J Clin Aesthet Dermatol. 2020;13(1):22–27
 by Juliano Borges, PhD; Luciana Araújo, MD; Tullia Cuzzi, PhD; Luis Martinez, MD; Yliana Gonzales, MD; and Mônica Manela-Azulay, PhD
by Juliano Borges, PhD; Luciana Araújo, MD; Tullia Cuzzi, PhD; Luis Martinez, MD; Yliana Gonzales, MD; and Mônica Manela-Azulay, PhD
Drs. Borges, Cuzzi, and Manela-Azulay are with Universidade Federal do Rio de Janeiro, UFRJ, in Rio de Janeiro-RJ, Brazil.Dr. Araújo is with Universidade Federal do Estado do Rio de Janeiro, UNIRIO, Brazil. Drs. Martinez and Gonzales are with Santa Casa de Misericórdia do Rio de Janeiro, Brazil.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Laser resurfacing is a common treatment for photoaging. This treatment creates skin incisions that initiate the wound healing cascade, including reorganization of the collagen matrix, in a dermal remodeling process that can last up to 12 months.
Objective. We compared the effects of nonablative and ablative Erbium fractional laser resurfacing on dermal content and arrangement of Collagen Types I and III, and on fibroblast activation.
Methods. Ten female patients (50–63 years) with Fitzpatrick Skin Types I–IV and clinical signs of photoaging underwent two types of Erbium fractional laser resurfacing (nonablative, 1540nm; ablative, 2940nm) on opposite sides of the face. Skin biopsies were obtained pretreatment and three months post-treatment. Morphometric analysis was performed using Picrosirius staining for overall collagen, and immunohistochemistry for Collagen Types I and III. Finally, hematoxylin and eosin staining was used to identify fibroblast activation.
Results. Both laser treatments induced reorganization of Collagen Types I and III and demonstrated signs of fibroblast activation. However, morphometric analysis of Picrosirius staining revealed that, after both treatments, there was a lower density of collagen fibers, which is characteristic of edema.
Conclusion. At three months after laser resurfacing, skin lifting in photoaged skin likely resulted from new collagen deposition but also from edema.
KEYWORDS: Erbium fractional laser resurfacing, ablative, nonablative, solar elastosis, Collagen Type I, Collagen Type III, photoaging, aging skin, rejuvenation
Skin aging can be subdivided into two groups: intrinsic and extrinsic. The latter group is characterized by skin changes associated with exposure to the outside world. Ultraviolet (UV) radiation is the most common factor underlying extrinsic aging, contributing to 80 percent of reported cases.1
Radiation modifies the dermis of the skin. Such modifications can be histologically observed using hematoxylin and eosin (H&E) staining and include collagen basophilic degeneration or solar elastosis. These changes result from an imbalance between production and degradation of the main proteins produced by fibroblasts.2 Among these proteins, the most prominent are Collagen Types I and III, which are responsible for 80 to 85 percent and 10 to 15 percent of total skin collagen, respectively.3
Clinically, photoaging, or the process by which UV radiation modifies the skin, is characterized by skin roughness, loss of elasticity, fine wrinkles, dyschromia, and melanosis.4 Dermatologists treat photoaging with a wide range of therapies, such as peelings, lasers, and biostimulators. However, the mechanisms underlying the effects of these treatments on solar elastosis are still being characterized.
The present study focused on the effects of fractional nonablative (1540nm) and ablative (2940nm) Erbium laser therapy on photoaging. These types of laser skin resurfacing create a skin incision that initiates the wound healing cascade, inducing a dermal remodeling process involving collagen matrix reorganization that can take up to 12 months.
Primarily, we investigated how this therapy treated solar elastosis and affected dermal collagen fiber organization and fibroblast activation. This analysis included morphometry of Picrosirius staining, immunohistochemical analysis of Collagen Types I and III, and H&E staining for fibroblast morphologic analysis using tissue sections collected three months after the interventions. The main objectives of this study were to histologically characterize how this procedure lifts the skin and determine the role of new collagen fiber deposition in this process.
