J Clin Aesthet Dermatol. 2025;18(4):51–56.
by Gary Goldenberg, MD; Ziv Schwartz, MD, MHA; and Faraz Yousefian, DO
Dr. Goldenberg is with the Icahn School of Medicine at Mount Sinai Hospital, Department of Dermatology in New York, New York. Dr. Schwartz is with Sidney Kimmel Medical College at Thomas Jefferson University, Department of Dermatology and Cutaneous Biology in Philadelphia, Pennsylvania. Dr. Yousefian is with the Philadelphia College of Osteopathic Medicine, Department of Dermatology and Mohs Surgery in Roswell, Georgia.
FUNDING: Funding was provided by Biofrontera Inc.
DISCLOSURES: The authors declare no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective: We evaluated the cosmetic outcome and clearance of actinic keratoses (AKs) using photodynamic therapy (PDT) with microneedling-assisted delivery of 10% aminolevulinic acid (ALA) gel (Ameluz®, Biofrontera, Woburn, MA) with 30-minute incubation followed by 10-minute illumination with a red light (BF-RhodoLED®, 635nm, 37 J/cm2). Methods: Five subjects were treated with red light PDT using microneedling-assisted delivery of 10% ALA gel. ALA gel was applied on the face and incubated for 30 minutes without occlusion, followed by illumination with a red light for 10 minutes (635nm, 37 J/cm2). Follow-up (FU) visits were made at Weeks 1, 2, 4, and 8. The primary endpoints were changes in subject- and investigator-graded Global Aesthetic Improvement Scale (GAIS) scores and assessment of quality in wrinkle, color evenness, texture, spot, and pore analyses with Canfield Visia-CR imaging system. Secondary endpoints were: 1) AK clearance as quantified by the count of AKs at eight-week FU versus baseline and 2) safety as measured by subject-reported pain (10-point VAS scale) during red-light illumination and adverse events. Results: Investigator- and subject-graded GAIS scores showed a sharp increase to “much improved” at two weeks and increased to “very much improved” at eight weeks. There was an average 24.93-percent improvement in texture and an average 10.30-percent improvement in skin tone (color) evenness. AK lesion clearance ranged from 70 to 100 percent, with the mean at 89.2±14.9 percent. Three subjects achieved 100-percent clearance. The mean pain score during red-light illumination was 3.2±1.6. All subjects completed the study. Limitations: The study included a small number of subjects (N=5). Conclusion: Our results indicate that red light PDT using microneedling-assisted delivery of 10% ALA gel and a short 30-minute incubation is a safe and tolerable procedure producing good cosmetic outcomes in several skin quality parameters, such as texture and skin tone evenness, as well as an AK lesion clearance rate of 89.2 percent at Week 8, relatively low pain scores, and a reduced PDT treatment time.
Keywords: Red-light, photodynamic therapy, aminolevulinic acid, actinic keratosis, safety, tolerability, cosmetic
Introduction
Chronic sun exposure results in ultraviolet (UV)-induced premature skin damage, including photoaging and photocarcinogenesis.1 UV radiation enhances the activity of matrix metalloproteinases (MMPs) in the skin to modify the various components of cellular extracellular matrix (ECM) proteins including collagen, fibronectin, proteoglycans, and elastin that play a huge role in functional and structural integrity of skin.2 The hallmark of photoaging is elastosis, composed of partially degraded elastin fibers in the upper cutaneous dermis.3,4 Photoaging can be manifested as rhytides, telangiectasia, dyspigmentation, increased fragility, and skin texture changes.5
Chronic exposure of the skin to solar UV radiation leads to photocarcinogenesis: the formation of precancerous and cancerous lesions.2 MMPs are known to have a crucial effect on regulating cellular processes leading to tumor progression.2 Among the possible pathological outcomes is actinic keratosis (AK), an epidermal keratinocyte dysplasia that can progress to squamous cell carcinoma (SCC).6 Because the malignant transformation of AK to SCC is unpredictable, removal of all AKs is a recommended preventative measure.7 Cryotherapy is a commonly used method to remove AKs, however, it is a lesion-directed therapy that does not address field cancerization (ie, the skin surrounding the clinically evident lesions) and that can lead to dyspigmentation (hypo- and hyper-), skin atrophy and even scarring, further aggravating the aging appearance of the skin. Photodynamic therapy (PDT) is a field-directed therapy treatment option that is minimally invasive and well-tolerated, with reported cosmetic benefits.8 However, drug penetration, prolonged incubation time, and pain during light exposure may be potential drawbacks to this field-directed therapy.
