 J Clin Aesthet Dermatol. 2022;15(11):30–36.
J Clin Aesthet Dermatol. 2022;15(11):30–36.
by Austin Ambur, DO; Abigale Clark, OMS-III; and Rajiv Nathoo, MD
All authors are with the Department of Dermatology at KCU-GME Advanced Dermatology and Cosmetic Surgery in Oviedo, Florida.
ABSTRACT: Objective. Keratoacanthomas are fast-growing cutaneous neoplasms that can be difficult to distinguish from squamous cell carcinoma, both clinically and histologically. The uncertain behavior of these neoplasms creates a challenge in management, and treatment choice often varies significantly between cases. The objective of this review is to discuss the most common and up-to-date treatment modalities used in the management of keratoacanthomas.
Methods. A literature search was performed using PubMed to access and review relevant keratoacanthoma treatment modalities published within the last 40 years. Keywords searched included “keratoacanthoma,” “Grzybowski syndrome,” “Ferguson-Smith syndrome,” “Witten-Zac syndrome,” and “Muir-Torre” syndrome.
Results. Our search resulted in 3,408 articles, of which 67 articles were ultimately included in this review.
Conclusion. Although surgical removal with excision or Mohs micrographic surgery remains the standard of therapy, there are many alternative therapeutic modalities that can be utilized.
Keywords: Keratoacanthoma, general dermatology, lasers, surgery, topical chemotherapy, dermatological therapy
Keratoacanthoma (KA) is a common cutaneous tumor characterized by rapid growth and possible spontaneous regression. It most commonly affects older, fair-skinned males with significantly sun damaged skin. Several variants have been described and are subdivided by solitary and multiple KA forms. These tumors have been postulated to originate from the hair follicle and possess a triphasic nature of rapid proliferation, stabilization, and regression mimics that of the hair cycle.1,2 Major signaling pathways that are potentially involved with KAs include Wnt/retinoic acid signaling, B-Raf, H-ras, p27, hedgehog, and p27.3–7
There are several morphological variants of KA. Solitary forms are the most common, are typically sporadic, and range in size from 1 to 2cm. Giant KAs are typically solitary lesions greater than 20cm commonly on the eyelid or nose. Keratoacanthoma centrifugum marginatum is a unique form of KA that is characterized by a peripheral expanding tumor with central healing that may be over 20cm in diameter.8
Genetic syndromes may predispose patients to the development of multiple KAs, including Muir-Torre syndrome, xeroderma pigmentosum, Ferguson-Smith, and Grzybowski. It is important to highlight on the later two genetic syndromes, as they involve multiple eruptive KAs. Ferguson-Smith syndrome is characterized by multiple KAs that develop in the third decade of life and regress over weeks to months. Witten-Zac syndrome is an autosomal dominant condition that classically develops in early childhood and characterized by the coexistence of features common to both Ferguson-Smith syndrome and Grzybowski syndrome, including multiple, small, miliary-type lesions, nodulo-ulcerative lesions, and large self-healing lesions. Grzybowski syndrome is characterized by thousands of eruptive KAs that may also resolve over several months, however lesions may also involve the airway. Grzybowski syndrome is sporadic and the individual lesions can resemble milia or eruptive xanthomas.
Several provoking factors that may be involved with these lesions include immunosuppression/immunodeficiency, radiation, trauma, chemicals, cell cycle modulating medications, and foreign bodies.9–17 The lesion has an uncertain behavior that lies on the border of benign and malignant and are commonly accepted as a variant of well-differentiated squamous cell carcinoma.
This nature poses clinicopathological challenges and controversies on how to appropriately manage the lesion. Surgical removal with conventional excision or Mohs micrographic surgery are the standard of therapy. Radiation, destructive therapies, topical therapies, systemic therapies, and observation have also been proposed. Eruptive keratoacanthomas are not an uncommon complication after treatment of solitary keratoacanthomas and presents another challenge that further complicates management of these lesions. No consensus guidelines on the alternative management of KAs exist. In this review, we will discuss an updated review of the most common treatment modalities in the management of KAs.
First-Line Therapy
Surgery. Standard excision is the treatment of choice for a majority of solitary KAs. This treatment modality is advantageous for rapid treatment, prevention of local invasion and tissue destruction, and cosmesis. Recurrence after standard excision may range from 4 to 8 percent.18
Mohs micrographic surgery is particularly useful for aggressive lesions, such as giant KAs and KA centrifugum, or locally destructive keratoacanthomas.19 Keratoacanthomas are known to have an unpredictable nature, are exceedingly difficult to distinguish from SCC, and are potentially destructive. Therefore, Mohs micrographic surgery should be considered for recurrent KAs and KAs in anatomically important areas where tissue sparing is vital.19,20 Recurrence after Mohs micrographic surgery may range from 2 to 8 percent.18
Alternative Therapy