Materials and Methods
Patient sample. A total of 10 female patients (age range 50–63 years) were recruited from the Dermatologic Service of Clementino Fraga Filho University Hospital, based on the following eligibility criteria: 1) Fitzpatrick Skin Types I–IV, 2) Glogau Classification II or III, and 3) clinical signs of photoaging (i.e., hyperpigmentation, wrinkles, altered skin texture, and sagging). Patients were excluded if they had undergone hormonal therapy or received topical or oral treatment for photoaging in the previous six months.
Pretreatment. Punch biopsies (3mm) of the skin were obtained from 10 patients (randomly selected), collecting bilateral preauricular samples at baseline (pretreatment) and three months after laser therapy. Skin tissue was fixed in formalin (10%), processed routinely, embedded in paraffin, and sectioned.
Ethics. Informed consent was received by all patients. The study protocol conformed to the guidelines outlined by the 1975 Declaration of Helsinki and was approved by the University Hospital Clementino Fraga Filho/Universidade Federal do Rio de Janeiro Ethics Committee.
Laser resurfacing. Each patient underwent both nonablative (1540nm) and ablative (2940nm) Erbium fractional laser resurfacing on opposite sides of the face. For each patient, the treatments were randomly applied by a single physician evaluator; however, all therapeutic parameters (e.g., fluency, pulse duration, number of passes, lateral and column overlap) were the same for each patient, using recommended treatment parameters stipulated by the literature as sufficient for clinical and histological improvement.5 Patients underwent a total of three resurfacing sessions, based also on stipulations in the literature that state the need for multiple nonablative (vs. ablative) sessions in fractional laser treatment of photoaging (5,6). Thus, the study began with the nonablative 1540nm laser resurfacing, reserving 2940nm resurfacing on the opposite side of the face for the third session (Table 1). Patients were instructed to refrain from using topical medications, apart from sunscreens, for the duration of the study (sun protection factor 30).

Collagen density morphometry. Morphometric analysis was used to quantify the density of collagen fibers before and after treatment. For this analysis, digital images were obtained from Picrosirius red-stained sections (JPEG format, 36-bit color, 1280 x 1024 pixels) using an LC Evolution camera (Media Cybernetics, Silver Spring, Maryland) and an Olympus BX51 microscope (Olympus Corp., Center Valley, Pennsylvania). The images were analyzed using Image Pro Plus software, Version 1.7 (Media Cybernetics, Silver Spring, Maryland) and grouped by shade to measure the collagen content. The histogram resulting from this grouping was assessed, and the results of the selected pixels were expressed as a percentage of the total image area.7,8 The amounts of collagen before and after treatment were compared using a Student’s t-test, with p<0.05 considered significant.
Immunohistochemical staining. Immunohistochemistry was performed for Collagen Types I and III using a monoclonal mouse anti-Collagen I antibody (Clone: COL-1, Dilution 1:50, ABICAN, Cod: ab90395) and a polyclonal rabbit anti-Collagen III antibody (Dilution 1:250, ABICAN, Cod: ab7778), respectively. Primary antibody labeling was visualized with the EnVision™ kit (Agilent, Santa Clara, California; FLEX cód: K8000 HRP, Rabbit/Mouse, low pH).
Observers compared Collagen Types I and III staining before and after the two treatments to identify the presence of new collagen fibers and characterize their organization.?
Cell analysis. H&E staining was used to characterize the appearance of dermal fibroblasts after the treatments to evaluate morphological changes and possible signs of fibroblast activation.?
Results
Morphometric studies. Morphometric analysis of Picrosirius red staining revealed that both laser treatments significantly lowered the density of collagen fibers relative to pretreatment levels.
The mean collagen densities before and three months after treatment using 1540nm were 69.56 and 68.64, respectively. The difference between the means was 0.92, which was considered statistically significant (p=0.016, Student’s t-test) (Figure 1).

The mean collagen densities before and three months after treatment using 2940nm were 71.25 and 51.63, respectively. The difference between the means was 23.58, which was considered statistically significant (p=0.0002, Student’s t-test) (Figure 1).
