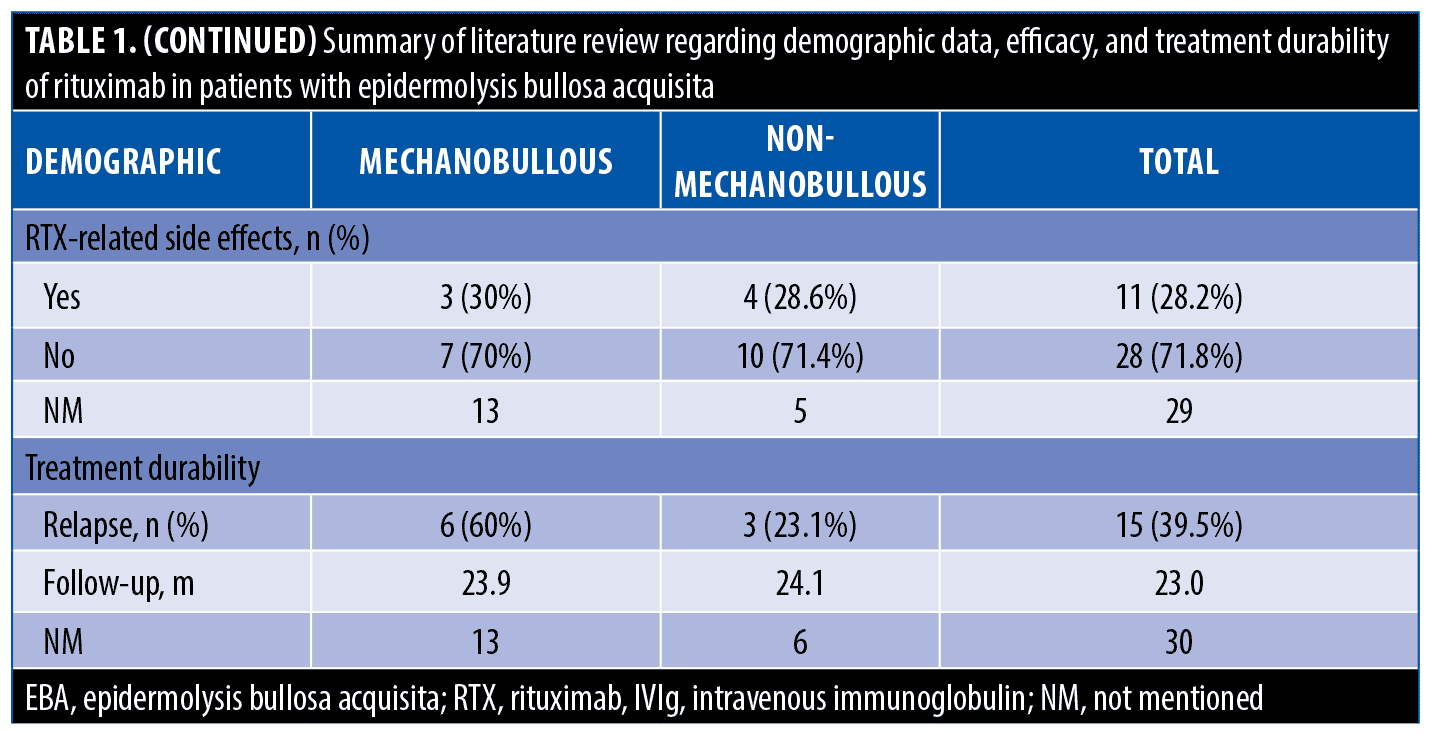
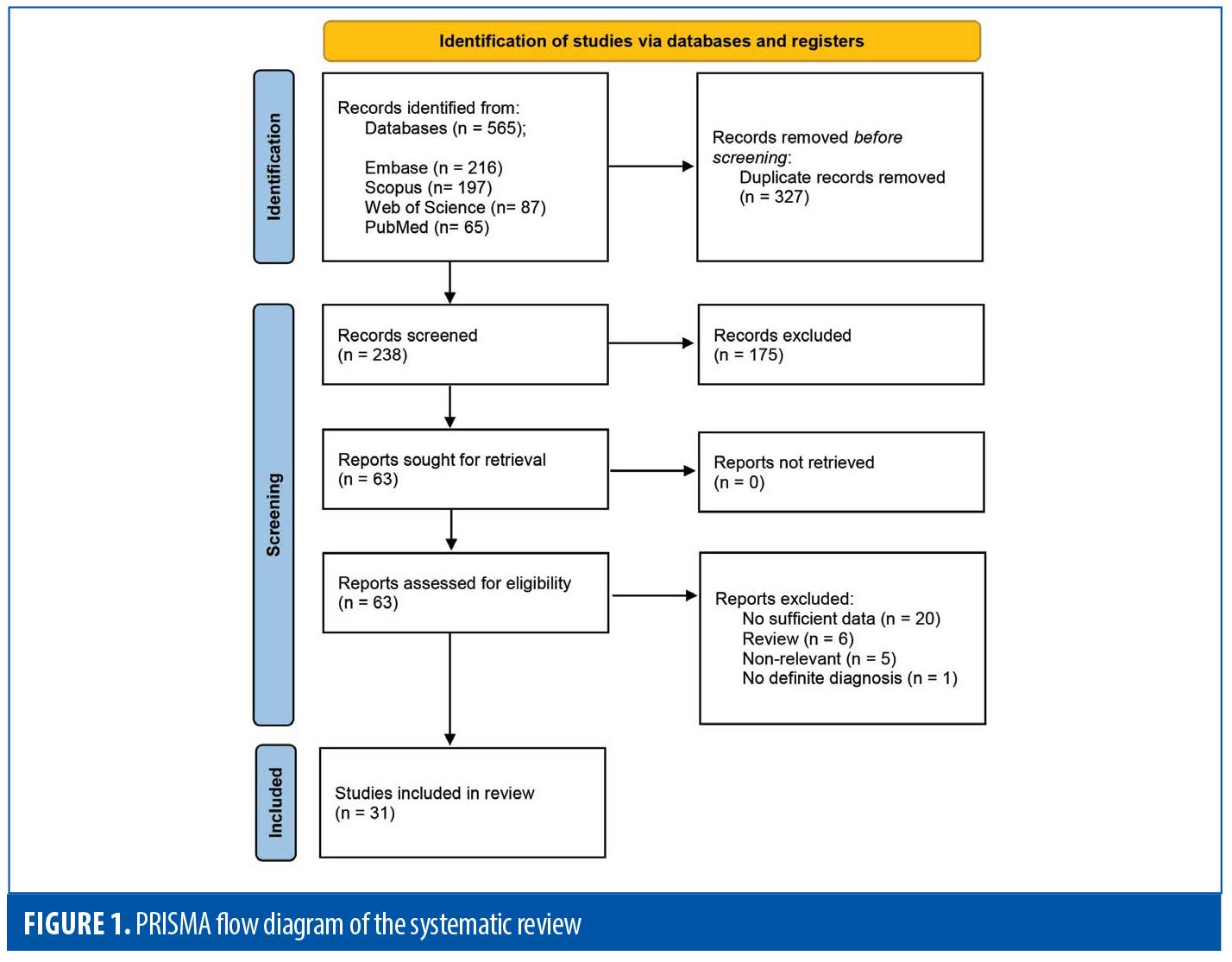
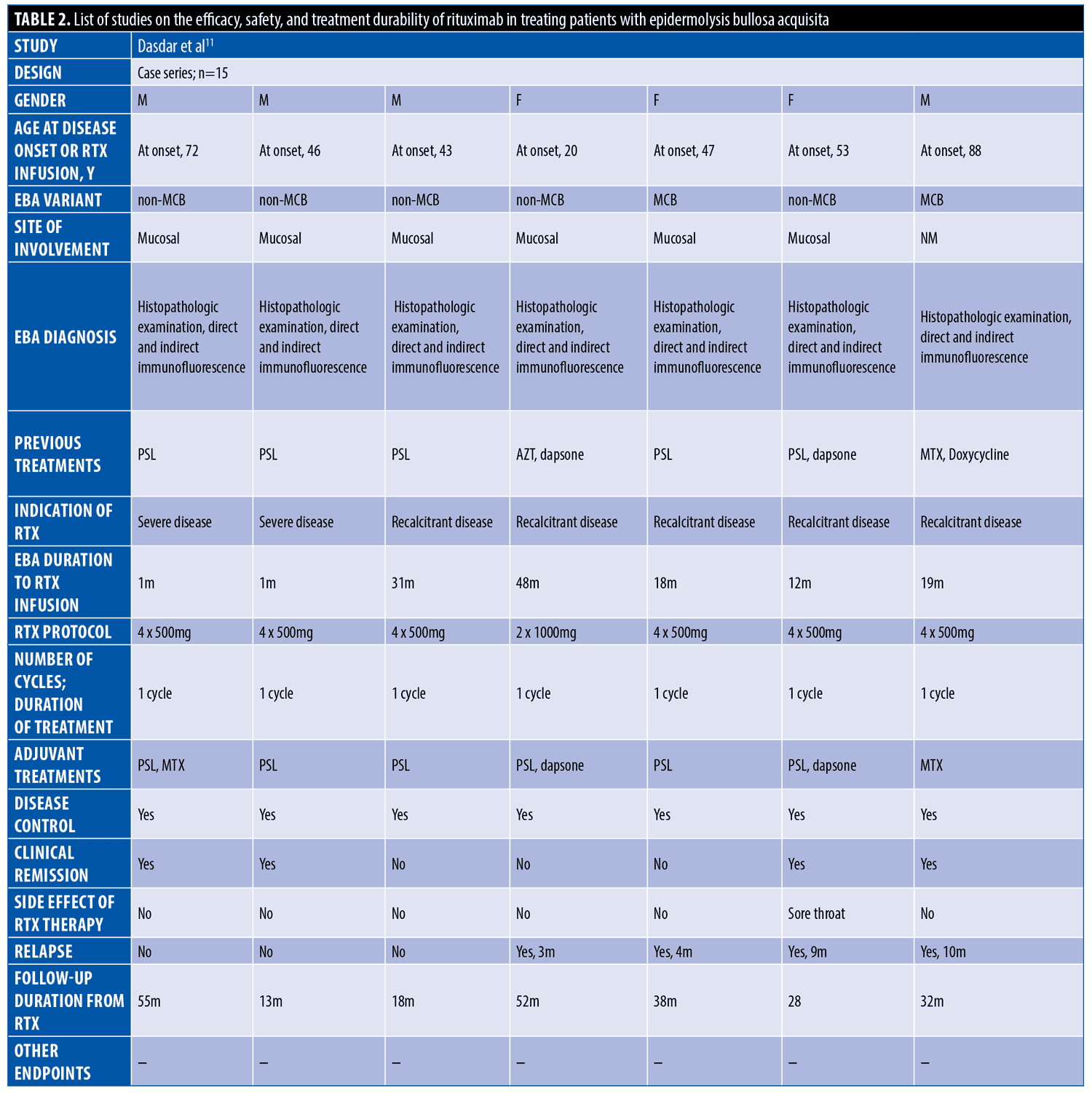
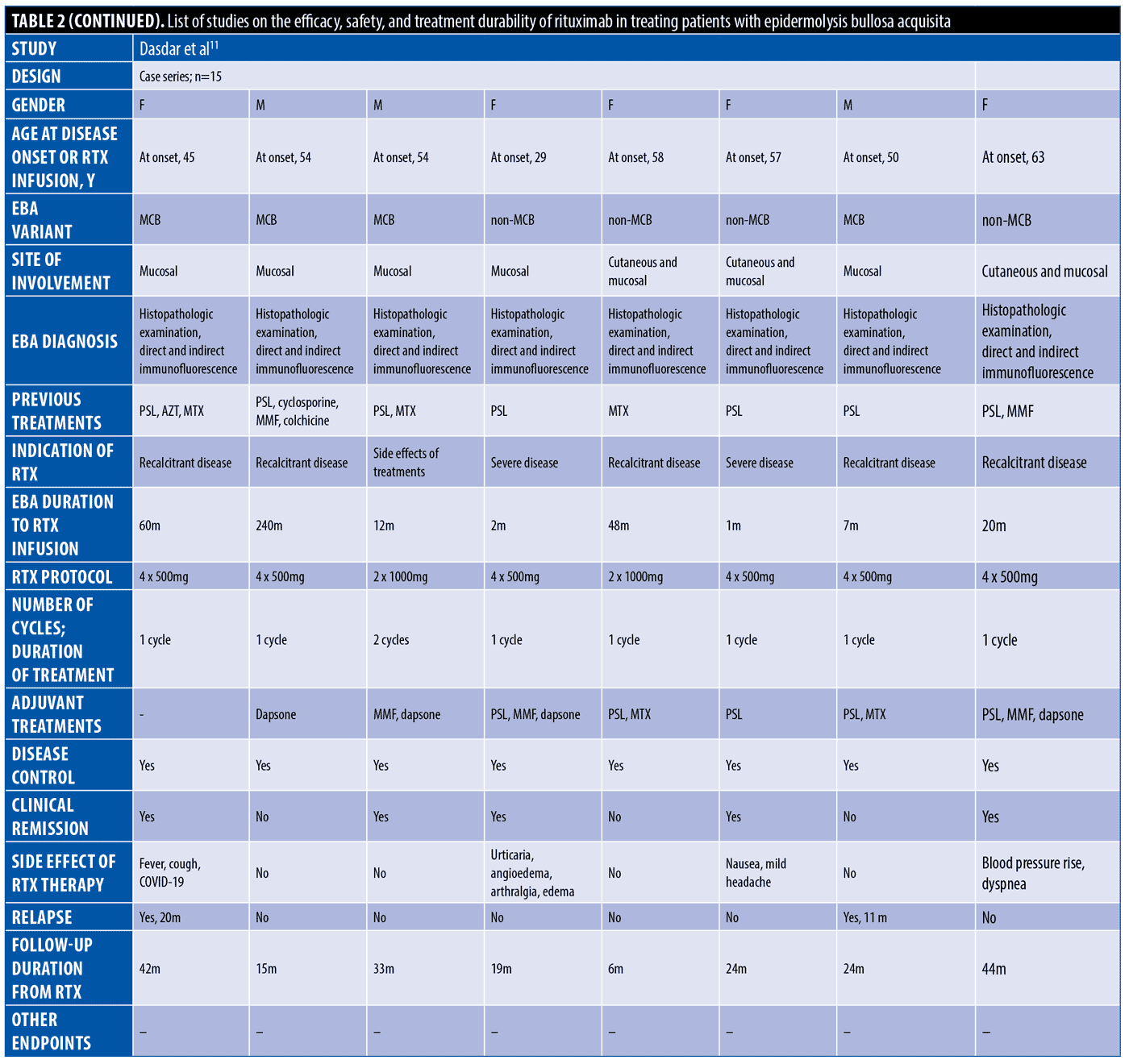
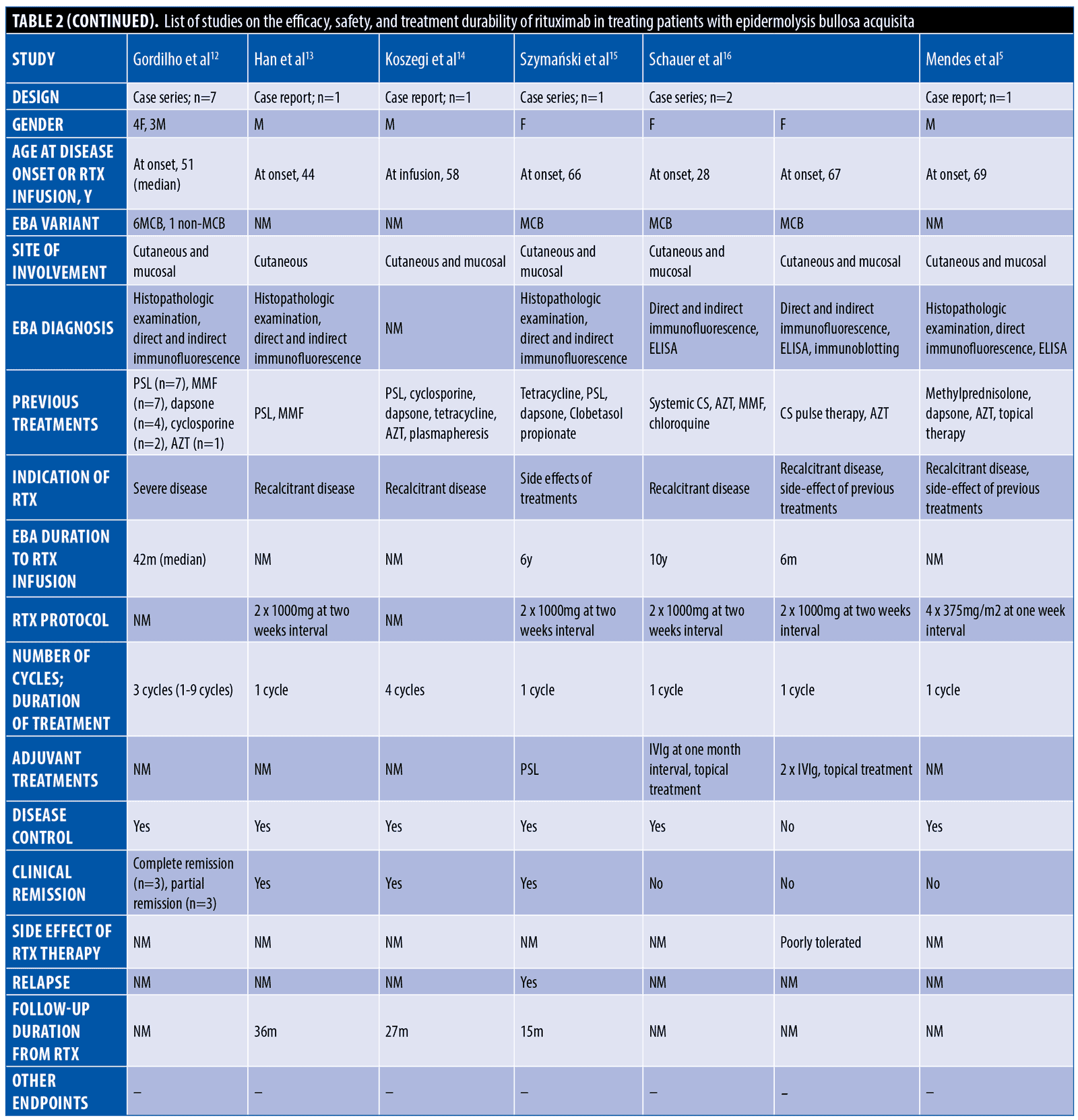
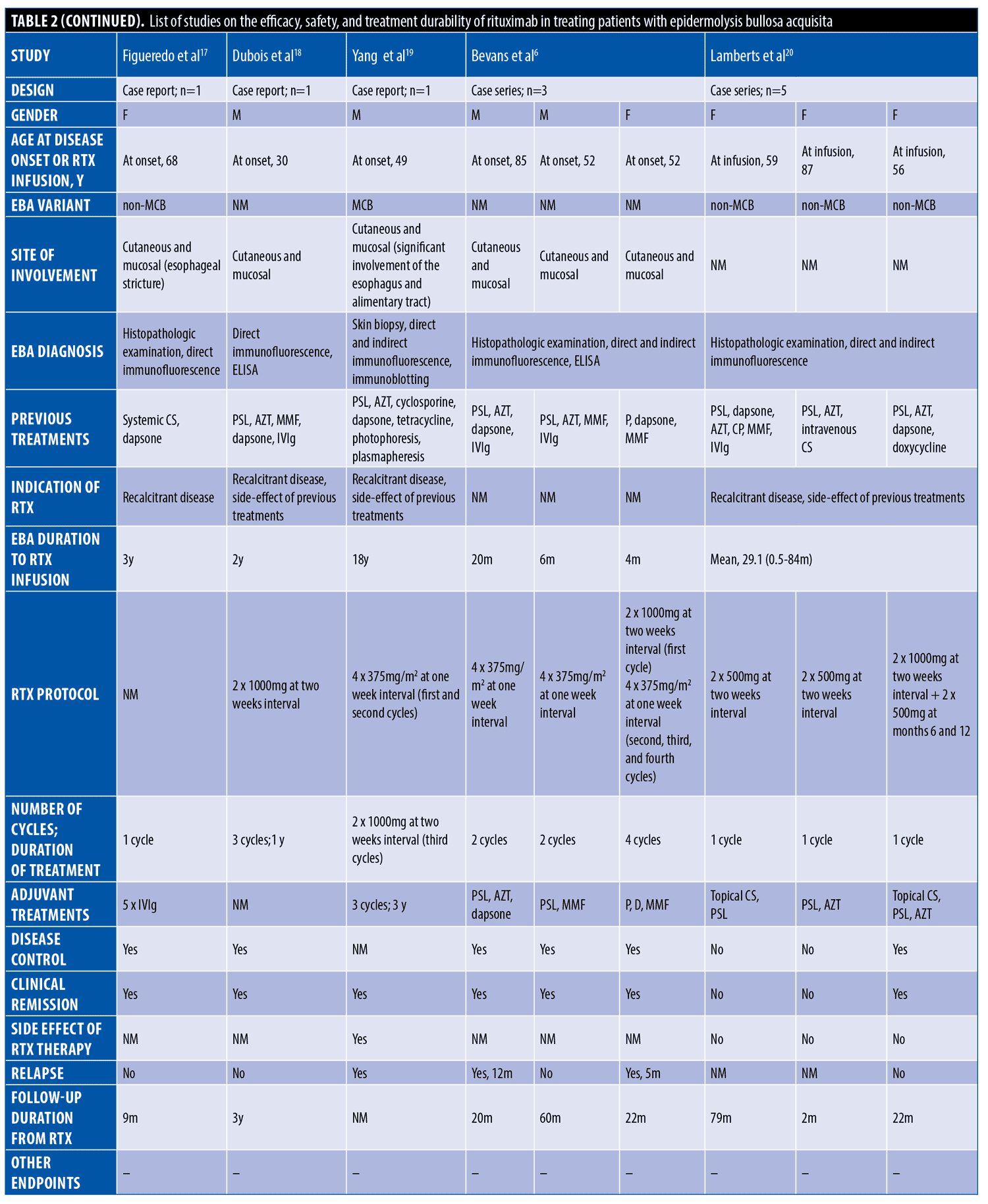
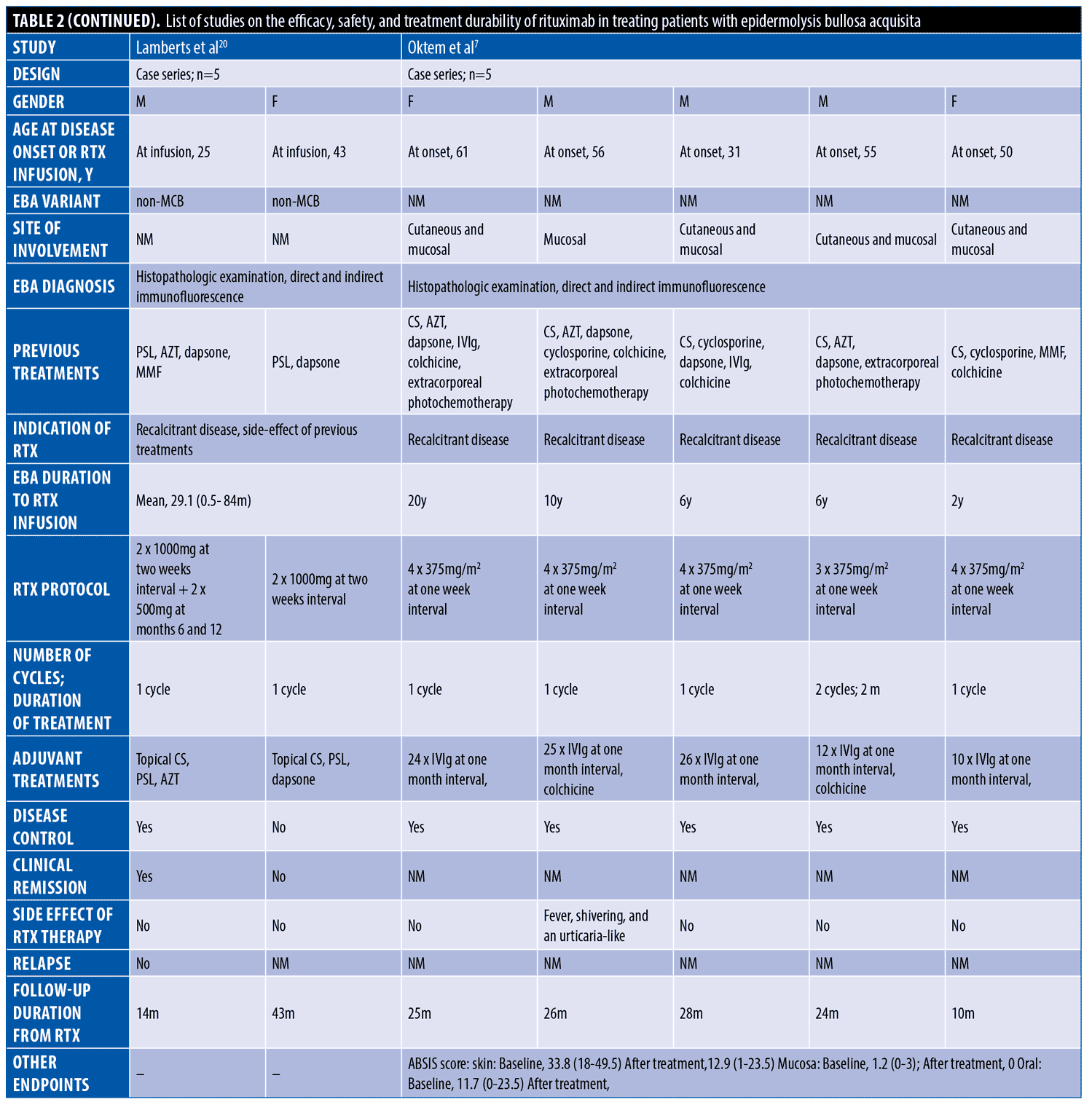
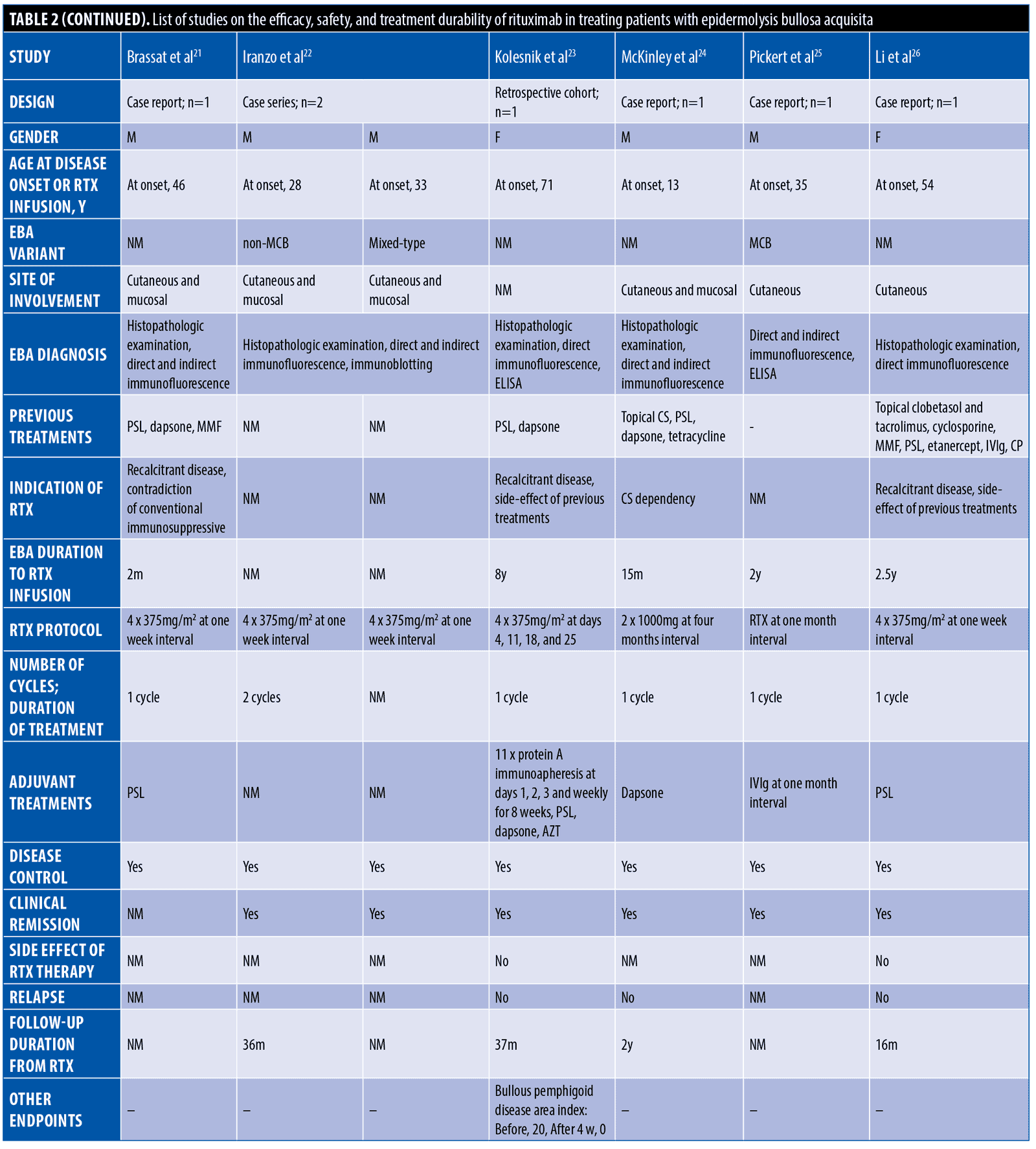
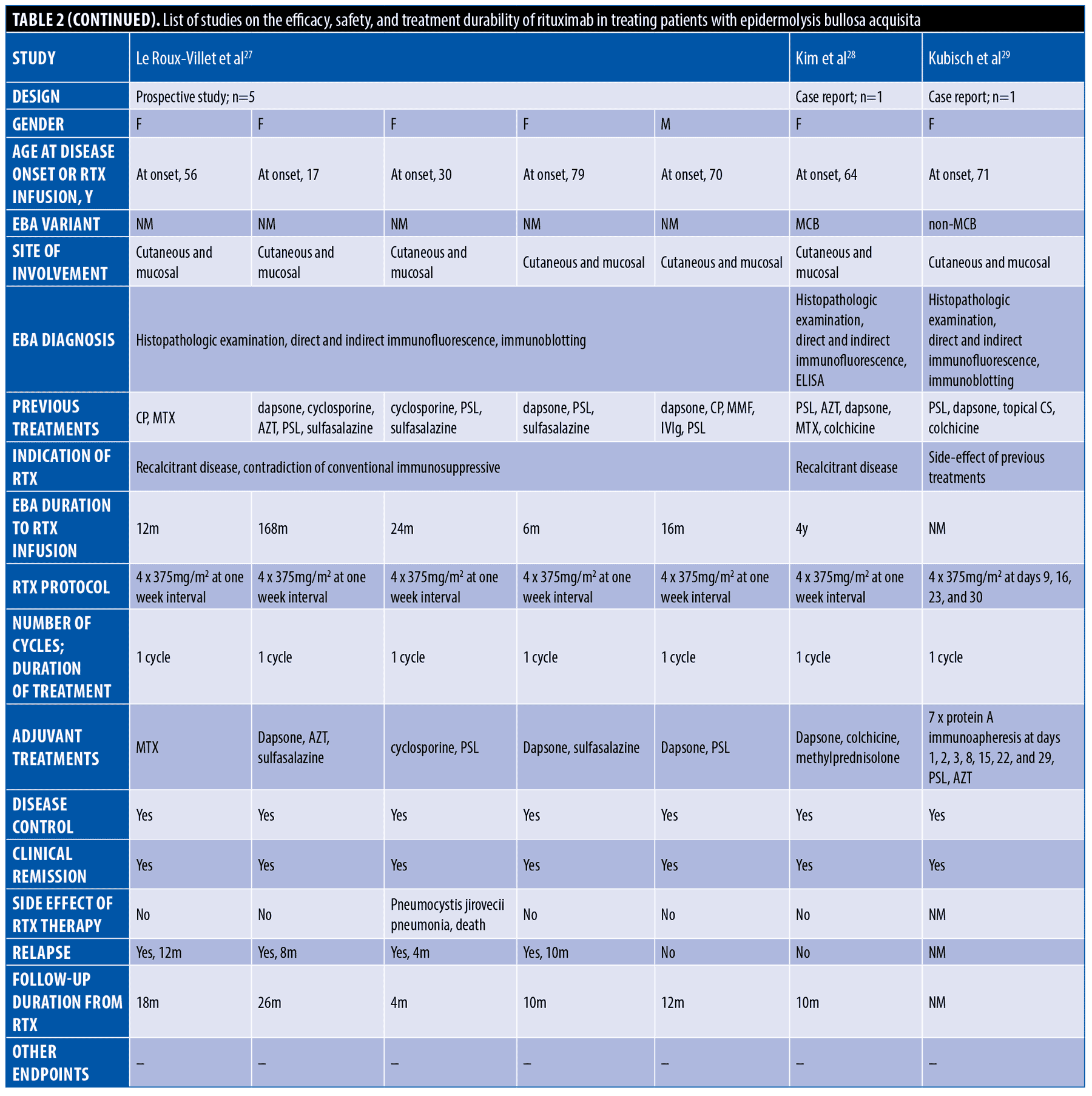
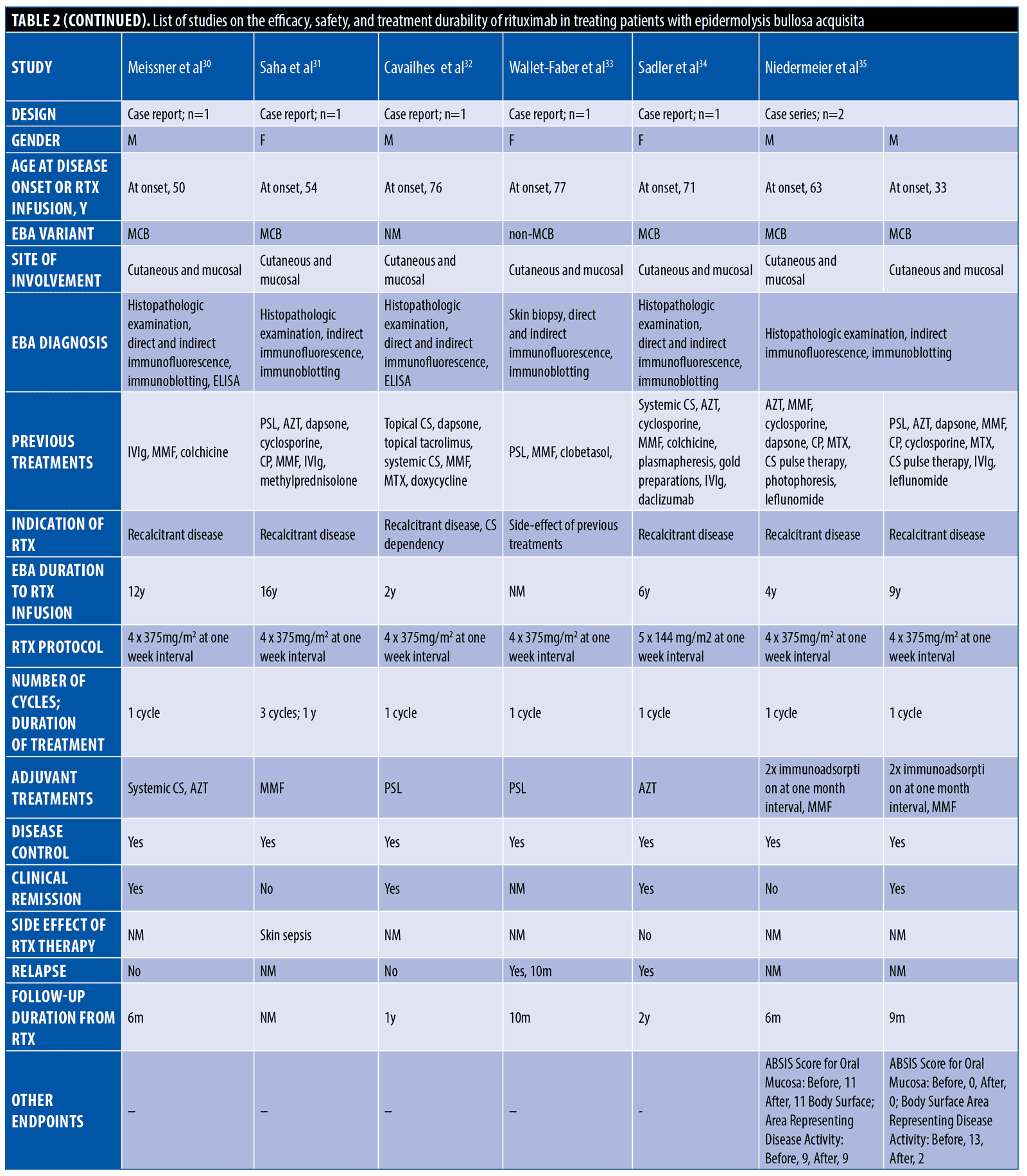
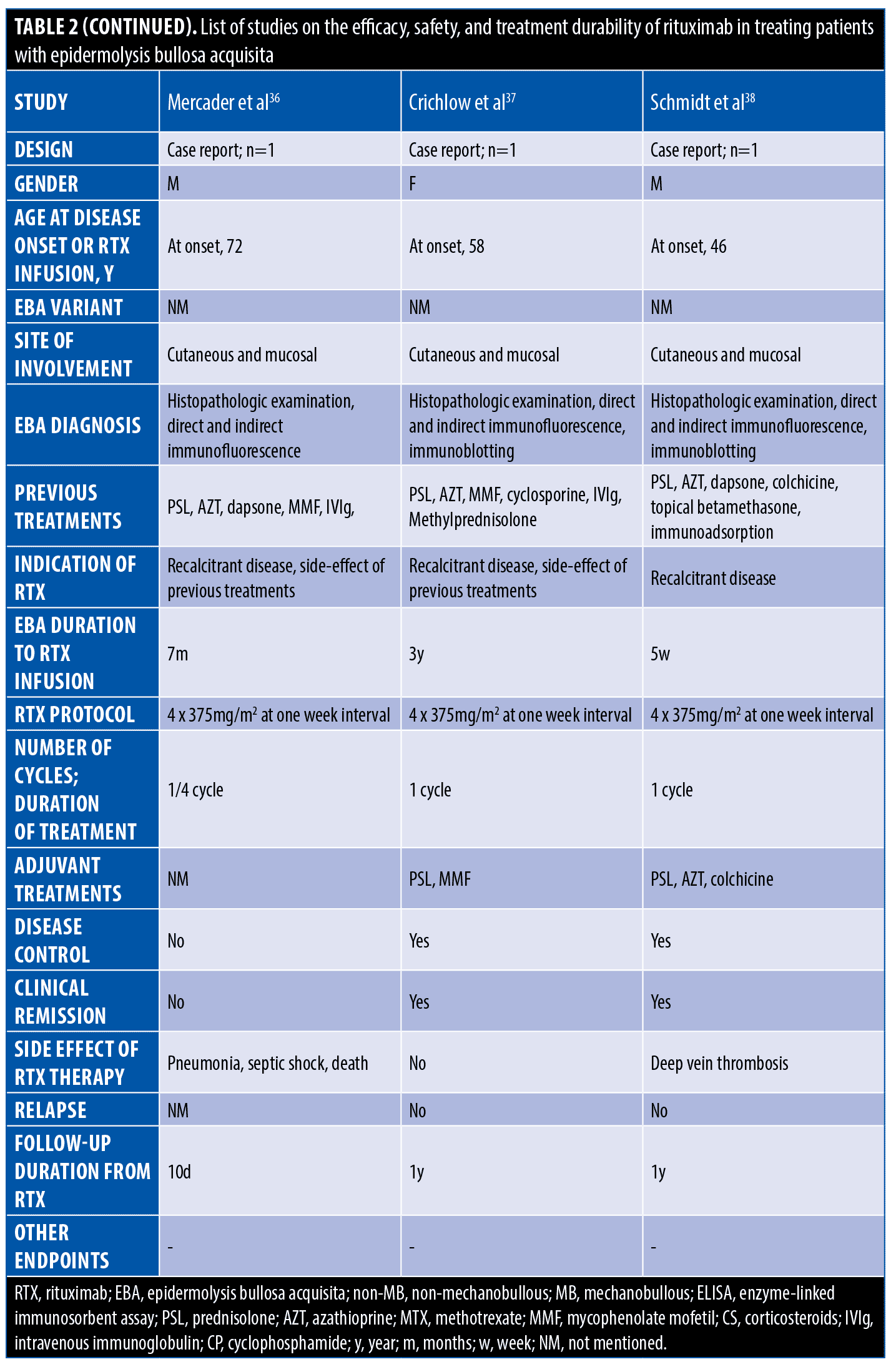
The systematic search resulted in 565 articles, 238 of which were non-duplicate reports. After the screening of title and abstracts, 63 studies were selected for full-text review. Accordingly, 31 articles including 68 patients with EBA were found eligible for data extraction (Figure 1).5–7,11–38 The mean age of the patients was 52.9 years old, with 35 (51.5%) women and 33 (48.5%) men. The EBA subtype was defined