Ionizing radiation. Radiotherapy is rarely used for the treatment of keratoacanthomas, though it is known that these lesions are extremely sensitive to this treatment modality. Several studies have demonstrated the effectiveness of radiotherapy on keratoacanthomas.12–24 Guidelines have not been established, but orthovoltage X-rays and electron radiotherapy are commonly used. Therapeutic regimens of orthovoltage X-rays and electron radiotherapy have been shown to be effective with excellent cosmetic results at a dose of 25Gy in five fractions on consecutive days.21 Increasing the time dose fractionation beyond 50 has not been found to further accelerate regression of the tumors.23 The mean time for disappearance of disease is 3 to 5 months.21 Others have found success with utilizing orthovoltage or electron radiotherapy at doses ranging from 3500cGy in 15 fractions to 5600cGy in 28 fractions, with full resolution in 1 to 3 months.22 A large scale study consisting of orthovoltage radiotherapy consisting of twice weekly fractions of 4Gy (total dose 40Gy) or 5Gy (total dose 50Gy) demonstrated success in complete resolution of KAs within one month.23 Radiotherapy with a 50-kV beryllium-windowed x-ray unit demonstrated success with a dosing regimen of five-times weekly fractions, with individual doses ranging from 4 to 5Gy and total dose ranging from 45 to 60Gy.24 All tumors resolved with satisfactory cosmetic results and without recurrence within a five-year observation period.24 Radiotherapy, if used, should be done before lesions progress too far, because cartilage destruction will result in less favorable cosmetic outcomes. It has been a proposed option for cosmetically sensitive, non-operable regions.24 Though radiotherapy is an effective treatment, its use does have limitations. It is unsuitable for patients younger than 60, and the multiple hospital visits that are required make it inconvenient. Further, radiation therapy may induce eruptive KAs.9,10
Topical Therapy
Treatment of KAs with topical 5-fluorouracil (5-FU) was first described more than 50 years ago and has continued to be an effective and well-tolerated treatment. Due to the various routes of administration, including intralesional, topical, and in combination with laser, 5-FU remains one of the first-line agents for treatment of KAs. In a retrospective chart review, complete resolution of KAs was noted in all patients who applied short contact topical 5-FU twice daily.25 Although the time to resolution varied from 4 to 6 weeks, all patients had complete resolution within six weeks. Due to minimal systemic uptake, 5-FU has an impressive safety profile and should always be considered in the initial treatment of KAs, particularly in locations where surgery may be less favorable, such as the face.25
Topical imiquimod is a topical immunomodulator that acts as a toll-like receptor agonist on toll-like receptors 7 and 8. Although more common treatment options include surgery, radiation, oral retinoids, and phototherapy, a few recent reports have described the effective treatment of KA with topical 5% imiquimod. In four cases reported by Jeon et al26, complete resolution of keratoacanthoma was observed after 9 to 11 weeks of topical imiquimod cream applied three times per week.26 Previously reported cases of KA treated with topical imiquimod have shown complete resolution of KA in as little as four weeks. Due to the risk of cosmetic deformity with surgical excision and the limitations of other treatment options, topical imiquimod may be a promising and effective alternative treatment option for keratoacanthomas.26
Destructive Therapy
A retrospective study of 111 keratoacanthomas in 106 patients treated with electrodesiccation and curettage (ED&C) demonstrated that four KAs (3.6%) reoccurred over a period of 3 to 26 months.27 The authors determined that ED&C was an effective therapy for KAs.27 Proposed advantages of this therapy are that it is cost-effective, provides preservation of surrounding uninvolved skin, and may be more convenient for patients who prefer fewer office visits.27 Disadvantages to this therapy are scarring and possible inadequate destruction of an underlying SCC, resulting in later progression.27 ED&C may be an appropriate treatment modality when the clinical features are typical and the tumor is located in a non-cosmetically sensitive area.
Cryotherapy consists of application of sub-zero temperatures to produce selective tissue destruction. Cryotherapy for KAs has been reported to have cure rates equivalent to ED&C, simple excision, or radiotherapy.28,29 Though studies are limited, cure rates may be as high as 87.5 percent.28 The procedure is typically used for lesions less than 20mms in diameter and consists of a pre-freeze shave or curettage followed by a double freeze-thaw cycle of 30 seconds with 3 to 5mm margins.28,29
Intralesional Therapy
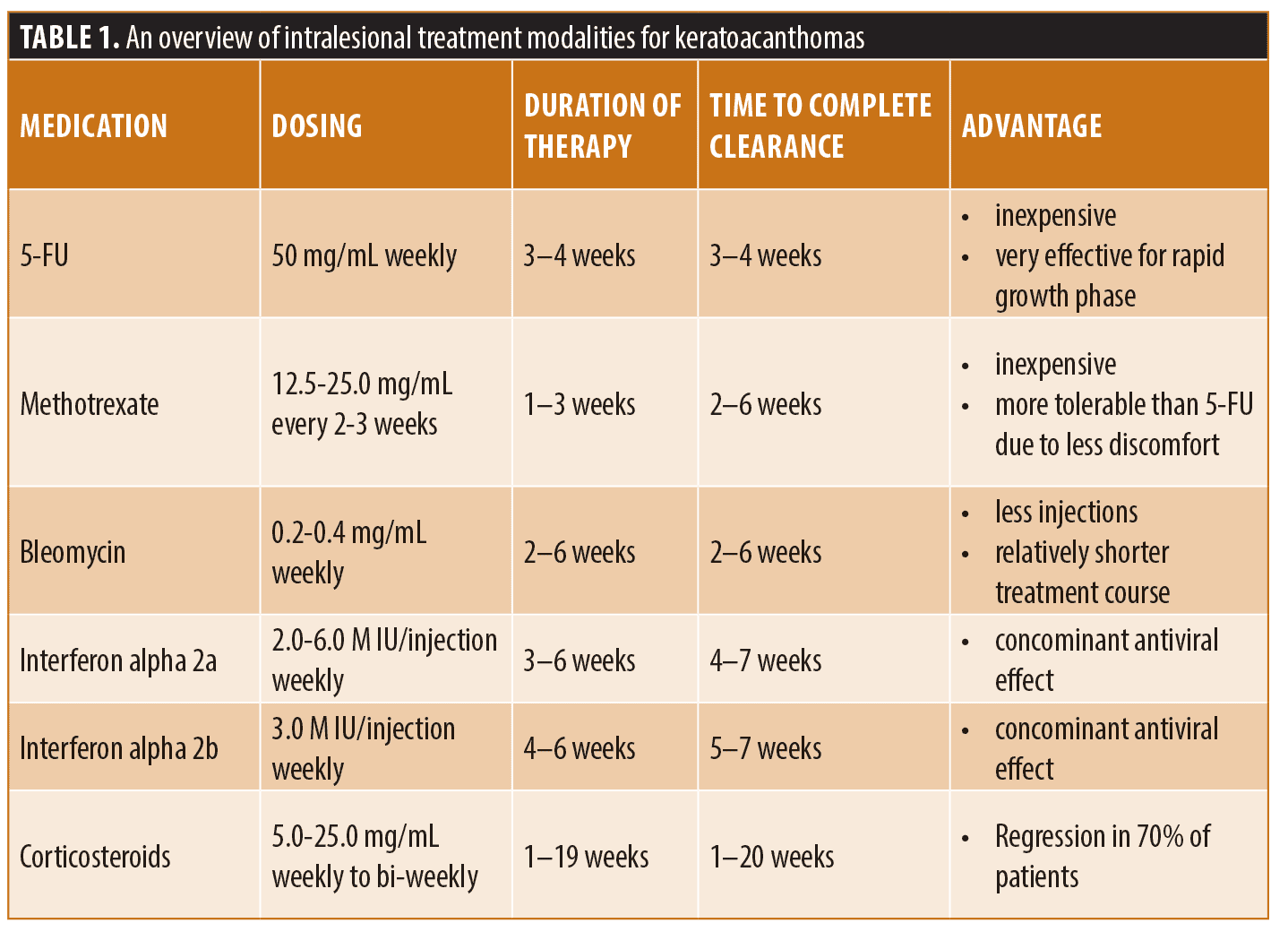
Intralesional 5-FU is a commonly used treatment modality for KAs. Typical dosing regimens consist of 50mg/mL of 5-FU injected weekly for 3 to 8 treatment sessions.30,31 Complete response rates may be as high as 98 percent.31 Intralesional 5-FU is a highly successful alternative to surgery in patients with large KAs, multiple KAs, recurrent KAs, and in invasive SCC or KA occurring in cosmetically sensitive areas.30 Combination therapy of intralesional 5-FU and systemic retinoids has been used successfully for the management of vemurafenib therapy.32 Though intralesional 5-FU appears to be a promising therapy, further large-scale studies should be completed, as its use is limited to case reports and case series.32
Intralesional methotrexate (MTX) may be considered for the treatment of KAs. MTX is a folic acid analog that works by binding to dihydrofolate reductase and prevents the conversion of dihydrofolate to tetrahydrofolate, thereby inhibiting DNA synthesis in actively dividing cells. It has been used successfully to treat KAs with cure rates that may be as high as 92 percent.33 It is important to note that its use is limited to case reports and case series.33 The standard therapy consists of injecting 1mL of 12.5 or 25mg/mL of MTX every 2 to 3 weeks over 1 to 4 sessions. Lesions typically resolve after 1 to 2 treatment sessions.30 When compared to 5-FU injections, there are several advantages of MTX, including fewer injections with a longer interval between injections, lower cost, and no requirement of anesthetic prior to injection.30
