J Clin Aesthet Dermatol. 2024;17(6):50–54.
J Clin Aesthet Dermatol. 2024;17(6):50–54.
by Kirsten Swenson, MS, MBA; Aliza Stern, MMSc, PA-C; and Emmy Graber, MD, MBA
Ms. Swenson is with The Dermatology Institute of Boston and Boston University School of Medicine in Boston, Massachusetts. Ms. Stern is with the Department of Dermatology at Boston University School of Medicine in Boston, Massachusetts. Dr. Graber is with The Dermatology Institute of Boston and Northeastern University in Boston, Massachusetts.
FUNDING: No funding was provided for this article.
DISCLOSURES: Dr. Graber served as a consultant for Digital Diagnostics, Almirall, Cutera, Hovione, Keratin Biosciences, La Roche Posay, Lipidor AB, Ortho Dermatologics, Sebacia, SolGel, Verrica, and WebMD. Dr. Graber has been provided with research grants from Almirall, Sebacia, and Ortho Dermatologics. Dr. Graber receives royalties from WoltersKleuwer Health and is a shareholder in Digital Diagnostics. The remaining authors have no conflicts of interest to disclose.
ABSTRACT: Periorificial dermatitis (POD) is a common, chronic, inflammatory facial skin rash that presents as tiny papules and papulopustules with underlying eczematous-like patches, typically confined to the perioral, perinasal, and periorbital areas. There is currently no Food and Drug Administration (FDA)-indicated treatment for POD; however, broad-spectrum antibiotics are efficacious as a treatment option. Broad-spectrum antibiotics negatively impact gut flora and lead to antibiotic resistance. Narrow-spectrum tetracyclines, such as sarecycline, have a low potential for promoting bacterial resistance and gastrointestinal issues.
Objective. We conducted a retrospective chart review in order to evaluate the efficacy of sarecycline in a cohort of patients diagnosed with POD that were treated with sarecycline.
Methods. A review of medical records was completed using an electronic medical record. Inclusion criteria included males and females aged 18 to 95 with a diagnosis of POD, treated with sarecycline with a documented follow-up.
Results. Six patients met inclusion criteria, all of which had shown improvement with no reported side effects. Of the six patients, four were female and two were male and the patient ages ranged from 26 to 58 years old (mean=41 years). The course of therapy ranged from 30 to180 days (median=90 days).
Conclusion. Based on the outcomes, there are many potential benefits to treatment of POD with sarecycline over the alternative tetracycline-class antibiotics. There is a need for more large-scale clinical studies evaluating treatment options for POD. Based on the efficacy and tolerability of sarecycline in large- scale acne studies, sarecycline may be an appropriate novel treatment option for POD and should be explored further.
Keywords: Periorificial dermatitis, sarecycline, antibiotics, tetracyclines, antibiotic resistance
Introduction
Periorificial dermatitis (POD, or perioral dermatitis) is a common chronic, inflammatory facial dermatosis. The typical presentation consists of erythematous papules or papulopustules around the perioral, perinasal, and/or periorbital area.1,2 The small pink papules and pustules with accompanying fine scale may recur over a course of weeks to months.2 Patients often report the affected areas are associated with symptoms of burning or stinging, as well as pruritus, in some cases.3