in 43 patients, including 23 (53.5%) with MB type, 19 (44.2%) with non-MB type, and one (2.3%) with mixed form. The indications of RTX administration in patients with EBA were mostly recalcitrant diseases (55.9 % in 38 patients). The appropriate clinical response to RTX was observed in 92.7 percent (63 patients) patients. Disease remission was considered the endpoint of treatment in 28 studies (61 patients), demonstrating an overall remission rate of 73.8 percent (45 patients). Treatment durability was assessed in 17 studies (38 patients); disease relapse rate was measured as 39.5 percent (15 patients) in the mean follow-up of 23.0 months. Of the patients, 28.2 percent (11 patients) experienced RTX-related side effects, which were primarily mild and transient. The severe side effects were pneumonia in two individuals (leading to death in both), and deep vein thrombosis in one. The results of the literature review are summarized in Tables 1 and 2.
Discussion
The treatment of EBA as a subtype of autoimmune bullous disease is a real challenge for dermatologists. Due to disease rarity, no randomized controlled trial is available to report on the efficacy and safety of medications on EBA. Patients are commonly refractory to conventional immunosuppressive agents, and many fail to attain disease remission.
According to a systematic review and meta-analysis on the efficacy of EBA treatments, only
intravenous immunoglobulin and RTX were associated with complete remission of the disease, among the others.1 Numerous studies have reported the beneficial use of RTX for treating EBA.5–7,11–38 RTX does not affect plasma cells secreting autoantibodies, causing a delay in the commencement of a clinical outcome.39