Bleomycin is a cytodestructive medication that exerts its action by binding to DNA during the M and G2 phases of the cell cycle, leading to single-strand breaks.34 It is directly cytotoxic to keratinocytes and eccrine epithelium.30 This chemotherapeutic medication is commonly used to treat lymphoma, testicular cancer, ovarian cancer, and cervical cancer. Its use to treat KAs is limited to case reports and case series.32,35–38 Dosing regimens consist of a single injection of 0.2 to 0.4mg of bleomycin. Occasionally, additional doses of the same regimen may be necessary if the lesion has not shown signs of involution on weekly follow-up visits. Full resolution of the lesions may occur within 2 to 6 weeks. Long-term follow-up has demonstrated an excellent cosmetic outcome and no recurrence after 18 months.37
Interferon alpha-2a is the recombinant form of endogenous interferon-a which acts by inducing release of intracellular enzymes, and inhibits protein synthesis.39 It also possesses immunomodulatory effects such as increased expression of major histocompatibility complex antigens, increased natural killer and cytotoxic T cell activity, cytokine induction, and enhanced production of endogenous interferons.39 Interferon alpha 2-b exerts similar anti-tumor effects by inhibition of the JAK-STAT pathway to directly inhibit tumor growth by cell cycle arrest, apoptosis, or differentiation.40 It indirectly functions to activate T cells and natural killer cell activity, and inhibits angiogenesis.40 The interferons are commonly used to treat lymphoma, viral hepatitis, and various cancers, including melanoma. Their use in treating KAs is limited to several case reports in which they were utilized as an alternative therapy to surgery.32,41,42 Dosing regimen of intralesional interferon alpha-2b has been shown to be successful with weekly injections of 3.0M IU/injection over 4 to 6 weeks.41 Complete regression may occur with an acceptable cosmetic outcome in 5 to 7 weeks.41 Dosing regimens for intralesional interferon alpha 2-a has shown to be successful with weekly injections of 2.0 to 6.0 M IU/injection until resolution of the lesions.42 Full resolution of the lesions may occur within 4 to 7 weeks.43
Intralesional corticosteroids is a treatment modality that is uncommonly utilized in the treatment of solitary, multiple, or recurrent keratoacanthomas. Its use is primarily discussed in older literature and is limited to case reports.32,43 It is not known how corticosteroids may induce regression of keratoacanthomas. It has been hypothesized that corticosteroids may inhibit KAs by affecting a cycle that mimics hair growth.43 Corticosteroids may also inhibit epidermal mitoses, DNA synthesis, and production of TGF-a, thereby inhibiting growth of KAs.43 Reportedly, intralesional corticosteroids may induce regression of up to 70 percent of keratoacanthomas with minimal scarring in 6 to 78 days.43 Triamcinolone has most commonly been used. Dosing regimens of various studies ranged from 5 to 25mg/mL intralesional injections weekly.43 Combination therapy of intralesional steroids with systemic retinoids has been utilized with good response for KAs arising in the setting of prurigo nodularis.44
Lasers
Argon lasers emit blue-green light between 488 and 514nm and have a selectivity to be absorbed by erythrocytes and melanocytes; thus, they are primarily used to treat vascular and pigmented disorders. This laser also creates a nonspecific localized thermal effect that may be used to generate precise destruction of non-melanocytic lesions. A study of argon laser therapy for KAs was completed on 17 patients with solitary KAs on the face and auricles.45 The continuous exposure technique was implemented with 4.5 watts and a beam diameter of 1mm.45 Excellent cosmetic results were attained in 65 percent of the patients. Mild scarring was noted in 35 percent of patients. No recurrence of disease as noted in a two year follow-up observational period.45 Given these results, we propose utilizing argon laser for management of small KAs in locations that are difficult to treat surgically.45
Er:YAG laser in combination with topical fluorouracil may be used for the treatment of KAs. Er:YAG lasers use an erbium-doped yttrium aluminum garnet medium to emit infrared light at a wavelength of 2940nm, which is absorbed by water present in cells. Literature on the use of this laser is limited to a case report in which a combination of ablative Er:YAG laser and topical 5-flurouracil was used to successfully treat a recurrent giant keratoacanthoma on the lower extremity of a 64-year-old Caucasian woman.46 The laser was used to increase the penetration and efficacy of topical 5% 5-FU. This therapy was demonstrated to be very effective and resulted in resolution of the tumor with no recurrence after six months. Though further studies are needed, Er:YAG laser treatments followed with topical 5-FU may be an effective treatment option for giant keratoacanthomas, even when recurrent.46
Photodynamic therapy (PDT) is a non-invasive treatment modality that combines a photosensitizing medication with light irradiation to create reactive oxygen species and lead to cellular death.47 Limited data is available on the use of PDT for KAs and is primarily limited to case reports and case series. Several studies have demonstrated effectiveness in treating KAs with PDT, while others have suggested that KAs might develop after its use.18,47 Further studies are required to elucidate the usefulness of PDT to treat keratoacanthomas.