In PDT, a topically applied ALA (a photosensitizer prodrug) permeates the actinic damaged skin, then converts to the photosensitizer which ultimately is activated by a light source of an appropriate wavelength to produce reactive oxygen species (ROS). The United States (US) Food and Drug Administration (FDA)-approved 10% ALA gel is an oil-in-water nanoemulsion formulation designed for improved penetration and ALA stability, thus reducing treatment time and enhancing efficacy for AK clearance9 as well as offering a potentially safe and efficacious treatment for non-melanoma skin cancer.10–12 When topically applied, ALA is converted to protoporphyrin IX (PpIX), which accumulates in the rapidly proliferating cells. In the presence of oxygen and red light illumination (635nm), PpIX produces free radicals (ie, reactive oxygen species) that destroy damaged and precancerous cells without damaging surrounding cells.9,13 In addition, PDT has been used off label for a variety of aesthetic conditions including photoaging demonstrating significant improvement in skin rejuvenation, reduced rhytides, and improving texture.14,15
Drug delivery via topical ALA has limited penetration through the skin, which has led to the use of adjuntive methods to enhance penetration, such as microneedling.16,17 Microneedling enables the drug to enter via micropuncture sites, thereby facilitating deeper penetration into the skin. Several clinical studies have shown enhanced cosmetic outcomes and AK clinical clearance when microneedling was performed before ALA incubation.18,19
Microneedling, also known as percutaneous collagen induction, is widely used as a minimally invasive procedure. Repeatedly puncturing the skin with thin needles leads to the stimulation of growth factors and the production of collagen and elastin which can be utilized in a variety of dermatological conditions including facial rejuvenation, abnormal pigmentation, and transdermal drug delivery.20–24
The present study aims to evaluate the safety, clinical efficacy, and cosmetic outcome of PDT for skin rejuvenation and AK clearance rate, using microneedling-assisted delivery of 10% ALA nanoemulsion gel with 30-minute incubation followed by red-light illumination.
Methods
Study design. A prospective single-arm study was conducted to evaluate facial cosmetic improvement and AK clearance relative to baseline in five subjects treated by PDT with microneedling-assisted delivery of 10% ALA gel and red-light illumination. Follow-up (FU) visits occurred at Weeks 1, 2, 4, and 8.
Subjects. Five subjects aged 18 to 75 years with photoaged skin and 4 to 8 mild to moderate (Olsen grade 1 and 2) facial AKs enrolled in the study. Subjects were of skin types I through IV, immunocompetent, did not take medications for AK prior to or during PDT, had stopped using topical therapies for AK at least three months before the study, and had limited sun exposure during the study. Exclusion criteria were pregnancy; breastfeeding; active skin infection in the treatment area; tendency for keloids; presence of tattoos, skin inflammation, or wounds close to or within treatment field that might interfere with assessments; intake of NSAIDs within seven days prior to or after PDT or systemic steroids 12 weeks before or after PDT, application of local steroids seven days before or after PDT; presence of hypertrophic AKs (Olsen grade 3), porphyria, photodermatoses, or hypersensitivity to treatment ingredients. All subjects provided signed informed consent.
Procedure. A history and physical examination was performed for each subject, including subject- and investigator-graded Global Aesthetic Improvement Scale (GAIS) scores, assessment of photoaged skin by the Canfield Visia-CR imaging System, the location, size, and number of AKs and extent of photodamage. To minimize pain associated with microneedling, topical numbing cream (compound of benzocaine, lidocaine, and tetracaine) was applied 30 minutes prior to the procedure and removed before treatment.
