J Clin Aesthet Dermatol. 2025;18(10):73–77.
by Margaryta Stoieva, MD, and Daniel Mettman, MD
Dr. Stoieva and Dr. Mettman are with the University of Kansas School of Medicine in Kansas City, Kansas.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors declare no conflicts of interest relevant to the content of this article.
Abstract: Objective: This study aims to assess the regression rate of basal cell carcinoma (BCC) in excision specimens following diagnostic biopsies obtained from patients in a veterans affairs facility. Methods: A retrospective review of biopsy-proven BCC excision cases was conducted. All included biopsy and corresponding excision reports were reviewed to determine the margin status of specimens. Proportion of residual carcinoma cases and regression rate of the tumor were determined. Results: Regression rate of BCC was estimated to be 47%. The most prevalent subtype was nodular BCC (46%), and the most prevalent location was the head. Tumors located on the trunk and upper extremities were more likely to regress, and those on the head were less likely to regress (p<0.05). Punch biopsy technique was associated with significantly lower regression rate (p<0.01). The time interval between the biopsy and excision and age were not significantly different between regressed and non-regressed tumors. Neither of sexes and neither of tumor subtypes had significantly different regression rates as compared to the general study population. Limitations: The limitation of our study is that the data may not be generalizable due to our unique study population. Conclusion: BCC is a tumor with the potential to regress. However, definitive management resides in complete surgical excision, which will most likely remain the mainstay of treatment in the near future. Keywords: Basal cell carcinoma, veteran, regression, biopsy, excision
Introduction
Basal cell carcinoma (BCC) is the most frequent cancer in adults with lighter skin tone.1 BCCs are slow growing, locally aggressive, malignant, epidermal skin tumors.2 The two major implications of BCCs for healthcare systems are their potential for multiple tumors occurrence in the same patient3 and their unpredictable clinical course,4 which precludes conservative management and makes surgical excision a first-line approach in management.5 A peripheral margin of 4 to 5mm is usually recommended for excision, a cutoff dictated by a tendency of asymmetrical subclinical extension beyond the clinically visible tumor.6 Surgical excision appears to be an effective treatment for BCC, with the five-year cure rate reported of 95.2% in one study.7 However, no suspected lesion should be treated without histopathologic confirmation,8 despite a 60 to 70% accuracy rate for clinical diagnosis.9 Two most widely employed techniques are the shave biopsy and the punch biopsy, both of which have shown comparable diagnostic accuracy.8 Multiple studies have examined the incidence of residual BCC following a diagnostic biopsy procedure with recurrence rates ranging from 19 to 85%.10–19 The two most recent studies, performed in 2023 and 2024, reported 53.8% and 39.4% residual tumor occurrence rate, respectively.14,17 Factors associated with residual tumor on excision have also been studied extensively.20–25 Among those, large tumor diameter, increased patient age, failure to achieve initial negative margin resection,23 and infiltrative and micronodular histopathological subtypes24 are considered the most significant.
Biopsy-induced tumor regression, an interesting phenomenon described by Swetter et al,26 was noted to be associated with the biopsy technique. However, despite an observable curative rate with diagnostic biopsy, the consensus is still to excise the histopathologically proven tumors. The major concern is that the traditional approach to excision specimen sectioning, involving transverse cross-sectioning (bread-loafing) at 4-mm intervals, has a low sensitivity (44%) in detecting residual tumor at the surgical margin.27 All this information needs to be incorporated carefully in the clinician’s decision. An enormous amount of evidence collected over more than five decades supported by an inflow of newer studies further strengthens the support of surgical management as the most effective treatment modality for BCC at this point.
To the authors’ knowledge, this is the largest study in the past ten years to assess the regression rate of BCC following diagnostic biopsy in excision specimens from the veteran patient population.
Methods
The retrospective review of biopsy-proven BCC excision cases was conducted at a large Veterans Affairs Medical Center. The management for all included cases consisted of the primary diagnostic biopsy (shave or punch technique) followed by the surgical excision. Excisional biopsies intended as a one-step complete tumor were not included in the study. Tumors referred for Mohs micrographic surgery were also not included. A total of 317 cases, in 196 patients seen from 2001-2024, were analyzed. The study population consisted of 309 male patients (97.5%) and eight female patients (2.5%). The median age at the diagnosis was 72 years (range: 35-94 years). The median time interval between the primary biopsy and surgical excision was 43 days (range: 2-812 days).