In some previous studies, RTX was used in combination with intravenous immunoglobulin or immunoadsorption. Accordingly, a study explored the combination therapy of RTX and intravenous immunoglobulin in five patients with recalcitrant EBA.7 All patients showed clinical response to the treatment contributing to a significant reduction in the mean skin surface involvement, mucosal involvement, and oral mucosal severity scores based on the Autoimmune Bullous Skin Disorder Intensity Score. There are also reports of four cases of EBA treated with RTX plus immunoadsorption, which led to complete disease remission of three.23,29,35 The intravenous immunoglobulin and immunoadsorption readily remove pathogenic autoantibodies. However, they have a short therapeutic effect, and the compensatory production of autoantibodies can lead to the rebound phenomenon.6 Combining these two treatment modalities with RTX would provide an immediate clinical effect with sustainable disease remission.
In the literature review, the rate of RTX-related adverse effects was estimated as 28.2 percent. A significant concern regarding RTX safety is the risk of infection. The prolonged B cell depletion and unselective removal of antibodies bear an increased risk of opportunistic infections to the patients. We detected two deaths from pneumonia in the systematic search of patients with EBA treated with RTX.27,36
Conclusion
In this study, we aimed to summarize the current evidence on the safety and efficacy of RTX in EBA. The only major safety concern is the higher risk of infection, which should be considered before administration in vulnerable patients. The combination therapy with intravenous immunoglobulin and immunoadsorption could provide