Systemic Therapy
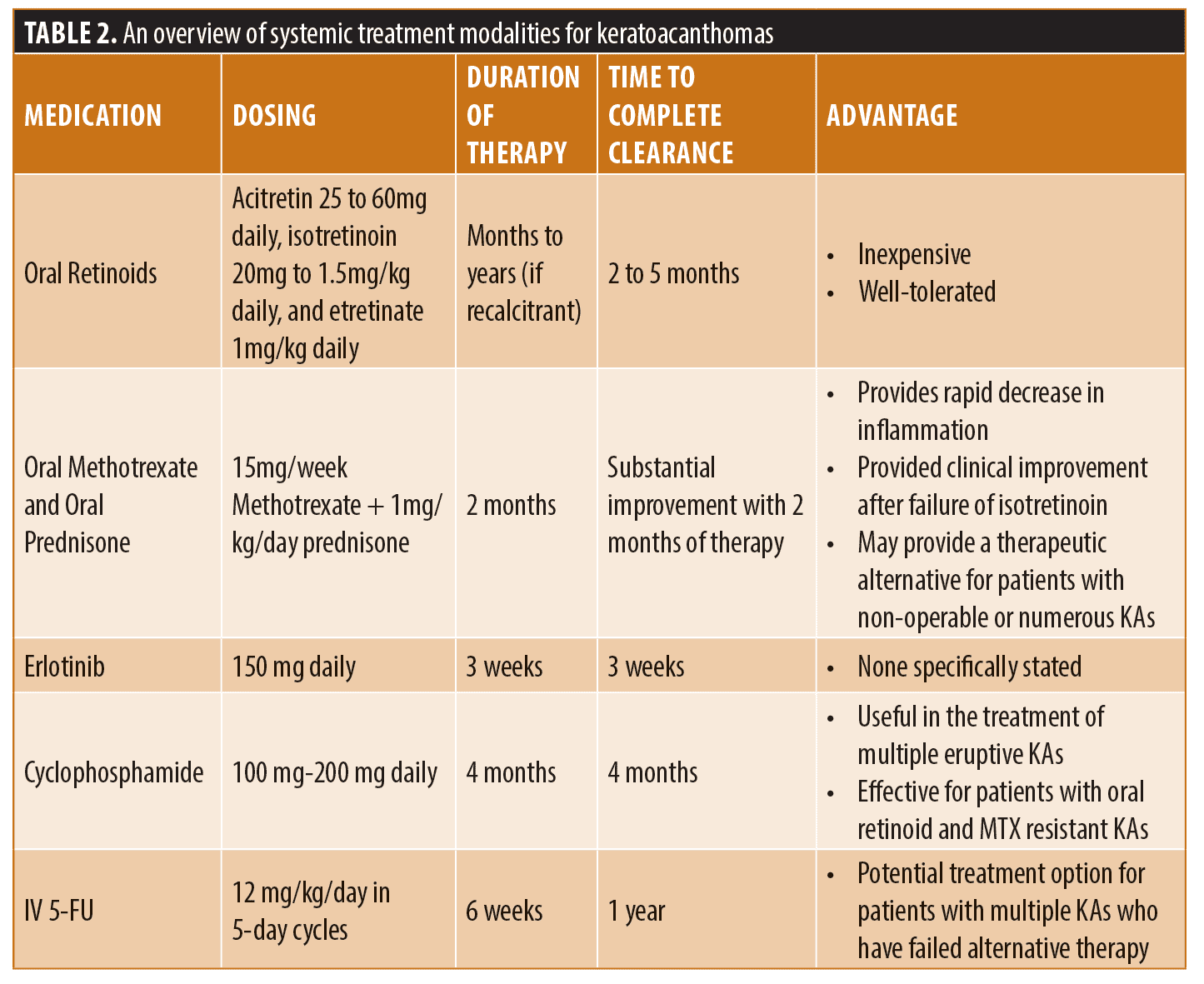
Oral retinoids have been used as systemic therapy for the treatment of KAs. Isotretinoin, etretinate, and acitretin are the most commonly used retinoids and have been associated with the best therapeutic efficacy within this class for the management of KAs. Retinoids exert their effect by binding to retinoic acid receptors and retinoid X receptors. The Wnt/retinoic acid signaling pathway is believed to be involved in the triphasic growth of KAs. Wnt is activated in the growth phase and inactivated in the regression phase. Systemic retinoids believed to reverse Wnt-related KA proliferation, inhibit keratinization, modulate terminal differentiation of epidermal cells, and increase both IL-2 production and mitogen-induced lymphocyte proliferation.3 Multiple case reports have demonstrated that retinoids are efficacious in patients with generalized eruptive keratoacanthoma of Grzybowski and Ferguson-Smith disease, however no large scale studies have been completed.48-60 The most common dosing regimens are acitretin 25 to 60 mg daily, isotretinoin 20mg to 1.5mg/kg daily, and etretinate 1mg/kg daily. Significant tumor regression with full remission is typically seen between 2 and 5 months. Recurrent disease upon discontinuation of etretinate has been successfully managed with a maintenance dose of 10mg daily.49 It is reasonable to consider an initial trial of oral isotretinoin in the treatment of KA in instances when surgical excision is not optimal, such as managing multiple eruptive KAs.48
Oral methotrexate is another potential therapeutic option to treat KAs. A case report of oral methotrexate 15mg/week was combined with oral prednisone at a dose of 1mg/kg/day for keratoacanthoma centrifugum marginatum with a substantial improvement after two months of therapy after failure of clearance with isotretinoin.61 The authors propose the combination of oral methotrexate with oral steroids for a short course for rapid decrease in inflammation.61 Though further large scale studies are required, this might provide a therapeutic alternative for patients with non-operable, or numerous KAs.