For statistical analysis, chi-square test, Fisher exact test, and t test were used. Chi-square and Fisher exact tests were used in intergroup comparisons of the count data for categorical variables (sex, biopsy technique, anatomic location, and tumor subtype) expressed as numbers, and percentages. In comparisons between residual tumor incidence, as for age and time interval between biopsy and excision, independent two sample t test was used. P-values lower than 0.05 were considered as statistically significant. The calculations were performed using GraphPad QuickCalcs website, MedCalc website, and Social Science Statistics website.28–30
Results
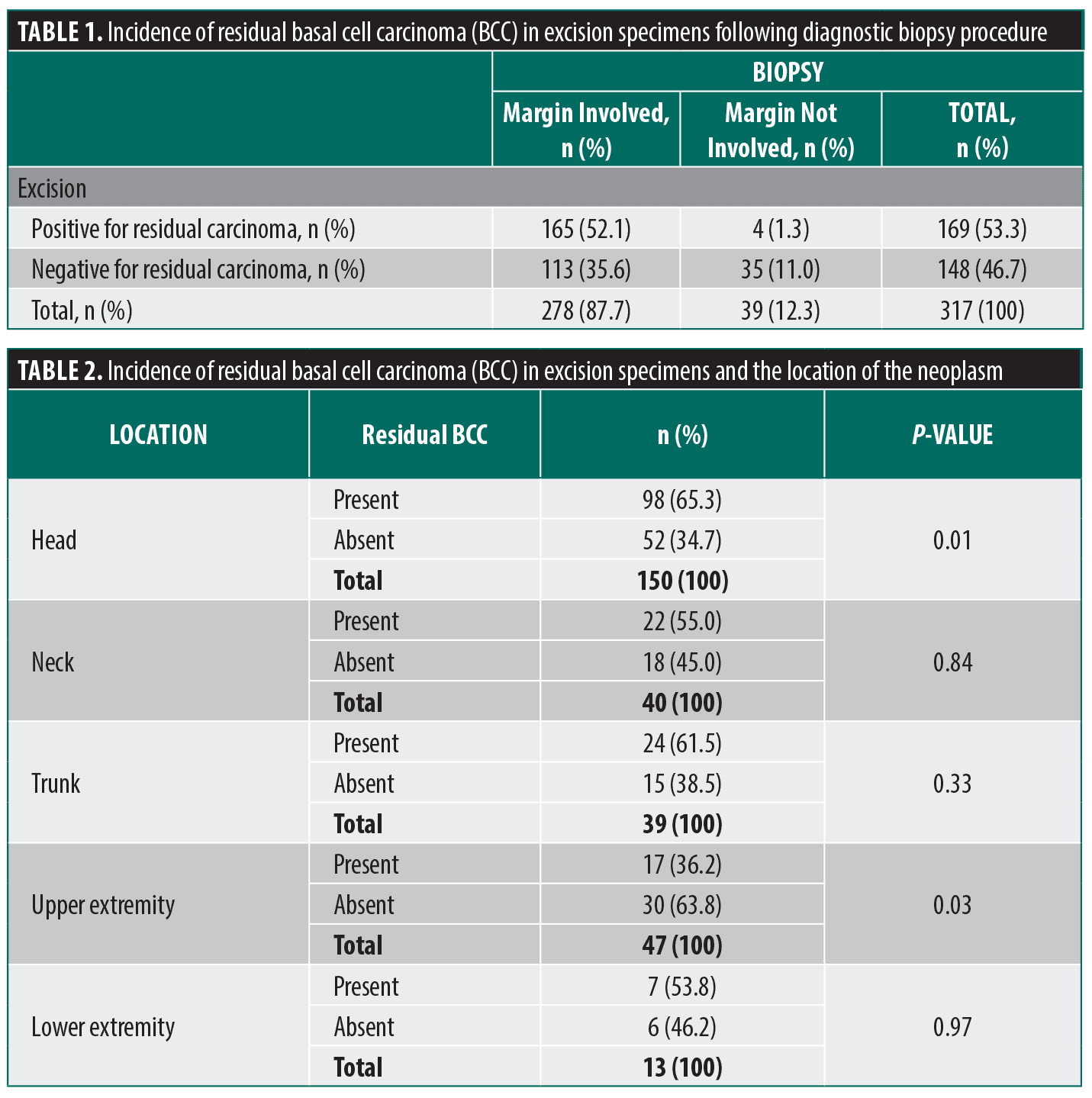
Among all excisions performed on biopsy-proven transected BCCs from August 2001 to July 2024, 317 cases were analyzed. Of those, 148 (46.7%) showed scar with no residual tumor in the excision specimens, and 169 (53.3%) had histologic evidence of residual BCC with accompanying biopsy site changes. Of the 148 BCCs with complete regression in the excision specimens, 113 (76.4%) cases had negative margin on biopsy, and 35 (23.6%) had at least one involved margin. Further, of the 169 BCCs with residual tumor present in the excision specimens, 165 (97.6%) had at least one involved margin, whereas 4 (2.4%) had negative margin on biopsy (Table 1).