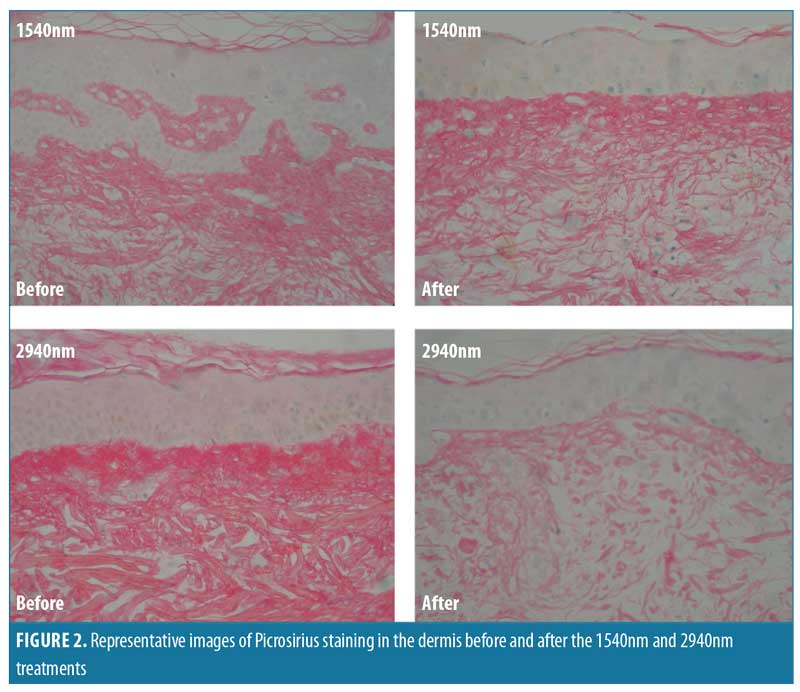
After both treatments, Picrosirius red staining was less homogenous and there was greater space between the fibers, indicative of edema. However, collagen density changes were more intense for the 2940nm treatment. These results correspond with our collagen morphometric analysis (Figure 2).
Immunohistochemistry. After both treatments (1540nm and 2940nm), immunohistochemistry revealed that Collagen Type I fibers (top row, Figures 3 and 4), stained in brown, had lost their homogeneity and assumed a fragmented, lumpy appearance and horizontal orientation in the subepithelial zone. New Collagen Type III fibers (bottom row, Figures 3 and 4), stained in brown, in the subepithelial area was horizontally oriented and regularly organized after both treatments.

Cell analysis. H&E staining for fibroblast activation revealed cells with fusiform and elongated nuclei that contrasted with cell morphology in pretreatment tissue sections (Figure 5). Both treatments induced the appearance of cells with fusiform and elongated nuclei contrary to morphology in pretreatment sections.

Discussion
The cutaneous architecture and physiological properties can be attributed to the extracellular matrix (ECM), which consists of many components, including collagen fibers. Dermal collagen is synthesized by fibroblasts such that the skin normally contains 80 to 85-percent Collagen Type I and 10 to 15 percent Type III. Reductions in Collagen Types I and III are seen in chronologically aged skin and can be exacerbated by photoaging.3
The main factor underlying extrinsic aging is exposure to UV radiation. Radiation activates an intricate cellular mechanism that leads to the clinical manifestations of photoaging,9,10 such as roughness, uneven pigmentation, telangiectasia, deep wrinkles, and neoplasia.1,11–13 This mechanism involves a phosphorylation cascade that stimulates the production of matrix metalloproteinases (MMPs), a class of enzymes that breakdown ECM components.14 The main MMPs involved in photoaging are MMPs 1, 3, and 9.15 MMP-1 initiates the cleavage of the central triple helix structure of Collagen Types I and III, while MMPs 3 and 9 induce subsequent cleavages. Together, activation of these enzymes lowers dermal integrity if the cleaved collagen is not replaced by new and properly organized fibers.16 Indeed, radiation-induced elastosis in the dermo–epidermal junction is characterized by the presence of disorganized and hyalinized collagen fibers.