Cyclophosphamide is an alkylating agent which acts by creating direct DNA damage via cross-linking. Literature is limited on the use of this cytotoxic agent to case reports involving treatment of multiple eruptive KAs.62,63 It has been shown to be effective for patients with oral methotrexate and acitretin-resistant multiple KAs.62,63 Successful dosing regimens consisted of 100mg was given daily for one month, followed by three months of treatment at 200mg daily or pulses of 1g per month.62,63 These regimens resulted in significant improvement and eventual remission of the patient’s keratoacanthomas without further treatment. It is important to note that cyclophosphamide can have significant hematologic side effects, including anemia, macrocytosis, and lymphopenia. Due to the various hematological side effects associated with cyclophosphamide, in addition to the numerous alternative therapies available, treatment of KAs with cyclophosphamide is not routinely recommended.62
Erlotinib is an epidermal growth factor receptor (EGFR) inhibitor that has been proposed to be utilized for the treatment of resistant, multiple KAs. There is a rarity of reported use in the literature and further studies are necessary. It has been hypothesized that KAs may exhibit increased activation of EGFR which could be targeted by an EGFR tyrosine kinase inhibitor, such as erlotinib.64 It has been used successfully to rapidly reduce the number, size, and symptoms patients affected by multiple KAs.64
Though intralesional 5-FU is more commonly used, intravenous 5-FU has occasionally been utilized in the treatment of KAs. A case series of three patients affected by multiple eruptive keratoacanthoma of Grzybowski and Ferguson-Smith who were unresponsive to oral methotrexate were treated with intravenous 5-FU at a dose of 12mg/kg/day in five-day cycles for six weeks.65 This therapy resulted in complete resolution of smaller lesions at Week 6 and full resolution of all lesions at one-year follow up. The authors propose intravenous 5-FU as a potential effective treatment modality for carefully selected patients affected by multiple KAs who fail alternative therapy.65 Further large-scale studies are required to determine the efficacy and safety of this treatment modality.
Discussion
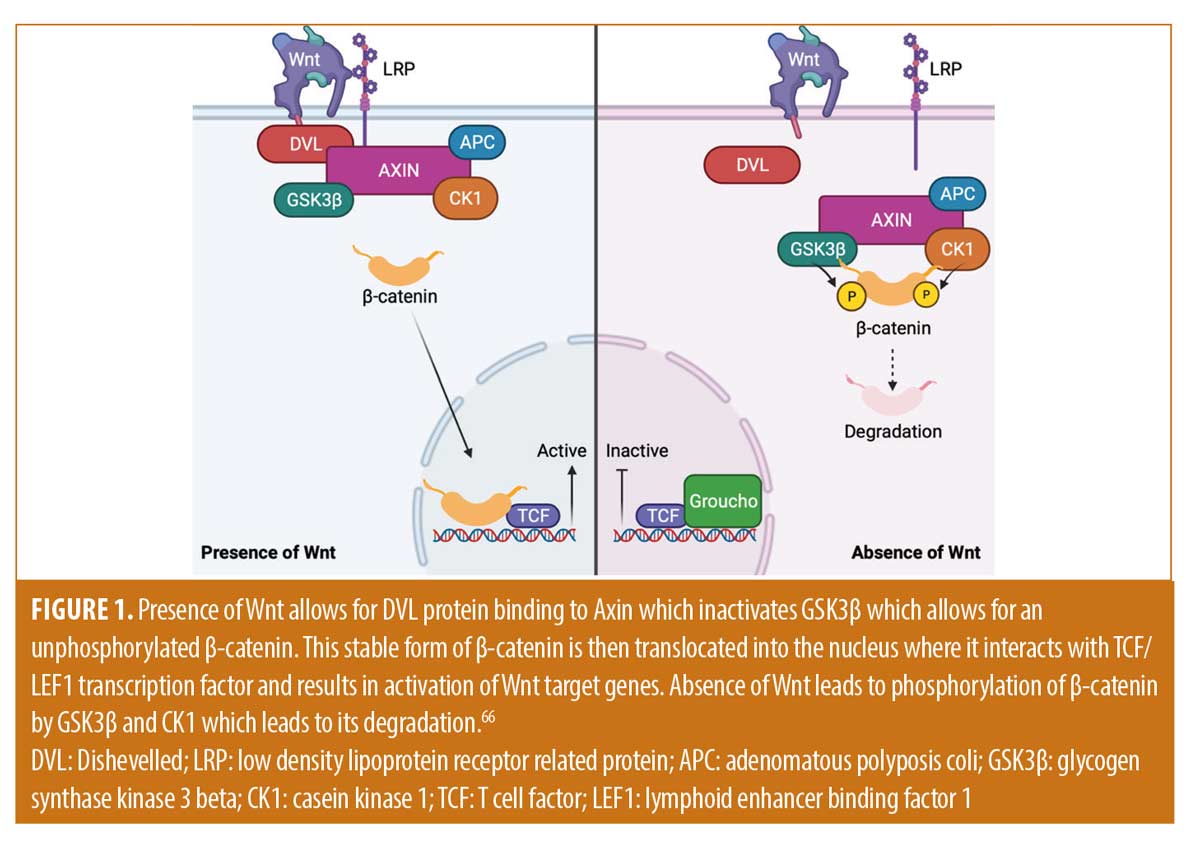
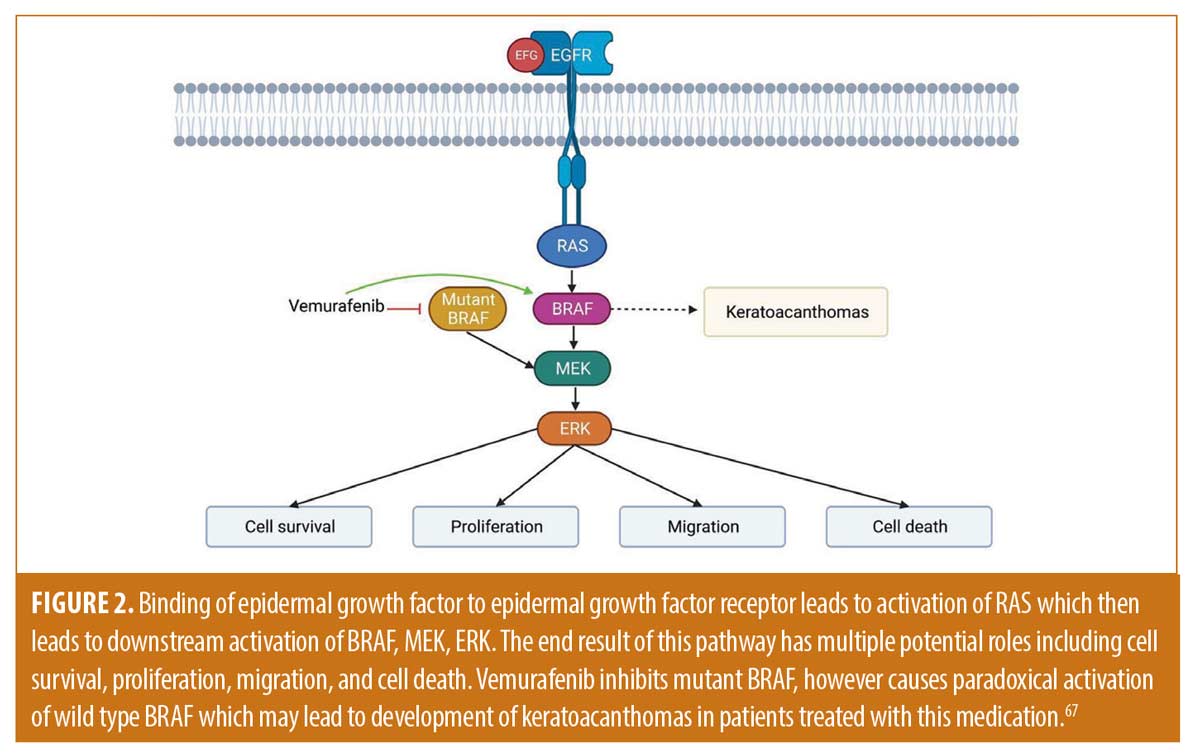
The clinical prospective of keratoacanthomas has changed throughout the years. There are numerous treatment modalities available for the management of KAs, however current treatment guidelines are lacking and there is no consensus on the most appropriate therapy for each subtype. Subtypes are divided into solitary and multiple forms. Solitary KAs are commonly treated with standard excision or Mohs micrographic surgery. Choosing the most appropriate therapy for the management of exceedingly large solitary KAs or multiple KAs may be more complicated. Understanding the biological nature of these tumors has allowed clinicians to develop alternative therapies to manage these complicated cases. KAs have been postulated to originate from the hair follicle. It is believed that they possess a triphasic growth