POD often affects women between the ages of 18 to 45 but can also affect men and children. Although the etiology is not well understood, topical corticosteroid use, among other agents, is a suspected trigger of POD. Therefore, a first-line treatment would be to discontinue any topical steroid use.3 Secondary treatment options include both topical and systemic medications, commonly antibiotics. However, there is no topical or oral medication with an FDA indication for POD. Although POD tends to respond quickly to treatment, it can be chronic and recurrent.3
Common treatments. Although there are many therapy options, both topical and oral, used favorably by clinicians for the treatment of POD, there is currently no FDA-approved treatment indicated for POD. In addition, there are very few randomized, controlled trials studying treatment options for POD. In mild cases of steroid-induced POD, discontinuation of the causative agent along with barrier repair moisturizers may be enough to treat the condition. The first-line therapies typically used for moderate presentations of POD consist of topical antibiotics (most commonly metronidazole, clindamycin, or erythromycin) and azelaic acid gel.4,5 Topical calcineurin inhibitors, including tacrolimus and pimecrolimus, have also shown to be effective in some studies.6,7
When topical therapies are not effective, or the presentation of POD is too severe, oral antibiotics are often used. Tetracycline class of antibiotics, particularly doxycycline 50 to 100mg twice daily and minocycline 50 to 100mg twice daily,2 are often the antibiotic of choice if the patient is over the age of eight years old, and not pregnant or breastfeeding. Antibiotics, particularly the tetracycline class, are very commonly indicated by dermatologists for the treatment of POD, and a typical course of oral tetracyclines for POD is approximately eight weeks.8
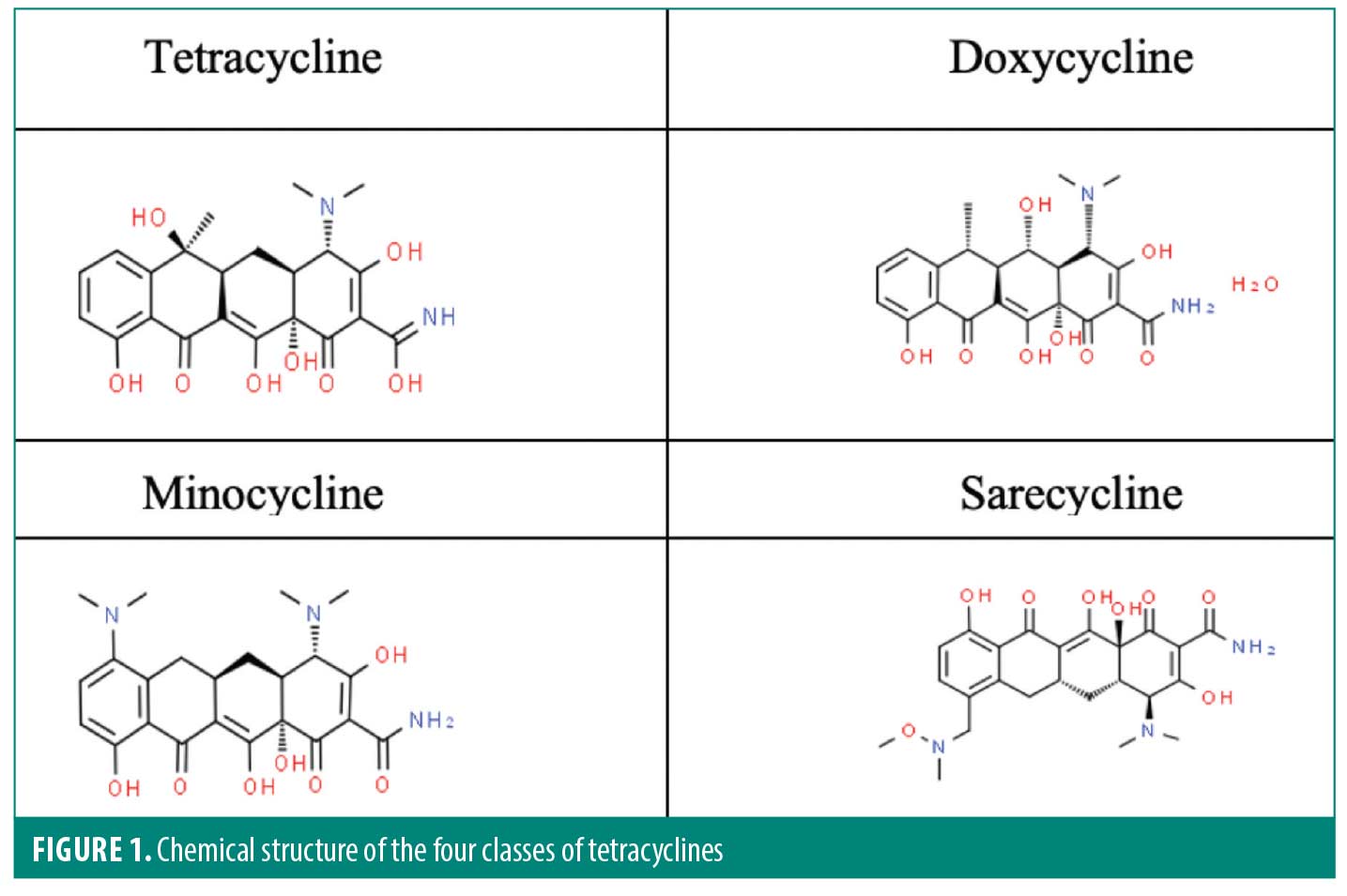
Sarecycline. The tetracycline class of antibiotics are the most prescribed antibiotics for inflammatory skin conditions, such as acne, rosacea, and POD. Sarecycline is the first-generation tetracycline-class antibiotic, doxycycline and minocycline are second-generation tetracycline-class antibiotics, and sarecycline (Seysara®, Almirall SA; Barcelona, Spain) is a third-generation tetracycline. Like the other tetracyclines, sarecycline is composed of four six-carbon rings, however sarecycline’s structure incorporates a longer C7 moiety which allows for greater stabilization and the ability to overcome tetracycline-resistant mechanisms, particularly the efflux pump (Figure 1).9
Sarecycline is an oral, narrow-spectrum tetracycline class drug that is currently approved by the FDA for the treatment of acne vulgaris in patients ages nine and older. Currently sarecycline is the only narrow-spectrum antibiotic approved to treat acne. Low propensity to develop resistance to sarecycline has been demonstrated in Cutibacterium acnes (C. acnes), Staphylococcus aureus (S. aureus), and Staphylococcus epidermidis (S. epidermidis).9,10 Although this medication is currently only FDA-indicated for the treatment of acne, there was a pilot study done recently capturing the efficacy and safety of sarecycline for the treatment of rosacea. The outcome of this study demonstrated sarecycline to be efficacious in the treatment of rosacea and suggested further study needed in a larger population.11 Sarecycline tablets come in three strengths; 60mg, 100mg, and 150mg, and the dosage is weight-based and should be taken once daily with or without food.