The treatment area was scrubbed with acetone-soaked gauze. Four pea-sized amounts of 10% ALA gel (Ameluz, Biofrontera, Inc., Woburn, MA) (approximately half of a 2-gram tube) were applied and evenly spread on the treatment area without occlusion. A single pass of microneedling (Eclipse MicroPen® Elite, Vitality Skin Bar, Calgary, AB) was performed with subject-dependent depths (0.8-1.0mm) at the discretion of the investigator. Blood, if present, was wiped with sterile water-soaked gauze and the bleeding stopped before proceeding. The remaining 10% ALA gel was applied and allowed to incubate for 30 minutes without occlusion. The treated area was illuminated with the narrow-band red light (635nm, 37 J/cm2, BF-RhodoLED, Biofrontera) for 10 minutes. Pain during illumination was graded as described below. Subjects were instructed to avoid sunlight for 48 hours post-treatment and apply a petrolatum ointment (Vaseline; Unilever) daily.
Endpoints. The primary endpoints were changes in photodamage/aging as quantified by the Canfield Visia-CR imaging System, and subject- and investigator-graded GAIS scores (GAIS score in which 3=very much improved, 2=much improved, 1=improved, 0=no change, -1=worse, -2=much worse, and -3=very much worse). Secondary endpoints were: 1) AK clearance as quantified by count of AKs at eight-week FU versus baseline and 2) safety as measured by subject-reported pain (VAS scale in which 0=no pain, 5=moderate pain, and 10=worst pain) during red-light illumination and adverse events documented at the time of treatment and at each FU visit.
Results
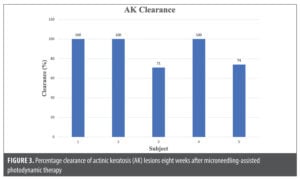
All five subjects completed the study. There was an improvement in qualitative, rhytides, color evenness, texture, spot, and pore analyses with Canfield Visia-CR imaging system at Baseline and post-treatment at Week 8 (Figure 1). In addition, there was an average 24.93-percent improvement in texture and an average 10.30-percent improvement in skin tone evenness (Table 1). Investigator- and subject-graded GAIS scores were in general agreement throughout the study, with both groups showing a sharp increase at two weeks (much improved) and continued increases up to very much improved at Week 8 (Figure 2). AK clearance ranged from 70 to 100 percent, with the mean at 89.2±14.9 percent and three subjects achieved 100-percent clearance (Figure 3). Lastly, the mean pain score during red-light illumination was 3.2±1.6, and four subjects tolerated the treatment well and reported pain levels at 2 or 3 (Figure 4).



Discussion
PDT has previously shown to be an efficacious treatment option for a variety of malignant and premalignant conditions, including AK, squamous cell carcinoma in situ, and superficial basal cell carcinoma.1,9,14,25 In recent years, the cosmetic outcome of PDT treatment has demonstrated promising effects in the improvement of a variety of conditions, including skin rejuvenation, scars, and acne vulgaris.26–28 The safety and efficacy of PDT with 10% ALA gel (referred to in previous publications as BF-200 ALA) and red-light illumination for the field-directed treatment of mild to moderate AKs on the face and/or scalp (MM1) has previously been demonstrated in several randomized controlled Phase 3 trials.8,29,30
Microneedling has been commonly used for transdermal drug delivery and a variety of dermatological purposes including skin tightening, skin rejuvenation, and scar remodeling.21–23,31 Transdermal delivery with microneedling has been previously studied with ALA in vitro and in vivo,24 ALA and methyl aminolevulinate (MAL) in human skin,32 MAL in human organ transplant recipients,33 and MAL in pigskin.17
Microneedling can be utilized in adjunct to ALA-PDT to further enhance drug delivery and cosmetic outcomes in patients. The combination of microneedling and PDT for the treatment of photoaging, AK, or both has been evaluated using ALA18,19,34–37 and MAL.16,38,39 In one study, ALA-PDT cosmetic improvement outcome in treating dyspigmentation, fine lines, and reductions in pore size was similar to 30% trichloroacetic acid chemical peel with lesser side effects and more rapid healing.14 In a split-face trial of 20% ALA-PDT with or without microneedling and one-hour 20% ALA incubation followed by blue light illumination, the mean percent reduction of AK lesions was 89.3 percent on the microneedling-PDT side and 69.5 percent on the PDT-alone side.19 A physician’s global cosmetic assessment was performed based on Canfield Visia photograph and 15 of the 19 patients showed improvement on one side of the face compared to the other, 13 of which were on the microneedling-PDT side. The procedure was safe and well tolerated with no significant side effects reported.