Currently, many treatments for the symptoms of photoaging, such as sagging and wrinkles, are designed to stimulate collagenogenesis. Some methods, such as intense pulsed light,17–19 promote a regenerative response to attain characteristics similar to those of healthy skin. Other treatments, such as fractional ablative laser resurfacing, induce a fibrotic scarring response that stimulates collagenogenesis by initiating wound healing.20–23 This controlled wound healing response underlies the benefits of such treatment, although overexposure can have secondary and detrimental effects, including hypertrophic scars and hyperchromia. The wound healing process comprises three distinct phases: inflammatory, proliferative, and remodeling. The inflammatory phase lasts up to four days after wounding and is characterized by hemostasis, while the proliferative phase can last up to 20 days and is characterized by the migration and proliferation of cells, including myofibroblasts. These cells are responsible for wound contractility and Collagen Type III production.24 Finally, the remodeling phase can persist for over a year and is characterized by collagen deposition and the transition of the initially deposited Collagen Type III to mature Collagen Type I.25,26 To date, the lifting effect associated with these treatment methods is thought to result from myofibroblast activation and new collagen deposition.
Previously, Orringer et al18 studied the molecular mechanisms of 1,550nm Erbium nonablative fractional lasers (NAFL) on 20 patients with cutaneous photoaging. Weekly biopsies were performed for a one-month period. At 24 hours after treatment, NAFL therapy decreased the expression of Collagen Types I and III, but these levels progressively increased over the next two weeks. Notably, the degree of increased collagen deposition was proportional to the energy of the laser used. However, higher laser intensity can also be detrimental. Fitzpatrick et al27 compared the effects of two types of ablative fractional lasers (AFL), CO2 and Erbium:yttrium-aluminum-garnet (Er:YAG) lasers. Ultrasonographic measurements and skin biopsies were performed monthly for six months after which, the authors concluded that skin improvements resulted from stretching of the collagen matrix caused by wound contraction. Importantly, in this study, the more intense the laser ablation, the greater the risk of scarring. Indeed, 41.43 percent of patients developed permanent scars, which were treated with blepharoplasty. In another study, El-Domyati et al28 objectively compared the ablative and the fractional modalities of Er:YAG laser using histology and immunohistochemistry. With serial biopsies, they demonstrated that, after four sessions, both treatment modalities induced quantitative increases in Collagen Types I, III, and VII. Thus, both modalities appear to affect collagen deposition. In another study, the same authors described similar findings in 10 patients after five sessions with Er:YAG AFL therapy.29 This finding coincides with a study by Laubach et al,30 who reported that AFL initially produced epidermal microinjuries and damaged dermal collagen that is subsequently replaced by Collagen Type III.
Data from our current study agrees with the literature in that we showed rearrangement of Collagen Types I and III after laser therapy, which suggests collagenogenesis. Contrary to previous reports, however, in our study, the deposited collagen fibers did not obviously establish a greater density after therapy. Thus, it is not clear whether increased collagen deposition was responsible for the increased skin firmness reported by patients. Alternatively, the space between the fibers in the papillary dermis demonstrated by Picrosirius red staining indicated late edema. Furthermore, our morphometric analysis showed a decreased density of collagen fibers after both laser treatments, of which the fractional ablative Erbium therapy was more intense. Together, these data indicate that edema was a relevant factor underlying the increased skin firmness reported three months after treatment. Interestingly, during normal wound healing, edema can be histologically observed acutely but completely resolves within seven days. It is also concentrated mainly in the basal layer rather than the papillary dermis, as seen in our current study.18,30,31
We also evaluated the effect of laser resurfacing on fibroblasts. Generally, photoaging alters collagen fibers, elastic fibers, and proteoglycans, modulating the tensile strength of fibroblasts and limiting their functionality. Laser resurfacing, on the other hand, is thought to enhance fibroblasts activity. Indeed, as fractional lasers incise the skin, they generate necrosis columns34 that remove damaged ECM material, allowing the resettlement of fibroblasts. Such resettled fibroblasts, under the influence of new tensile strength, resume production of ECM components, such as collagen, elastin, and proteoglycans. Our analysis corroborated this idea, as both laser treatments activated fibroblasts. Fibroblast activation can also affect dermal hydration levels, which directly correlate with radiation-induced collagen degeneration.35–39 Increased dermal remodeling likely underlies the edema we observed in the current study.