Pharmacodynamics and pharmacokinetics. Sarecycline is a ribosomal protein inhibitor of the tetracycline class that displays potent activity against Gram-positive bacteria as well as anti-inflammatory properties in vitro studies.12 The exact mechanism of action as to how the drug displays these anti-inflammatory properties is not well understood. However, the longer C7 moiety has been shown to increase stabilization of the ribosome due to its direct interaction with the messenger RNA (mRNA), giving it a stronger inhibitory effect compared to other tetracyclines.9
Photosensitivity is a potential side effect of sarecycline, along with the other tetracycline class antibiotics, but in clinical studies has been reported in only 0.2 percent of the patients, which is significantly lower than doxycycline and minocycline.12,13 Another side effect of tetracyclines, specifically minocycline, are adverse vestibular effects. A study evaluating the blood-brain barrier penetration of sarecycline displayed sarecycline’s inability to cross the blood-brain barrier compared to minocycline.14 This corresponded to sarecycline’s lower lipophilicity, which could be a potential explanation for the lower percentage of adverse vestibular effects in clinical trials.14
Mechanism of action. Tetracyclines inhibit protein synthesis by inhibiting the association of aminoacyl-tRNA with a bacterial ribosome, particularly the 30S ribosomal subunit, which blocks the tRNA at the acceptor (A) site halting the elongation of the polypeptide chain.17 Sarecycline inhibits bacterial ribosomes through interactions with the mRNA because of C7 optimization.17,18 All tetracyclines interact with the 70S bacterial ribosome, which is composed of the 30S and 50S ribosomal subunits that come together during protein synthesis. However, the distinct feature of the C7 moiety extends into the mRNA channel to directly interact with the A-site codon.17 This potentially interferes with the movement of the mRNA, tethering it to the 70S ribosomal subunit. Based on a study investigating the functional role of the C7 extension, it was concluded that sarecycline appeared to be a more potent inhibitor based on the interaction with the mRNA.17
Side effects of sarecycline. The most common side effect associated with sarecycline is nausea (2.1%), observed in a Phase III clinical trial.9,19 Other side effects of tetracycline class antibiotics, both broad and narrow spectrum, include vestibular effects (e.g., lightheadedness, dizziness, vertigo), intracranial hypertension leading to headache, blurred vision, papilledema, and photosensitivity (i.e., increased likelihood to sunburn).16 However, sarecycline has demonstrated few of these side effects, with 0.2 percent of patients experiencing sunburn and no reports of vestibular side effects.
Sarecycline and antibiotic resistance. Due to the growing concern of antibiotic resistance and emergence of new resistant strains, there was an interest in creating a semi-synthetic drug that could be classified into a new generation of tetracyclines.20 Sarecycline was developed specifically for the treatment of acne and was designed to have high selectivity against the bacteria C. acnes. The higher level of selectivity makes it less likely to contribute to antibiotic resistance, especially when compared to the other tetracycline-class antibiotics.18,20 This study aims to provide an alternative treatment option to broad spectrum antibiotics for the treatment of POD and reduce their contribution to antibiotic resistance.