The mean patient ages for regressed and nonregressed BCCs were 72 years (range: 48-92 years) and 72 years (range: 35-94 years), respectively.
A majority of BCCs occurred on the head area (n=150; 47.3%), with highest number of tumors arising on the cheek (n=42, 28%), followed by the forehead (n=29, 19.3%), the temple (n=26, 17.3%), the nose (n=13, 8.7%), the chin and ear (each, n=10, 6.7%), the nasolabial fold (n=7, 4.7%), the nasal ala and vertex scalp (each, n=6, 4.0%), and the cutaneous lip (n=1, 0.7%). The second most frequent location was the trunk (n=67, 21.1%). Of those, 39 cases occurred on the back (58.2%) and 28 cases occurred on the chest (41.8%). Other locations included the upper extremity (n=47, 14.8%) and neck (n=40, 12.6%). The least common location was the lower extremity (n=13, 4.1%). Regression rate was the highest among the tumors arising on the upper extremity (63.8%). This site showed significantly less residual tumors in excision specimens (Table 2).
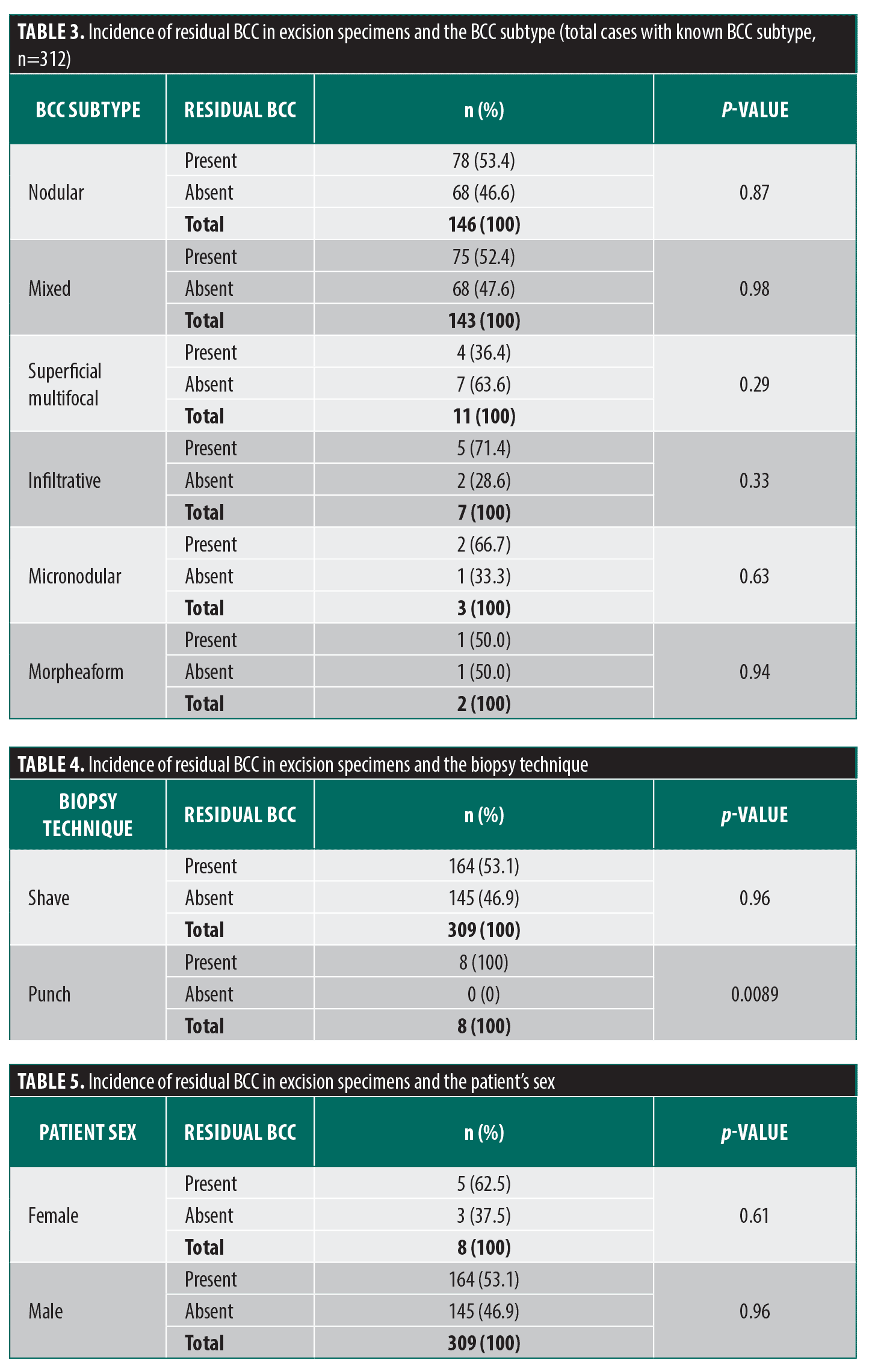
Subtypes of BCC diagnosed on the primary biopsy were as follows: nodular (n=146, 46%); mixed (n=143, 45%); superficial (n=11, 3%); micronodular (n=3, <1%); and morpheaform (n=2, <1%). The subtype was not specified in five cases (1%). As demonstrated, the mixed subtype constituted a significant proportion of cases. Nonetheless, a nodular component was identified in 116 of mixed subtype cases (81.1%). Superficial multifocal and morpheaform (sclerosing) subtypes demonstrated the highest regression rate of all subtypes (63.6% and 50.0%, respectively), followed by mixed and nodular subtypes (47.6% and 46.6%, respectively). However, no statistically significant difference in regression rate was noted between the aforementioned types and the total study population (Table 3).
Time from biopsy to surgical excision ranged from 2 to 812 days (mean: 60 days) for nonregressed tumors and from 2 to 329 days (mean: 49 days) for regressed tumors. There was no statistically significant difference between time intervals at this study power.
Two types of biopsy technique were utilized in examined cases: shave and punch biopsy. Shave technique was used in 309 (97.5%) cases, and punch technique was used in 8 (2.5%) cases. All excisions following punch biopsy showed residual tumor (0% regression rate), which was significantly different from the total study tumor population regression rate (p<0.05) (Table 4).
Regression rates of BCC among men or women (46.9% and 37.5%, respectively) did not differ significantly from the total study population tumor regression rate (Table 5).