pattern of rapid proliferation, stabilization, and regression which mimics that of the hair cycle.1,2 Major signaling pathways that may be involved with KAs includes include Wnt/retinoic acid signaling, B-Raf, H-ras, hedgehog, and p27.3-7 Wnt is activated in the growth phase and inactivated in the regression phase (Figure 1).66 Systemic retinoids are utilized in this pathway by reversing Wnt-related KA proliferation.3 H-ras mutations are common in KAs and are postulated to be involved with the change from KA proliferation to the regression phase. The Hedgehog pathway is hypothesized to be involved with the development of KAs as they can develop in patients being treated with vismodegib.7 Similarly, B-Raf signaling pathways may be involved given that KAs can appear in melanoma patients being treated with B-Raf inhibitors (Figure 2).6,7,67 Cyclin-dependent kinase inhibitor p27 is expressed during the regression phase and is not expressed during the growth phase.4 KAs also may demonstrate increased activation of EGFR which could be targeted by an EGFR tyrosine kinase inhibitor, such as erlotinib. Superficial ionizing radiation may be considered in cases where surgical or other therapeutic options are contraindicated, may result in cosmetic complications, or with aggressive subtypes. Destructive therapy has been utilized with success and includes use of Argon lasers, Er:YAG lasers, cryotherapy, and ED&C. Intralesional therapy with 5-FU, methotrexate, bleomycin, interferon alpha 2a and 2b, bleomycin, and corticosteroids have all been successfully utilized (Table 1). Systemic therapy may include retinoids, methotrexate, cyclophosphamide, erlotinib, and intravenous 5-FU (Table 2). Topical therapy may include 5-FU or imiquimod. Each of these treatment modalities have been used with variable success alone or in combination therapy. It should be emphasized that double-blind randomized control studies are needed to truly compare various treatment modalities presented. Conclusions of superiority cannot be reached based on the numbers presented. It is important to recognize that several provoking factors have been associated with the development of KAs, including immunosuppression/immunodeficiency, radiation, trauma, chemicals, cell cycle modulating medications, and foreign bodies.9-17 These factors may impact the treatment modality most appropriate for the specific patient. Eruptive keratoacanthomas are not an uncommon problem to occur after treatment of solitary keratoacanthomas and have been reported after surgical excision, laser therapy, and radiation. Further trauma to the skin with most available standard therapies risks the development of additional eruptive KAs and some KAs may be too large for surgical therapy. Guidelines and large-scale studies for the most appropriate alternative therapy when surgery is not feasible are lacking. A patient specific approach that focuses on evidence, efficacy, safety, cost effectiveness, and patient preference is needed. In this article, we reviewed the most common standard and alternative treatment modalities for KAs to better help clinicians to work with their patients in choosing the optimal therapy.