further clinical benefits. Future studies could improve our insights into the effectiveness and action mechanisms of RTX therapy in patients with EBA by providing further immunological details.
Supplemental Materials
References:
- Iwata H, Vorobyev A, Koga H, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis. 2018;13(1):153.
- Buijsrogge JJ, Diercks GF, Pas HH, et al. The many faces of epidermolysis bullosa acquisita after serration pattern analysis by direct immunofluorescence microscopy. Br J Dermatol. 2011;165(1):92–98.
- Santi CG, Gripp AC, Roselino AM, et al. Consensus on the treatment of autoimmune bullous dermatoses: bullous pemphigoid, mucous membrane pemphigoid and epidermolysis bullosa acquisita – Brazilian Society of Dermatology. An Bras Dermatol. 2019;94(2 Suppl 1):33–47.
- Koga H, Prost-Squarcioni C, Iwata H, et al. Epidermolysis Bullosa Acquisita: The 2019 Update. Front Med (Lausanne). 2018;5:362.
- Mendes SR, Coutinho I, Cardoso JC. Epidermolysis bullosa acquisita treated with rituximab. BMJ Case Reports. 2021;14(7):e243432.
- Bevans SL, Sami N. The use of rituximab in treatment of epidermolysis bullosa acquisita: Three new cases and a review of the literature. Dermatol Ther. 2018;31(6):e12726.
- Oktem A, Akay BN, Boyvat A, et al. Long-term results of rituximab-intravenous immunoglobulin combination therapy in patients with epidermolysis bullosa acquisita resistant to conventional therapy. J Dermatolog Treat. 2017;28(1):50–54.
- Kianfar N, Dasdar S, Daneshpazhooh M, et al. A systematic review on efficacy, safety and treatment durability of intravenous immunoglobulin in autoimmune bullous dermatoses: Special focus on indication and combination therapy. Exp Dermatol. 2023;32(7):934–944.