Discussion
Spontaneous tumor regression is a rare phenomenon observed in many types of tumors, one of them being BCC. In a study by Curson and Weedon,31 20% of 400 tumors showed at least some evidence of regression characterized predominantly by disruption of the palisaded outline of some tumor islands with penetration by a lymphocytic infiltrate and the presence of apoptotic tumor cells. Immune system activation appears to correlate with successful eradication of BCC, and it is the basic mechanism of topical agents, such as imiquimod, used to treat a subset of BCC.32
Tumor microenvironment appears to be different in tumors with tendency toward regression. In contrast to nonregressing tumors, regressing tumors exhibit markedly increased numbers of CD31 and CD4-positive T cells, as well as dendritic cells.33 Inflammation may play a dual role in tumor development and progression. Chronic inflammation caused by persistent infections and metabolic disorders is thought to contribute to increased cancer risk and accelerated cancer progression. Oppositely, acute inflammation induced by bacteria-based vaccines or that is occurring after cancer selectively inhibits cancer progression and metastasis.34 Stress introduced by disruption of the skin surface during biopsy can be an inciting event of initiation of anti-tumoral response. As demonstrated by Swetter et al,26 the biopsy technique itself has impact on the rate of tumor regression. The authors speculated that some event in the interval between biopsy and excision may lead to the eradication of residual tumor. Our study demonstrated the same results with regards to biopsy technique. Although there were only eight punch biopsy cases, all had residual carcinoma in the excision specimen, in comparison to shave biopsy cases, nearly half of which (47%) showed no residual tumor on subsequent excision. These results suggest that surface area involved in acute inflammation and repair may play a role in triggering tumor regression. The time interval itself did not seem to influence the regression rate, being comparable between regressed and nonregressed tumors. Neither sex alone was associated with significantly higher regression rate than the general study population. The same was true for histological subtypes. The only tumor characteristic associated with significantly different regression rate was location on the trunk and upper extremity. These tumors demonstrated significantly higher regression rate, compared to the general study population.