Limitations. Other factors are described in the literature as potential explanations for the lifting effect of laser resurfacing. These factors include stretching resulting from dermal heating,32 which involves the dissociation of intermolecular peptide bonds within the collagen triple helix, thereby increasing skin tension. Another potential factor is neoelastogenesis, or the reconstitution of oxitalanic and elauninic fibers that are destroyed by UV radiation. We did not evaluate the contributions of these factors in the current study, which limits our conclusion; however, the presence of edema in the tissues collected three months after therapy was clear. One key question remains unanswered: After six months when edema subsides, will the collagen fibers continue to accumulate and organize in a way that sustains the lifting effect observed in this study? Wound healing studies report that dermal remodeling can take up to 12 months, so future studies should include longer time points that span the entire remodeling process. Other limitations of this study include the small sample size and lack of investigation into the composition of the edema observed. Future studies should determine if this edema is a sign of fibroblast activation and potentially incorporate an Alcian blue stain to determine if it is composed of glycosaminoglycans such as hyaluronic acid.
Conclusion
Both ablative and nonablative Erbium fractional laser resurfacing stimulate collagen reorganization and fibroblast activation in photoaged skin. However, the benefits of these treatments might also result from edema. Future studies should continue to histologically characterize the effects of laser resurfacing on the skin to optimize this procedure as a treatment for photoaging.
References
- Azulay MM, Lacerda CAM, Perez MA, et al. Vitamina C [Vitamin C]. An Bras Dermatol. 2003;78(3):265–272.
- Mauch C, Hatamochi A, Scharffetter K, Krieg T. Regulation of collagen synthesis in fibroblasts within a three-dimensional collagen gel. Exp Cell Res. 1988;178(2):493–503.
- El-Domyati M, Attia S, Saleh F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11(5):
398–405. - Uitto J, Bernstein EF. Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J Invest Dermatol Symp Proc. 1998;3:41–44.
- Borges J, Cuzzi T, Mandarim-de-Lacerda CA, Manela-Azulay M. Fractional Erbium laser in the treatment of photoaging: randomized comparative, clinical and histopathological study of ablative (2940nm) vs. nonablative (1540nm) methods after 3 months. An Bras Dermatol. 2014;89(2):250–258.
- Ribé A, Ribé N. J Neck skin rejuvenation: histological and clinical changes after combined therapy with a fractional nonablative laser and stabilized hyaluronic acid-based gel of non-animal origin. J Cosmet Laser Ther. 2011;13:154–161.
- Chan NP, Ho SG, Yeung CK, et al. The use of nonablative fractional resurfacing in Asian acne scar patients. Lasers Surg Med. 2010;42:710–715.
- Mandarim-de-Lacerda CA, Fernandes-Santos C, Aguila MB. Image analysis and quantitative morphology. Methods Mol Biol. 2010;611:
211–225. - Manela-Azulay M, Cuzzi T, Pinheiro J, et al. Objective methods for analyzing outcomes in research studies on cosmetic dermatology. An Bras Dermatol. 2010;85:65-71.
- Kraft DC, Deacaris CC, Rattan SI. Proteasomal oscillation during mild heat shock in aging human skin fibroblasts. Ann N Y Acad Sci. 2006;1067:
224–247. - Bravo BF, Azulay DR, Luiz RR, et al. Oral isotretinoin in photoaging: objective histological evidence of efficacy and durability. An Bras Dermatol. 2015;90(4):479–486.
- Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989;21:510–513.
- Oikarinen A. The aging of skin: chronoaging versus photoaging. Photodermatol Photoimmunol Photomed. 1990;7(1):3–4.
- Chung JH, Seo JY, Choi HR, et al. Modulation of skin collagen metabolism in aged and photoaged human skin in vivo. J Invest Dermatol. 2001;117(5):1218–1224.