- Thomas RM, Colon A, Motaparthi K. Rituximab in autoimmune pemphigoid diseases: Indications, optimized regimens, and practice gaps. Clin Dermatol. 2020;38(3):384–396.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89.
- Dasdar S, Kianfar N, Tavakolpour S, et al. M. Rituximab for the treatment of epidermolysis bullosa acquisita: A large case series of 15 patients. J Dtsch Dermatol Ges. 2023;21(7):779–784.
- Gordilho JO, Miyamoto D, Maruta CW, et al. Persistence of IgG4 as a potential serological marker of disease activity in patients with epidermolysis bullosa acquisita treated with rituximab. J Eur Acad Dermatol Venereol. 2023.
- Han JH, Yook HJ, Bang CH, et al. Refractory Epidermolysis Bullosa Acquisita with Chronic Graft-versus-Host Disease Successfully Treated with Rituximab. Ann Dermatol. 2023;35(Suppl 1):S129–S131.
- Koszegi B, Stone C, Murrell M. A case of sustained clinical remission for 6 years in Epidermolysis Bullosa Acquisita following rituximab infusions. Aust J Dermatol. 2023;64(S1):31–35.
- Szymański K, Kowalewski C, Pietrzyk E, et al. Case Report: Biological treatment of epidermolysis bullosa acquisita: report on four cases and literature review. Front Immunol. 2023;14:1214011.
- Schauer F, Nyström A, Kunz M, et al. Case Report: Diagnostic and Therapeutic Challenges in Severe Mechanobullous Epidermolysis Bullosa Acquisita. Front Immunol. 2022;13:883967.
- Figueredo C, Boroda K, Hertan H. Epidermolysis bullosa acquisita: an uncommon cause of esophageal stricture. Oxf Med Case Reports. 2021;2021(4):omab010.
- Dubois C, Hampton P. A case of epidermolysis bullosa acquisita in sustained remission for over 3 years following rituximab treatment. Poster presented at: British Association of Dermatologists 2019; July 2-4, 2019; Liverpool, Eng.
- Yang A, Kim M, Craig P, et al. A Case Report of the Use of Rituximab and the Epidermolysis Bullosa Disease Activity Scoring Index (EBDASI) in a Patient with Epidermolysis Bullosa Acquisita with Extensive Esophageal Involvement. Acta Dermatovenerol Croat. 2018;26(4):325–328.
- Lamberts A, Euverman HI, Terra JB, et al. Effectiveness and Safety of Rituximab in Recalcitrant Pemphigoid Diseases. Front Immunol. 2018;9:248.
- Brassat S, Fleury J, Camus M, et al. [Epidermolysa bullosa acquisita and graft-versus-host disease]. Ann Dermatol Venereol. 2014;141(5):369–373.
- Iranzo P, Herrero-González JE, Mascaró-Galy JM, et al. Epidermolysis bullosa acquisita: a retrospective analysis of 12 patients evaluated in four tertiary hospitals in Spain. Br J Dermatol. 2014;171(5):1022–1030.
- Kolesnik M, Becker E, Reinhold D, et al. Treatment of severe autoimmune blistering skin diseases with combination of protein A immunoadsorption and rituximab: a protocol without initial high dose or pulse steroid medication. J Eur Acad Dermatol Venereol. 2014;28(6):771–780.
- McKinley SK, Huang JT, Tan J, et al. A case of recalcitrant epidermolysis bullosa acquisita responsive to rituximab therapy. Pediatr Dermatol. 2014;31(2):241–244.
- A case of epidermolysis bullosa acquisita treated with rituxmab with comparisons to other epidermolysis bullosa acquisita reports and pemphiguis. J Am Acad Dermatol. 2013;68(4):AB110.
- Li Y, Foshee JB, Sontheimer RD. Sustained clinical response to rituximab in a case of life-threatening overlap subepidermal autoimmune blistering disease. J Am Acad Dermatol. 2011;64(4):773–778.
- Le Roux-Villet C, Prost-Squarcioni C, Alexandre M, et al. Rituximab for patients with refractory mucous membrane pemphigoid. Arch Dermatol. 2011;147(7):843–849.
- Kim JH, Lee SE, Kim SC. Successful treatment of epidermolysis bullosa acquisita with rituximab therapy. J Dermatol. 2012;39(5):477–479.
- Kubisch I, Diessenbacher P, Schmidt E, et al. Premonitory epidermolysis bullosa acquisita mimicking eyelid dermatitis: successful treatment with rituximab and protein A immunoapheresis. Am J Clin Dermatol. 2010;11(4):289–293.
- Meissner C, Hoefeld-Fegeler M, Vetter R, et al. Severe acral contractures and nail loss in a patient with mechano-bullous Epidermolysis bullosa acquisita. Eur J Dermatol. 2010;20(4):543–544.
- Saha M, Cutler T, Bhogal B, et al. Refractory epidermolysis bullosa acquisita: successful treatment with rituximab. Clin Exp Dermatol. 2009;34(8):e979–980.
- Cavailhes A, Balme B, Gilbert D, et al. [Successful use of combined corticosteroids and rituximab in the treatment of recalcitrant epidermolysis bullosa acquisita]. Ann Dermatol Venereol. 2009;136(11):795–799.
- Wallet-Faber N, Franck N, Batteux F, et al. Epidermolysis bullosa acquisita following bullous pemphigoid, successfully treated with the anti-CD20 monoclonal antibody rituximab. Dermatology. 2007;215(3):252–255.
- Sadler E, Schafleitner B, Lanschuetzer C, et al. Treatment-resistant classical epidermolysis bullosa acquisita responding to rituximab. Br J Dermatol. 2007;157(2):417–419.
- Niedermeier A, Eming R, Pfütze M, et al. Clinical response of severe mechanobullous epidermolysis bullosa acquisita to combined treatment with immunoadsorption and rituximab (anti-CD20 monoclonal antibodies). Arch Dermatol. 2007;143(2):192–198.
- Mercader P, Rodenas JM, Peña A, et al. Fatal Pseudomona pneumonia following rituximab therapy in a patient with epidermolysis bullosa acquisita. J Eur Acad Dermatol Venereol. 2007;21(8):1141–1142.
- Crichlow SM, Mortimer NJ, Harman KE. A successful therapeutic trial of rituximab in the treatment of a patient with recalcitrant, high-titre epidermolysis bullosa acquisita. Br J Dermatol. 2007;156(1):194–196.
- Schmidt E, Benoit S, Bröcker EB, Zillikens D, Goebeler M. Successful adjuvant treatment of recalcitrant epidermolysis bullosa acquisita with anti-CD20 antibody rituximab. Arch Dermatol. 2006;142(2):147–150.
- Cragg MS, Walshe CA, Ivanov AO, et al. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174.