It is important to emphasize the possibility of encountering residual carcinoma in an excision specimen despite negative margins on the preceding biopsy. Such phenomenon was encountered in 1% of specimens in our study. This fact may be of particular importance when dealing with cases of superficial multifocal subtype of BCC. In fact, two of four cases that demonstrated residual carcinoma on excision specimen in our study, had a component of superficial multifocal subtype on a biopsy. In addition, three of four cases showed residual carcinoma of superficial multifocal subtype. One potential explanation is that superficial multifocal BCCs characteristically tend to spread widely with “skip areas” in which no tumor is present, rendering widely accepted micrographic surgery not applicable for management.36 Clinicians are generally well aware of this fact, and may choose a noninvasive approach for small superficial multifocal subtype tumors. Depending on tumor characteristics and patient desire, dermatologists may perform curettage as a primary procedure, followed by topical treatment. However, the possibility of residual carcinoma at the curettage site cannot be completely excluded without histological evaluation of the excision specimen.
Recently, a new noninvasive approach demonstrated the potential to reduce invasive management of superficial tumors. Reflectance confocal microscopy (RCM) is a technique that allows for in vivo visualization of the skin with quasihistologic resolution. In one recent study, it demonstrated strong sensitivity (92.8%) in detecting residual carcinoma.35 However, the design of the device itself permits accurate assessment of only low histological risk tumors (ie, superficial multifocal subtype).
Tumors that invade deeply into the dermis in an infiltrative manner were not included in the study and may not be detected by this modality with high level of reliability. In addition, the specificity of the method was estimated at only 68.4%. Lastly, this method may require specially trained personnel and experience in interpreting imaging findings, at least at this point of development.
Conclusion
The results of our study are consistent with the accumulated body of evidence, showing that a proportion of BCCs regress following diagnostic biopsy. This indicates that the surgery remains the mainstay of treatment for BCC regardless of subtype, and permits not only to make a definitive diagnosis, but to improve patient outcomes.
References
- Basset-Seguin N, Herms F. Update in the Management of Basal Cell Carcinoma. Acta Derm Venereol. 2020;100(11):adv00140.
- Roewert-Huber J, Lange-Asschenfeldt B, Stockfleth E, Kerl H. Epidemiology and aetiology of basal cell carcinoma. Br J Dermatol. 2007;157 Suppl 2:47-51.
- Marghoob A, Kopf AW, Bart RS, et al. Risk of another basal cell carcinoma developing after treatment of a basal cell carcinoma. J Am Acad Dermatol. 1993;28:22-28.
- Franchimont C, Pierard GE, Van Cauwenberge D, Damseaux M, Lapiere CH. Episodic progression and regression of basal cell carcinomas. Br J Dermatol. 1982 Mar;106(3):305-10.
- Kimyai-Asadi A, Alam M, Goldberg LH, Peterson SR, Silapunt S, Jih MH. Efficacy of narrow-margin excision of well-demarcated primary facial basal cell carcinomas. J Am Acad Dermatol. 2005;53(3):464-8.
- Kim JYS, Kozlow JH, Mittal B, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78(3), 540-559.
- Silverman MK, Kopf AW, Bart RS, Grin CM, Levenstein MS. Recurrence rates of treated basal cell carcinomas. Part 3: Surgical excision. J Dermatol Surg Oncol. 1992;18(6):471-6.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88(2):167-79.
- Presser SE, Taylor JR. Clinical diagnostic accuracy of basal cell carcinoma. J Am Acad Dermatol. 1987;16:988-90.
- Gooding CA, White G, Yatsuhashi M. Significance of marginal extension in excised basal-cell carcinoma. N Engl J Med. 1965;273(17):923-4.
- Pascal RR, Hobby LW, Lattes R, Crikelair GF. Prognosis of “incompletely excised” versus “completely excised” basal cell carcinoma. Plast Reconstr Surg. 1968;41(4):328-32.
- Taylor GA, Barisoni D. Ten years’ experience in the surgical treatment of basal-cell carcinoma. A study of factors associated with recurrence. Br J Surg. 1973;60(7):522-5.