Methods
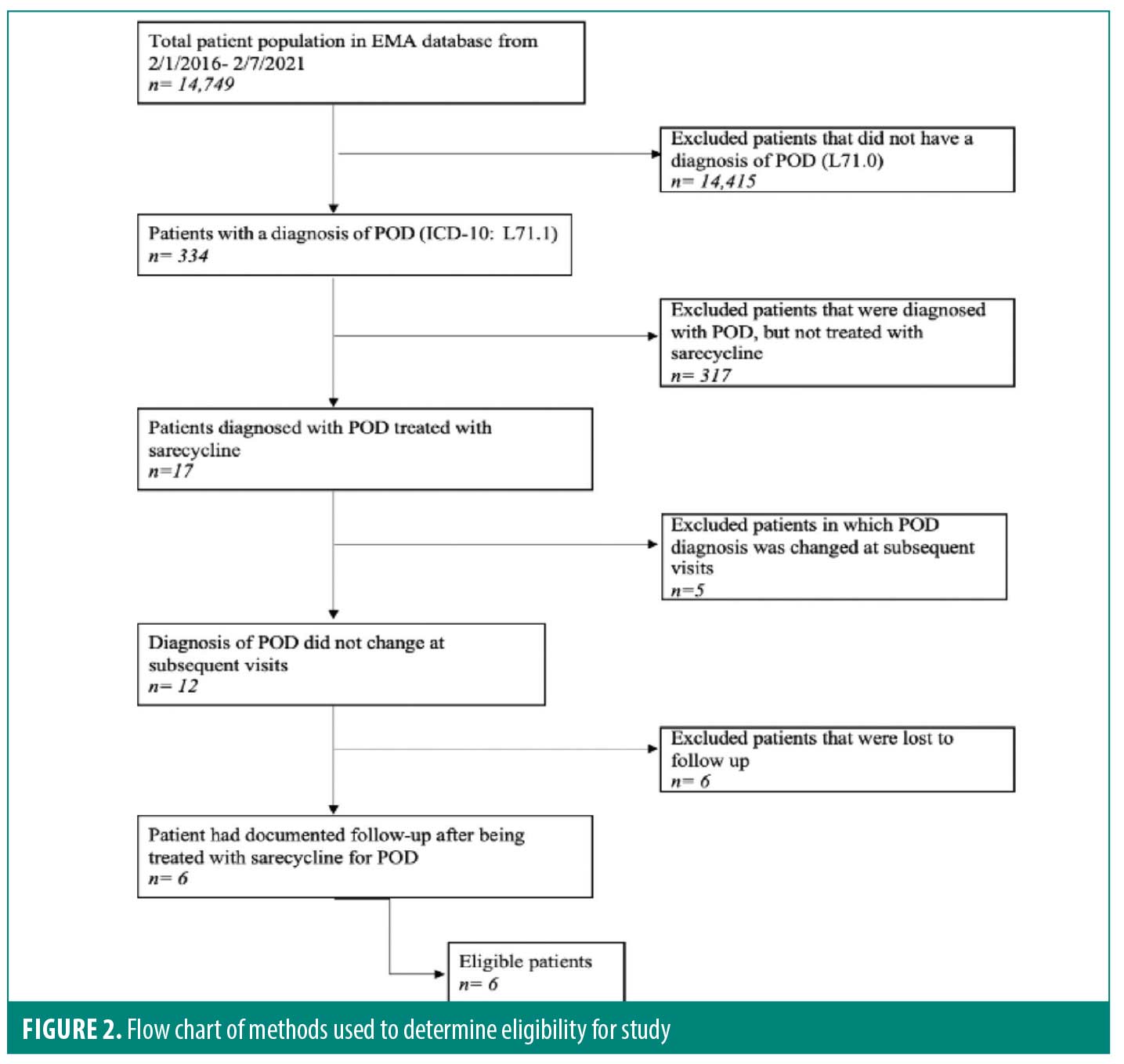
After approval was granted by the Institutional Review Board (IRB), the medical charts of patients at the Dermatology Institute of Boston were searched using the electronic medical record, EMA® (Modernizing Medicine®). To locate the patients who had a diagnosis of POD and were prescribed sarecycline, an advanced patient search was done using the International Classification of Disease, tenth revision (ICD-10) code L71.0 for POD, as well as “Seysara”™ (Almirall LLC; Malvern, Pennsylvania) in the medication search box. Using the two search terms, a total of 17 patient charts were identified and reviewed. Information extracted from the patient charts included date of birth, sex, duration of therapy, sarecycline dosage (60mg, 100mg, or 150mg), if POD resolved following treatment with sarecycline, and if recurrence occurred, how long after sarecycline was discontinued. Other information reviewed in the charts included side effects, whether a biopsy was performed to confirm the diagnosis of POD, and if there was a concomitant topical therapy used throughout treatment with sarecycline. It was also noted if POD was recurrent for the patient, and what prescriptions had been used in the past, in addition to side effects, if any, from previous treatments. Inclusion criteria for this study included patients who had a diagnosis of POD, were prescribed sarecycline, and had a documented follow up. Patients who had a diagnosis of POD and were prescribed sarecycline but did not have documented follow-up or the diagnosis was changed at subsequent visits were excluded from the study (Figure 2).