Microneedling-enhanced PDT with the 10% ALA gel has been previously reported in three patients—one with a solitary AK lesion on the upper lip, another with multiple AK lesions on the forehead, and a third with a single lesion of Bowen’s disease on the cheek.35 The patients had failed to show significant improvement after five PDT alone treatments and the addition of microneedling prior to PDT, for four additional treatments, resulted in complete healing of all lesions and excellent cosmesis without relapse three months later. The authors concluded that pretreatment with microneedling was a simple, rapid, and low-cost method to improve PDT of hyperkeratotic AKs and Bowen’s disease.
The present study suggests that PDT using microneedling-assisted delivery of 10% ALA and red-light illumination without curettage and with a much shorter incubation period (83% less) is well tolerated and results in very cosmesis, including parameters such as wrinkles, color evenness, texture, spot, and pore and nearly 90-percent clearance of AK lesions.
Limitations. Limitations of the present study are the small number of patients and the short follow-up time. The results are encouraging and call for a larger study that would include a control group in which PDT is performed without microneedling for comparison, as well as follow-up times up to six months to evaluate long-term cosmetic benefits and AK lesion recurrence.
Conclusion
PDT using microneedling-assisted delivery of 10% ALA nanoemulsion gel with 30-minute incubation followed by red-light illumination is a well-tolerated, efficacious, and time-efficient procedure that results in improved cosmesis including wrinkle, color evenness, texture, spot, and pore and AK clearance.
References
- Fernandez Figueras MT. From actinic keratosis to squamous cell carcinoma: pathophysiology revisited. J Eur Acad Dermatol Venereol. 2017;31 Suppl 2:5–7.
- Pittayapruek P, Meephansan J, Prapapan O, et al. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17(6):868.
- Gilchrest BA. Photoaging. J Invest Dermatol. 2013;133(E1):E2–E6.
- Rittié L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5(1):a015370.
- Quan T, Qin Z, Xia W, et al. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14(1):20–24.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177(2):350–358.
- Schmitz L, Kahl P, Majores M, et al. Actinic keratosis: correlation between clinical and histological classification systems. J Eur Acad Dermatol Venereol. 2016;30(8):1303–1307.
- Reinhold U, Dirschka T, Ostendorf R, et al. A randomized, double-blind, phase III, multicentre study to evaluate the safety and efficacy of BF-200 ALA (Ameluz(®) ) vs. placebo in the field-directed treatment of mild-to-moderate actinic keratosis with photodynamic therapy (PDT) when using the BF-RhodoLED(®) lamp. Br J Dermatol. 2016;175(4):696–705.
- Nestor MS, Berman B, Patel J, et al. Safety and efficacy of aminolevulinic acid 10% topical gel versus aminolevulinic acid 20% topical solution followed by blue-light photodynamic therapy for the treatment of actinic keratosis on the face and scalp: a randomized, double-blind study. J Clin Aesthet Dermatol. 2019;12(3):32–38.
- Cervantes JA, Zeitouni NC. Photodynamic therapy utilizing 10% ALA nano-emulsion gel and red-light for the treatment of squamous cell carcinoma in-situ on the trunk and extremities: pilot study and literature update. Photodiagnosis Photodyn Ther. 2021;35:102358.
- Shim PJ, Zeitouni NC. Long-term follow up of ALA 10% gel and red-light photodynamic therapy for the treatment of squamous cell carcinoma in situ. Photodiagnosis Photodyn Ther. 2023;41:103211.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179(2):309–319.
- Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14(4):275–292.
- Gilbert DJ. Incorporating photodynamic therapy into a medical and cosmetic dermatology practice. Dermatol Clin. 2007;25(1):111–118.
- Philipp-Dormston WG. Photodynamic therapy for aesthetic-cosmetic indications. G Ital Dermatol Venereol. 2018;153(6):817–826.
- Osman-Ponchet H, Gaborit A, Sevin K, et al. Pretreatment of skin using an abrasive skin preparation pad, a microneedling device or iontophoresis improves absorption of methyl aminolevulinate in ex vivo human skin. Photodiagnosis Photodyn Ther. 2017;20:130–136.