- Shanoff LB, Spira M, Hardy SB. Basal cell carcinoma: a statistical approach to rational management. Plast Reconstr Surg. 1967;39(6):619-24.
- Daviti M, Lallas K, Dimitriadis C, et al. Real-life data on the management of incompletely excised basal cell carcinoma. Dermatology. 2023;239(3):429-435.
- Gurunluoglu R, Kubek E, Arton J, Olsen A, Bronsert M. Nonpersistence of basal cell carcinoma after diagnostic shave biopsy: reconstruction when specimen is negative during surgery. Ann Plast Surg. 2015;74(6):695-8.
- Alcalay J, Alkalay R. Histological evaluation of residual basal cell carcinoma after shave biopsy prior to Mohs micrographic surgery. J Eur Acad Dermatol Venereol. 2011;25(7):839-41.
- Cecil AJ, McClure SP, Seger EW, Sultana N, Tate JA. A large percentage of excision specimens show residual basal cell carcinoma: A Retrospective Chart Review. Dermatol Surg. 2024.
- Stewart CM, Garlick J, McMullin J, et al. Surgical excision of non-melanoma skin cancer in an elderly veteran’s affairs population. Plast Reconstr Surg Glob Open. 2015;2(12):e277.
- Holmkvist KA, Rogers GS, Dahl PR. Incidence of residual basal cell carcinoma in patients who appear tumor free after biopsy. J Am Acad Dermatol. 1999;41(4):600-605.
- Dixon AY, Lee SH, McGregor DH. Factors predictive of recurrence of basal cell carcinoma. Am J Dermatopathol. 1989;11:222-32.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-7.
- Boriani F, Marconi F. Basal cell carcinomas of the inner canthus: incidence of incomplete excision according to topographical localization of tumours. Br J Dermatol. 2007;157:1301-2.
- Troeltzsch M, Probst FA, Knosel T, et al. Clinical and pathologic parameters predicting recurrence of facial basal cell carcinoma: a retrospective audit in an advanced care center. Int J Dermatol. 2016;55:1281-8.
- Armstrong LTD, Magnusson MR, Guppy MPB. Risk factors for recurrence of facial basal cell carcinoma after surgical excision: a follow-up analysis. J Plast Reconstr Aesthet Surg. 2017;70:1738-45.
- Koplin L, Zarem HA. Recurrent basal cell carcinoma. Plast Reconstr Surg. 1980;65:656–64.
- Swetter SM, Boldrick JC, Pierre P, Wong P, Egbert BM. Effects of biopsy-induced wound healing on residual basal cell and squamous cell carcinomas: rate of tumor regression in excisional specimens. J Cutan Pathol. 2003;30(2):139-46.
- Kimyai-Asadi A, Goldberg LH, Jih MH. Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen. J Am Acad Dermatol. 2005;53:469-74.
- MedCalc Software Ltd. Comparison of proportions calculator. https://www.medcalc.org/calc/comparison_of_proportions.php (Version 23.0.2; accessed September 25, 2024)
- https://www.graphpad.com/quickcalcs/ttest1/
- Fisher Exact Test Calculator. Social Science Statistics. https://www.socscistatistics.com/tests/fisher/default2.aspx.
- Curson C, Weedon D. Spontaneous regression in basal cell carcinomas. J Cutan Pathol. 1979;6(5):432–7.
- Kaporis H, Del Rosso J. Peritumoral inflammation in basal cell carcinoma: fundamentals, clinical significance, and changes after topical imiquimod therapy. Cos Dermatol. 2008; 21(1):38-43.
- Hunt MJ, Halliday GM, Weedon D, et al. Regression in basal cell carcinoma: an immunohistochemical analysis. Br J Dermatol. 1994;130:1–8.
- Liu X, Yin L, Shen S, Hou Y. Inflammation and cancer: paradoxical roles in tumorigenesis and implications in immunotherapies. Genes Dis. 2021 Oct 18;10(1):151-164.
- Navarrete-Dechent C, Cordova M, Aleissa S, et al. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery: A prospective study. J Am Acad Dermatol. 2019;81(2):417-426.
- Jadotte YT, Sarkissian NA, Kadire H, Lambert WC. CASE REPORT: Superficial spreading basal cell carcinoma of the face: A surgical challenge. Eplasty. 2010;10:e46.