Results
From February 1, 2016, through February 7, 2022, there were a total of 14,749 charts reviewed to determine eligibility in the study. Of the 334 patients diagnosed with POD, 17 were prescribed sarecycline for the treatment of POD. Of the 17 patients, five patients were excluded due to the diagnosis being changed at subsequent visits and six patients were lost to follow up. Six patients were eligible for the study, as they met the inclusion criteria of having a diagnosis of POD, treated with sarecycline, and having documented follow up.
Of the six patients, four were female and two were male. The patient ages ranged from 26 to 58 years (mean=41 years). The course of therapy ranged from 30 to 180 days (median=90 days). The strengths of sarecycline used were 100mg and 150mg tablets and this was determined by the patients’ weight at the time the prescription was given. All six patients in the study had resolution of POD after being treated with a course of sarecycline. Two of the patients reported a recurrence of POD six months and 18 months after discontinuing sarecycline. None of the patients reported any adverse side effects while being treated with sarecycline. One patient had a biopsy to prove the diagnosis of POD, however the other five patients did not, and their diagnosis was based on clinical presentation. One patient was using topical metronidazole 0.75% concomitantly with sarecycline.
Discussion
POD is a common inflammatory facial skin condition that affects many patients. Given the lack of FDA-approved treatments indicated for POD, the need to explore alternative treatment approaches is warranted. Of the six subjects in this study, all had shown improvement after treatment with sarecycline. Although the sample size was small, the use of sarecycline for the treatment of POD was shown to be efficacious for all patients, which suggests the use of sarecycline as a potential treatment option for POD that should be further explored. Aside from a single patient case report, this is the first documented case series of patients treated with sarecycline for periorificial dermatitis.
Sarecycline versus doxycycline. Tetracyclines are effective in treating inflammatory skin conditions due to their antimicrobial and anti-inflammatory properties.13 The antimicrobial effect of tetracyclines is demonstrated by the tetracycline reversibly binding to the bacterial ribosome, specifically at the 30S subunit, preventing acyl-transfer RNA from binding to the ribosome, therefore halting protein synthesis.9,21 Tetracyclines show a variety of biological actions in addition to their antimicrobial activity, including anti-inflammatory activity, anti-apoptotic activity, inhibition of proteolysis, and suppression of angiogenesis and tumor metastasis.22,23
Doxycycline has demonstrated efficacy in the treatment of POD and is often the preferred oral medication for POD.24 Prescribing broad-spectrum antibiotics for inflammatory skin conditions may not be preferable due to the side effects associated with these treatments and the potential for antibiotic resistance. Gastrointestinal distress (e.g., nausea, vomiting, and diarrhea) are the leading adverse events associated with the broad-spectrum members of the tetracycline class of antibiotics. A study done by Leyden et al14 in 2013 reported that gastrointestinal adverse events ranged from 9.4 to 22.9 percent for patients taking doxycycline. It has also been noted that doxycycline may contribute to the development of irritable bowel syndrome and inflammatory bowel disease and is contraindicated in that patient population. Although sarecycline is a part of the tetracycline class and has potential for side effects, it is generally very well tolerated. A case study in 2021 published by Graber25 demonstrated the tolerability of sarecycline in a patient diagnosed with Crohn’s disease, who was unable to tolerate doxycycline in the past.