References
- Ramselaar CG, Ruitenberg EJ, Kruizinga W. Regression of induced keratoacanthomas in anagen (hair growth phase) skin grafts in mice. Cancer Res. 1980;40(5):1668–1673.
- Iyengar B, Ramesh V. Hair cycle and the histogenesis of pillar tumors. Indian J Cancer. 1989;26(1):1–9.
- Zito G, Saotome I, Liu Z, et al. Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross-talk. Nat Commun. 2014;5:3543. Published 2014 Mar 26.
- Hu W, Cook T, Oh CW, et al. Expression of the cyclin-dependent kinase inhibitor p27 in keratoacanthoma. J Am Acad Dermatol. 2000;42(3):473–475.
- Corominas M, Kamino H, Leon J, et al. Oncogene activation in human benign tumors of the skin (keratoacanthomas): is HRAS involved in differentiation as well as proliferation? Proc Natl Acad Sci USA. 1989;86(16):6372–6376.
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–215.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149(2):242–243.
- Miedzinski F, Kozakiewicz J. Keratoacanthoma centrifugum-a special variety of keratoacanthoma [in German]. Hautarzt. 1962;13:348–352.
- Robertson SJ, Bashir SJ, Pichert G, et al. Severe exacerbation of multiple self-healing squamous epithelioma (Ferguson-Smith disease) with radiotherapy, which was successfully treated with acitretin. Clin Exp Dermatol. 2010;35(4):e100–e102.
- Shaw JC, Storrs FJ, Everts E. Multiple keratoacanthomas after megavoltage radiation therapy. J Am Acad Dermatol. 1990;23(5 Pt 2):1009–1011.
- Pattee SF, Silvis NG. Keratoacanthoma developing in sites of previous trauma: a report of two cases and review of the literature. J Am Acad Dermatol. 2003;48(2 Suppl):S35–S38.
- Mohr B III, Fernandez MP, Krejci-Manwaring J. Eruptive keratoacanthomas after Jessners and trichloroacetic acid peel for actinic keratosis. Dermatol Surg. 2013;39:331–333.
- Cox S. Rapid development of keratoacanthomas after a body peel. Dermatol Surg. 2003;29:201–203.
- Mamelak AJ, Goldberg LH, Marquez D, et al. Eruptive keratoacanthomas on the legs after fractional photothermolysis: report of two cases. Dermatol Surg. 2009;35:513–518.
- Goldberg LH, Silapunt S, Beyrau KK, et al. Keratoacanthoma as a postoperative complication of skin cancer excision. J Am Acad Dermatol. 2004;50:753–758.
- Gewirtzman A, Meirson DH, Rabinovitz H. Eruptive keratoacanthomas following carbon dioxide laser resurfacing. Dermatol Surg. 1999;25:666–668.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603–611.
- Tran DC, Li S, Henry S. An 18-year retrospective study on the outcomes of keratoacanthomas with different treatment modalities at a single academic centre. The British Journal of Dermatology. 2017;177(6):1749–1751.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. Journal of the American Academy of Dermatology. 1998;39(1):79–97
- Garcia-Zuazaga J, Ke M, Lee P. Giant keratoacanthoma of the upper extremity treated with mohs micrographic surgery: a case report and review of current treatment modalities. The Journal of Clinical and Aesthetic Dermatology. 2009;2(8):22–25
- Shimm DS, Duttenhaver JR, Doucette J, et al. Radiation therapy of keratoacanthoma. International Journal of Radiation Oncology, Biology, Physics. 1983;9(5):759–761.
- Donahue B, Cooper JS, Rush S. Treatment of aggressive keratoacanthomas by radiotherapy. Journal of the American Academy of Dermatology. 1990;23(3 Pt 1):489–493.
- Caccialanza M, Sopelana N. Radiation therapy of keratoacanthomas: results in 55 patients. International Journal of Radiation Oncology, Biology, Physics. 1989;16(2):475–477.
- Goldschmidt H, Sherwin WK. Radiation therapy of giant aggressive keratoacanthomas. Archives of Dermatology. 1993;129(9):1162–1165.
- Thompson BJ, Ravits M, Silver DN. Clinical efficacy of short contact topical 5-Fluorouracil in the treatment of keratoacanthomas: a retrospective analysis. The Journal of Clinical and Aesthetic Dermatology. 2014;7(11):35–37.
- Jeon HC, Choi M, Paik SH, et al. Treatment of keratoacanthoma with 5% imiquimod cream and review of the previous report. Annals of Dermatology. 2011;23(3): 357–361.
- Nedwich JA. Evaluation of curettage and electrodesiccation in treatment of keratoacanthoma. The Australasian Journal of Dermatology. 1991;32(3):137–141.
- Holt PJ. Cryotherapy for skin cancer: results over a 5-year period using liquid nitrogen spray cryosurgery. The British Journal of Dermatology. 1988;119(2):231–240.
- Kuflik EG. Cryosurgery for cutaneous malignancy. An update. Dermatologic Surgery. 1997;23(11):1081–1087.
- Good LM, Miller MD, High WA. Intralesional agents in the management of cutaneous malignancy: A review. Journal of the American Academy of Dermatology. 2011;64(2):413–422.
- Goette DK, Odom RB. Successful treatment of keratoacanthoma with intralesional fluorouracil. Journal of the American Academy of Dermatology. 1980;2(3):212–216.
- Kiss N, Avci P, Bánvölgyi A, et al. Intralesional therapy for the treatment of keratoacanthoma. Dermatologic Therapy. 2019;32(3):e12872.
- Yoo MG, Kim IH. Intralesional methotrexate for the treatment of keratoacanthoma: retrospective study and review of the Korean literature. Annals of Dermatology. 2014;26(2):172–176.
- Landis L. Topical and intralesional antiviral agents. In: Wolverton SE, Wu JJ. Comprehensive Dermatologic Drug Therapy. 4th edition. Elsevier, Philadelphia, PA, 2021, pp. 499–500.
- Andreassi A, Pianigiani E, Taddeucci P, et al. Guess what! Keratoacanthoma treated with intralesional bleomycin. European Journal of Dermatology. 1999;9(5):403–405.