- Kraft DC, Deacaris CC, Rattan SI. Proteasomal oscillation during mild heat shock in aging human skin fibroblasts. Ann N Y Acad Sci. 2006;1067:
224–247. - Almeida Issa MC, Piñeiro-Maceira J, et al. Immunohistochemical expression of matrix metalloproteinases in photodamaged skin by photodynamic therapy. Br J Dermatol. 2009;161(3):647–653.
- Uitto J, Fazio MJ, Olsen DR. Molecular mechanisms of cutaneous aging: age associated connective tissue alterations in the dermis. J Am Acad Dermatol. 1989;21:614–622.
- Erol OO, Gurlek A, Agaoglu G, et al. Treatment of hypertrophic scars and keloids using intense pulsed light (IPL). Aesthetic Plast Surg. 2008;32(6):902–909.
- Orringer JS, Rittié L, Baker D, et al. Molecular mechanisms of nonablative fractionated laser resurfacing. Br J Dermatol. 2010;163(4):757–768.
- Haedersdal M, Moreau KE, Beyer DM, et al. Fractional nonablative 1540nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41(3):189–195
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36(3):299–306.
- Varani J, Spearman D, Perone P, et al. Inhibition of type I procollagen synthesis by damaged collagen in photo-aged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001;158(3):
931–942. - Hedelund L, Haak CS, Togsverd-Bo K, et al. Fractional CO2 laser resurfacing for atrophic acne scars: a randomized controlled trial with blinded response evaluation. Lasers Surg Med. 2012;44(6):447–452.
- Costa FB, El Ammar ABPC, Campos VB, Kalil CLPV. Complicações com o uso de lasers. Parte II: laser ablativo fracionado e não fracionado e laser não ablativo fracionado. Surg Cosmet Dermatol. 2011;3(2):135–146.
- Shaw TJ, Martin P: Wound repair at a glance. J Cell Sci. 2009;122:3209–3213.
- Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg 2006;117(7 Suppl):12S–34S.
- Broughton G, 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S–32e-S.
- Fitzpatrick RE, Rostan EF, Marchell N. Collagen tightening induced by carbon dioxide laser versus erbium: YAG laser. Lasers Surg Med. 2000;27(5):395–403.
- El-Domyati M, Abd-El-Raheem T, Abdel-Wahab H, et al. Fractional versus ablative erbium:yttrium-aluminum-garnet laser resurfacing for facial rejuvenation: an objective evaluation. J Am Acad Dermatol. 2013;68(1):103–112.
- El-Domyati M, Abd-El-Raheem T, Medhat W, et al. Multiple fractional erbium: yttrium-aluminum-garnet laser sessions for upper facial rejuvenation: clinical and histological implications and expectations. J Cosmet Dermatol. 2014;13(1):
30–37. - Laubach HJ, Tannous Z, Anderson RR, Manstein D. Skin responses to fractional photothermolysis. Lasers Surg Med. 2006;38(2):142–149.
- Prignano F, Bonciani D, Campolmi P, et al. A study of fractional CO2 laser resurfacing: the best fluences through a clinical, histological, and ultrastructural evaluation. J Cosmet Dermatol. 2011;10:210–216.
- Lipozencic J, Mokos ZB. Will nonablative rejuvenation replace ablative lasers? Facts and controversies. Clin Dermatol. 2013;31(6):718–724.
- Uitto J. Understanding of cutaneous aging. New Engl J Med. 1997;337:1463–1465.
- Campos V, Mattos R, Fillippo A, Torezan LA. Laser no rejuvenescimento facial [Laser in facial rejuvenation]. Surg Cosme Dermatol. 2009;1(1):29–36.
- Varani J, Warner RL, Gharaee-Kermani M, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinase and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486.
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470.
- Röck K, Grandoch M, Majora M, et al. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB). J Biol Chem. 2011;286:18268-76.
- Oh JH, Kim YK, Jung JY, et al. Intrinsic aging and photoaging dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J Dermatol Sci. 2011;62:192–201.
- Borges J, Manela-Azulay M, Cuzzi T. Photoaging and the clinical utility of fractional laser. Clin Cosmetic Invest Dermatol. 2016;9:107–114.