Adverse events associated with sarecycline. There were no adverse events or side effects documented in this case series. During the follow-up appointments, the treating clinicans documented whether the patients had experienced any side effects while taking sarecycline. Although there were no side effects reported at the visits, the clinicians did not specifically ask if the subject experienced every potential side effect that has been reported with the use of sarecycline. Most commonly, it was asked if the patients experienced nausea, as this is the most common adverse event which is reported less than one percent of the subjects while taking sarecycline.16 Side effects that were not specifically inquired about included less common side effects, including vaginal candidiasis and phototoxicity. Although these side effects are rare (<1%), they have been reported in sarecycline clinical studies.
Antibiotic resistance. Antibiotic resistance is a wide scale concern to public health that requires attention, as each year 2.8 million people are infected with antibiotic- resistant microorganisms, and over 35,000 of those infected people die.26 Dermatologists prescribe more antibiotics than any other specialty, which can put these patients at a higher risk of developing antibiotic-resistant infections.24 For nearly all antibiotics that have been created, there is some degree of bacterial resistance.27
Broad-spectrum antibiotics and resistance. Broad-spectrum, tetracycline-class antibiotics can treat both gram-positive and gram-negative bacteria, which makes them a common prescription to treat an array of bacterial infections.21 However, unlike broad-spectrum antibiotics, narrow-spectrum antibiotics target a specific type of bacteria rather than indiscriminately acting against both gram-positive and gram-negative bacteria. However, broad spectrum antibiotics can put patients more at risk for antimicrobial resistance as due to the larger, more diverse microbiome that they target.21 Narrow- spectrum antibiotics, such as sarecycline, discriminately target a smaller subset of bacteria, lessening the risk of antimicrobial resistance. The development of antibiotics that are more selective in the bacteria they target could be a potential solution to contribute to a decline in antibiotic resistance.
There are several ways in which bacteria may develop resistance to antibiotics. Common modes of antibiotic resistance to the tetracycline class of antibiotics include export by efflux pumps, ribosomal protection proteins, ribosomal mutations, and chemical inactivation.17,21,28 In addition to being a narrow-spectrum antibiotic, the unique chemical structure of sarecycline evades the development of resistance by resisting the bacterial efflux pump mechanism and through ribosomal protection.
Sarecycline’s role in antibiotic resistance. Sarecycline was developed specifically for the treatment of acne with high selectivity against the bacteria C. acnes. This higher level of selectivity makes it less likely to contribute to antibiotic resistance, especially when compared to the other tetracycline-class antibiotics.18,20 A study by Zhanel et al10 in 2019 that measured the activity of sarecycline and other tetracycline-class antibiotics against both gram-positive and gram-negative bacteria. Where there was not much of a difference with gram-positive bacteria compared to the other tetracycline-class drugs, sarecycline had little to no activity against gram-negative bacteria commonly found within the gut microbiome. Based on these findings, sarecycline was shown to be 4-to 8-fold less active than doxycycline against the bacteria that compose the normal intestinal microbiome.10
Proposed sarecycline mechanism of action for POD. There are a few proposed theories explaining why sarecycline may be efficacious in treating POD. Being in the tetracycline class of antibiotics, sarecycline is shown to have anti-inflammatory properties. Although the mechanism of these properties is not entirely understood, it has demonstrated efficacy in treating inflammatory lesions of acne and rosacea. This may suggest that POD is a subset of acne or rosacea, as the inflammatory skin conditions share similar morphologies and respond to similar treatments. The mechanism of action of the inflammatory skin conditions with the treatment of antibiotics should be further explored.
Conclusion
The observations gleaned from this case series highlight the many potential benefits to treatment with sarecycline over the alternative tetracycline-class antibiotics. There is an absolute need for more large-scale clinical studies evaluating treatment options for POD, with special attention to the impact on antibiotic resistance and its implications on public health. Based on the efficacy and tolerability of sarecycline in large-scale acne studies, sarecycline may be an appropriate novel treatment option for POD and should be explored further with larger randomized, controlled studies.
References
- Tempark T, Shwayder TA. Perioral Dermatitis: A Review of the Condition with Special Attention to Treatment Options. Am J Clin Dermatol. 2014 Apr;15(2):101–113.
- Lipozencic J, Ljubojevic S. Perioral dermatitis. Clinics in Dermatology. 2011;29(2):157–161.
- Tolaymat L, Hall MR. Perioral dermatitis. StatPearls [Internet]. 2021.