- Jhanker Y, Mbano MN, Ponto T, et al. Comparison of physical enhancement technologies in the skin permeation of methyl amino levulinic acid (mALA). Int J Pharm. 2021;610:121258.
- Petukhova TA, Hassoun LA, Foolad N, et al. Effect of expedited microneedle-assisted photodynamic therapy for field treatment of actinic keratoses: a randomized clinical trial. JAMA Dermatol. 2017;153(7):637–643.
- Spencer JM, Freeman SA. Microneedling prior to levulan pdt for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15(9):1072–1074.
- Sitohang IBS, Sirait SAP, Suryanegara J. Microneedling in the treatment of atrophic scars: a systematic review of randomised controlled trials. Int Wound J. 2021;18(5):577–585.
- Iosifidis C, Goutos I. Percutaneous collagen induction (microneedling) for the management of non-atrophic scars: literature review. Scars Burn Heal. 2019;26;5:2059513119880301.
- Ahmed Saeed Al-Japairai K, Mahmood S, Hamed Almurisi S, et al. Current trends in polymer microneedle for transdermal drug delivery. Int J Pharm. 2020;587:119673.
- Juhasz MLW, Cohen JL. Microneedling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997–1003.
- Donnelly RF, Morrow DIJ, McCarron PA, et al. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: potential for enhanced topical photodynamic therapy. J Control Release. 2008;129(3):154–162.
- Queirós C, Garrido PM, Maia Silva J, et al. Photodynamic therapy in dermatology: beyond current indications. Dermatol Ther. 2020;33(6):e13997.
- Tosa M, Ogawa R. Photodynamic therapy for keloids and hypertrophic scars: a review. Scars Burn Heal. 2020;6:2059513120932059.
- Chen D, Zhang Y, Long W, et al. Visible light-driven photodynamic therapy for hypertrophic scars with MOF armored microneedles patch. Front Chem. 2023;11:1128255.
- Boen M, Brownell J, Patel P, et al. The role of photodynamic therapy in acne: an evidence-based review. Am J Clin Dermatol. 2017;18(3):311–321.
- Szeimies RM, Radny P, Sebastian M, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a prospective, randomized, double-blind, placebo-controlled phase III study. Br J Dermatol. 2010;163(2):386–394.
- Dirschka T, Radny P, Dominicus R, et al. Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo. Br J Dermatol. 2012;166(1):137–146.
- Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268.
- Mikolajewska P, Donnelly RF, Garland MJ, et al. Microneedle pre-treatment of human skin improves 5-aminolevulininc acid (ALA)- and 5-aminolevulinic acid methyl ester (MAL)-induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharm Res. 2010;27(10):2213–2220.
- Bencini PL, Galimberti MG, Pellacani G, et al. Application of photodynamic therapy combined with pre-illumination microneedling in the treatment of actinic keratosis in organ transplant recipients. Br J Dermatol. 2012;167(5):1193–1194.
- Clementoni MT, B-Roscher M, Munavalli GS. Photodynamic photorejuvenation of the face with a combination of microneedling, red light, and broadband pulsed light. Lasers Surg Med. 2010;42(2):150–159.
- Kolde G, Rowe E, Meffert H. Effective photodynamic therapy of actinic keratoses and Bowen’s disease using microneedle perforation. Br J Dermatol. 2013;168(2):450–452.
- Taub AF, Schieber AC. New methods for the clinical enhancement of photodynamic therapy. J Drugs Dermatol. 2015;14(11):1329–1334.
- Lev-Tov H, Larsen L, Zackria R, et al. Microneedle-assisted incubation during aminolaevulinic acid photodynamic therapy of actinic keratoses: a randomized controlled evaluator-blind trial. Br J Dermatol. 2017;176(2):543–545.
- Torezan L, Chaves Y, Niwa A, et al. A pilot split-face study comparing conventional methyl aminolevulinate-photodynamic therapy (PDT) with microneedling-assisted PDT on actinically damaged skin. Dermatol Surg. 2013;39(8):1197–1201.
- Bento CO, Pantaleão L, de Souza MB, et al. Comparison of clinical and histologic findings in daylight photodynamic therapy for skin field cancerization: A randomized controlled four-arm study on physical methods-assisted delivery of methyl aminolevulinate. Photodiagnosis Photodyn Ther. 2021;35:102404.