- Sayama S, Tagami H. Treatment of keratoacanthoma with intralesional bleomycin. The British Journal of Dermatology. 1983;109(4):449–452.
- De la Torre C, Losada A, Cruces MJ. Keratoacanthoma centrifugum marginatum: treatment with intralesional bleomycin. Journal of the American Academy of Dermatology. 1997;37(6):1010–1011.
- Rapaport J. Giant keratoacanthoma of the nose. Archives of Dermatology. 1975;111(1):73–75.
- Aria M, Benfield P. Interferon-alpha-2a. A review of its pharmacological properties and therapeutic use in the management of viral hepatitis. Drugs. 1995;50(5):873–896.
- Ningrum AR. Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica. 2014;2014:970315.
- Oh CK, Son HS, Lee JB, et al. Intralesional interferon alfa-2b treatment of keratoacanthomas. Journal of the American Academy of Dermatology. 2004;51(5 Suppl):S177–S180.
- Grob JJ, Suzini F, Richard MA, et al. Large keratoacanthomas treated with intralesional interferon alfa-2a. Journal of the American Academy of Dermatology. 1993;29(2 Pt 1):237–241.
- Sanders S, Busam, KJ, Halpern AC, et al. Intralesional corticosteroid treatment of multiple eruptive keratoacanthomas: case report and review of a controversial therapy. Dermatologic Surgery. 2002;28(10):954–958.
- Nofal A, Assaf M, Ghonemy S, et al. Generalized eruptive keratoacanthoma: a diagnostic and therapeutic challenge. International Journal of Dermatology. 2015;54(2):160–167.
- Neumann RA, Knobler RM. Argon laser treatment of small keratoacanthomas in difficult locations. International journal of dermatology. 1990;29(10):733–736.
- Thiele, JJ, Ziemer M, Fuchs S, et al. Combined 5-fluorouracil and Er:YAG laser treatment in a case of recurrent giant keratoacanthoma of the lower leg. Dermatologic Surgery. 2004;30(12 Pt 2):1556–1560.
- Tampa M, Sarbu MI, Matei C, et al. Photodynamic therapy: A hot topic in dermato-oncology. Oncology Letters. 2019;17(5):4085–4093.
- Wong WY, Kolbusz RV, Goldberg LH, et al. Treatment of a recurrent keratoacanthoma with oral isotretinoin. International Journal of Dermatology. 1994;33(8):579–583.
- Yoshikawa K, Hirano S, Kato T, et al. A case of eruptive keratoacanthoma treated by oral etretinate. The British Journal of Dermatology. 1985;112(5):579–583.
- Barysch MJ, Kamarashev J, Lockwood LL, et al. Successful treatment of multiple keratoacanthoma with topical imiquimod and low-dose acitretin. The Journal of Dermatology. 2011;38(4):390–392.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. International journal of dermatology. 2007;46(1):77–79.
- Vandergriff T, Nakamura K, High WA. Generalized eruptive keratoacanthomas of Grzybowski treated with isotretinoin. Journal of Drugs in Dermatology. 2008;7(11):1069–1071.
- Aydin F, Senturk N, Sabanciler E, et al. A case of Ferguson-Smith type multiple keratoacanthomas associated with keratoacanthoma centrifugum marginatum: response to oral acitretin. Clinical and Experimental Dermatology. 2007;32(6):683–686.
- Kato N, Ito K, Kimura K, et al. Ferguson Smith type multiple keratoacanthomas and a keratoacanthoma centrifugum marginatum in a woman from Japan. Journal of the American Academy of Dermatology. 2003;49(4):741–746.
- Benoldi D, Alinovi A. Multiple persistent keratoacanthomas: treatment with oral etretinate. Journal of the American Academy of Dermatology. 1984;10(6):1035–1038.
- Haydey RP, Reed ML, Dzubow LM, et al. Treatment of keratoacanthomas with oral 13-cis-retinoic acid. The New England journal of medicine. 1980;303(10):560–562.
- Ogasawara Y, Kinoshita E, Ishida T, et al. A case of multiple keratoacanthoma centrifugum marginatum: response to oral etretinate. Journal of the American Academy of Dermatology. 2003;48(2):282–285.
- Blitstein-Willinger E, Haas N, Nürnberger F, et al. Immunological findings during treatment of multiple keratoacanthoma with etretinate. The British Journal of Dermatology. 1986;114(1):109–116.
- Cherif F, Mebazaa A, Kort R, et al. Kératoacanthomes centrifuges marginés multiples [Multiple keratoacanthoma centrifugum marginatum]. Annales de Dermatologie et de Venereologie. 2002;129(4 Pt 1):413–415.
- Schaller M, Korting HC, Wolff H, et al. Multiple keratoacanthomas, giant keratoacanthoma and keratoacanthoma centrifugum marginatum: development in a single patient and treatment with oral isotretinoin. Acta Dermato-venereologica. 1996;76(1):40–42.
- Mangas C, Bielsa I, Ribera M, et al. A case of multiple keratoacanthoma centrifugum marginatum. Dermatologic Surgery. 2004;30(5):803–806.
- Oakley A, Ng S. Grzybowski’s generalized eruptive keratoacanthoma: remission with cyclophosphamide. The Australasian Journal of Dermatology. 2005;46(2):118–123.
- Nofal A, Assaf M, Ghonemy S, et al. Generalized eruptive keratoacanthoma: a diagnostic and therapeutic challenge. International Journal of Dermatology. 2015;54(2):160–167.
- Reid DC, Guitart J, Agulnik M, et al. Treatment of multiple keratoacanthomas with erlotinib. International Journal of Clinical Oncology. 2010;15(4):413–415.
- Agarwal M, Chander R, Karmakar S, et al. Multiple familial keratoacanthoma of Witten and Zak – A report of three siblings. Dermatology. 1999;198(4):396–399.
- Lee, Jae Ho. Wnt Signaling in Hair Follicle Development. Asian Journal of Beauty and Cosmetology. 2017; 15(2):242–246.
- Wan CJ, Brownell I. BRAF Inhibitors for the Treatment of Papulopustular Eruptions from MAPK Pathway Inhibitors. American Journal of Clinical Dermatology. 2020;21(6):759–764.