- Veien NK, Munkvad JM, Nielsen AO, et al. Topical metronidazole in the treatment of perioral dermatitis. J Am Acad Dermatol.1991 Feb;24(2 Pt 1):258–260.
- Jansen T. Azelaic acid as a new treatment for perioral dermatitis: Results from an open study. Br J Dermatol. 2004 Oct;151(4):933–934.
- Goldman, D. Tacrolimus ointment for the treatment of steroid-induced rosacea: A preliminary report. J Am Acad Dermatol. 2001 Jun;44(6):995–998.
- Chu CY. The use of 1% pimecrolimus cream for the treatment of steroid‐induced rosacea. Br J Dermatol. 2005 Feb;152(2):396–399.
- Reichenberg J, Dahl MV, and Ofori AO. Perioral (periorificial) dermatitis. UpToDate. 2017; Topic,13622.
- Graber EM. Treating acne with the tetracycline class of antibiotics: A review. Dermatological Reviews. 2021,1-10.
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018 Dec 21;63(1):e01297-18.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the scientific panel on antibiotic use in dermatology of the American Acne and Rosacea Society: art 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016 Apr; 9(4): 18–24.
- Deeks ED. Sarecycline: First global approval. Drugs. 2019; 79(3): 325–329.
- Armstrong AW, Hekmatjah J, and Kircik LH. Oral Tetracyclines and Acne: A Systematic Review for Dermatologists. J Drugs Dermatol. 2020 Nov 1;19(11):s6–s13.
- Stein-Gold L, Moore A Tanaka SK, et al. Reduced Bloodbrain Barrier Penetration of Sarecycline Relative to Minocycline in Rats Corresponds with Lipophilicity and Low Vestibular Side Effects. SKIN The Journal of Cutaneous Medicine. 2020: 4(6), s107–s107.
- Moore AY, Del Rosso JQ, Johnson JL, et al. Sarecycline: A review of preclinical and clinical evidence. Clin Cosmet Investig Dermatol. v.13; 2020.
- Almirall Pharmaceuticals International Limited. Seysara (Sarecycline) tablets for oral use: US prescribing information. 2018. https://www.accessdata.fda.gov/.
- Batool Z, Lomakin IB, Polikanov YS, et al. Sarecycline interferes with tRNA accommodation and tethers mRNA to the 70S ribosome. Proceedings of the National Academy of Sciences, 2020: 117(34), 20530-20537.
- Rusu A and Buta EL. The Development of Third-Generation Tetracycline Antibiotics and New Perspectives. Pharmaceutics. 2021 Dec 5;13(12):2085.
- Pariser DM, Green LJ, Lain EL, et al. Safety and tolerability of sarecycline for the treatment of acne vulgaris: results from a Phase III, multicenter, open-label study and a Phase I phototoxicity study. J Clin Aesthet Dermatol. 2019 Nov; 12(11):E53–E62.
- Haidari W, Bruinsma R, Cardenas-de la Garza, et al. Sarecycline review. Annals of Pharmacotherapy. 2020. 54(2):164–170.
- Bunick CG, Keri, J Tanaka SK, et al. Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation. Antibiotics (Basel). 2021 Apr 15;10(4):439.
- Sapadin AN and Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006 Feb;54(2):258–265.
- Satyawan I, Oranje AP and Van Joost TH. Perioral dermatitis in a child due to rosin in chewing gum. Contact Dermatitis. 1990 Mar;22(3):182–183.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in Oral Antibiotic Prescription in Dermatology, 2008 to 2016. JAMA Dermatol. 2019 Mar; 155(3):290–297.
- Graber E and Kay CR. Successful Treatment of Periorificial Dermatitis with Novel Narrow Spectrum Sarecycline. J Drugs Dermatol. 2021 Jan 1;20(1):98–100.
- Centers for Disease Control and Prevention, Office of Infectious Disease. Antibiotic resistance threats in the United States, 2013. April 2013. Available at: http://www.cdc.gov/drugresistance/ threat-report-2013.
- Ventola CL. The antibiotic resistance crisis: Part 1: causes and threats. Pharmacy and Therapeutics. 2015 Apr;40(4):277–283.
- Hobson C, Chan AN and Wright GD. The antibiotic resistome: A guide for the discovery of natural products as antimicrobial agents. Chem Rev. 2021 Mar 24;121(6):3464–3494.