 J Clin Aesthet Dermatol. 2021;14(1):21–23
J Clin Aesthet Dermatol. 2021;14(1):21–23
by Stephen Ansah-Addo, MD, and Andrew F. Alexis, MD, MPH
Mr. Ansah-Addo is with the Albert Einstein College of Medicine in the Bronx, New York, New York. Dr. Alexis is with the Department of Dermatology at Mount Sinai St. Luke’s and Mount Sinai West in New York, New York.
FUNDING: No funding was provided for this article.
DISCLOSURES: Dr. Alexis has received grant/research support from Leo, Novartis, Almirall, Bristol-Myers-Squibb, Celgene, Menlo, Galderma, SkinMedica, and Valeant (Bausch Health) and is a consultant for Leo, Novartis, Menlo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Unilever, Celgene, Beiersdorf, Valeant, L’Oreal, BMS, Menlo, Scientis, Valeant. Mr. Ansah-Addo reports no conflicts of interest relevant to the content of this article.
ABSTRACT: Pretibial myxedema (PM) is a rare extrathyroid condition seen in about 0.5 to 4.3 percent of individuals with hyperthyroidism due to Graves’ disease, often presenting with associated thyroid orbitopathy. In most cases, patients with PM have elevated levels of thyroid antibodies, such as thyroid peroxidase (TPO), thyroglobulin, and—most especially—thyroid-stimulating hormone receptor antibodies. We present a rare case of biopsy-proven PM in a euthyroid patient with no history of Graves’ disease or Hashimoto’s disease. TPO and thyroglobulin antibody counts were slightly elevated but less than what is typically seen in PM and thyroid-stimulating hormone receptor antibodies (thyrotrophin-binding inhibitor immunoglobulin) were negative.
KEYWORDS: Pretibial, myxedema, Graves, hyperthyroidism, extrathyroid, nodular, hyaluronic acid
Pretibial myxedema (PM) is a rare extrathyroid condition seen in about 0.5 to 4.3 percent of individuals with hyperthyroidism due to Graves’ disease, often presenting with associated thyroid orbitopathy.1,2 In most cases, patients with PM have elevated levels of thyroid antibodies such as thyroid peroxidase (TPO), thyroglobulin, and—most especially—thyroid-stimulating hormone receptor antibodies.3–6 We present a rare case of biopsy-proven PM in a euthyroid patient with no history of Graves’ disease or Hashimoto’s disease.
Case report. A 63-year-old African American man presented with multiple hyperpigmented nodules on the lower legs. The nodules had been progressively enlarging since he first noticed them one year prior to visiting our clinic. Initially, they were pruritic, but were asymptomatic at the time of diagnosis. His past medical history was significant for type II diabetes mellitus, asthma, hypertension, and nummular eczema. He reported no history of thyroid disease. His medications included hydrochlorothiazide, amlodipine-benazepril, pioglitazone, sitagliptin, pravastatin, breo ellipta, crisaborole, and fluocinonide 0.05% cream. He denied heat intolerance, palpitations, anxiety, hand tremor, shortness of breath, increased frequency of bowel movement, loss or gain of weight, cold intolerance, constipation, fatigue, and change in voice.
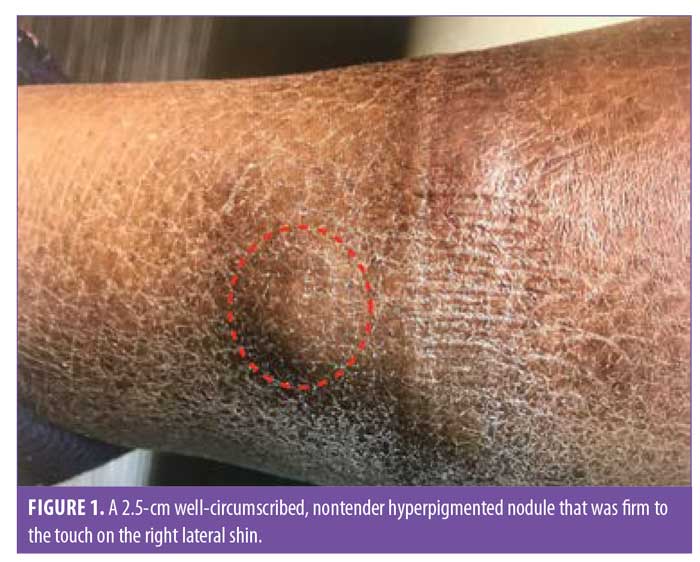
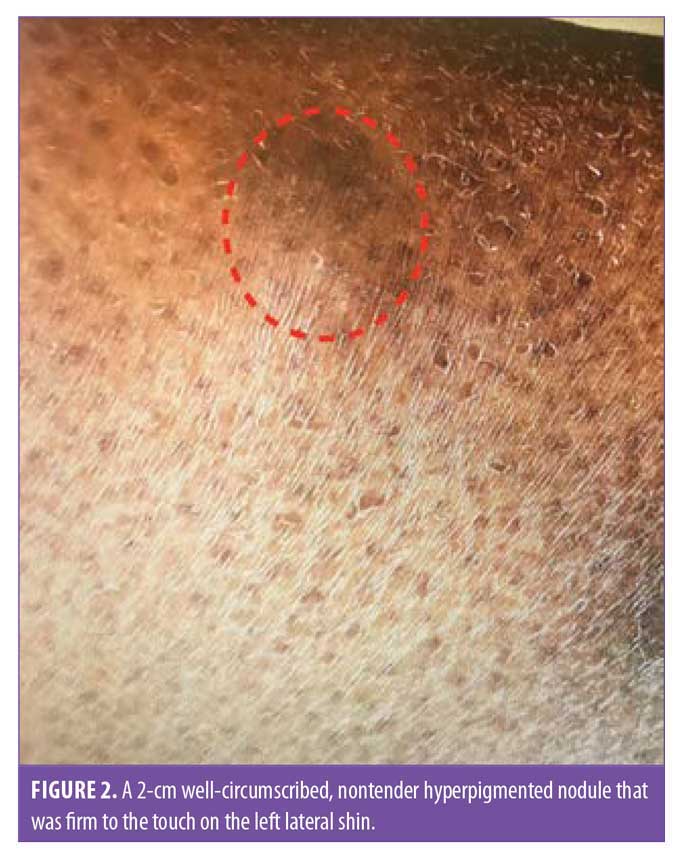
Examination of the patient’s skin showed well-circumscribed, nontender nodules that were firm to the touch on both lateral shins—two on the left and three on the right—measuring between 2 and 2.5cm in diameter (Figures 1 and 2). There was no hyperhidrosis, acropachy, or thyromegaly.
Laboratory examination results included a free serum triiodothyronine level of 2.3 pg/mL (normal range: 2.0–4.4 pg/ml), total triiodothyroinine level of 81 ng/dL (normal range: 71–180 ng/dL), free serum thyroxine level of 0.95 ng/dL (normal range: 0.82–1.77 ng/dL), thyroid-stimulating hormone level of 1.670 uIU/mL (normal range: 0.450–4.500 ulU/mL), thyroid peroxidase antibody level of 48 IU/mL (normal range: 0–34 IU/mL), and thyroglobulin antibody level of 6.3 IU/mL (normal range: 0.0–0.9 IU/mL). He was negative for thyroid-stimulating hormone receptor antibodies and positive for antinuclear antibodies.
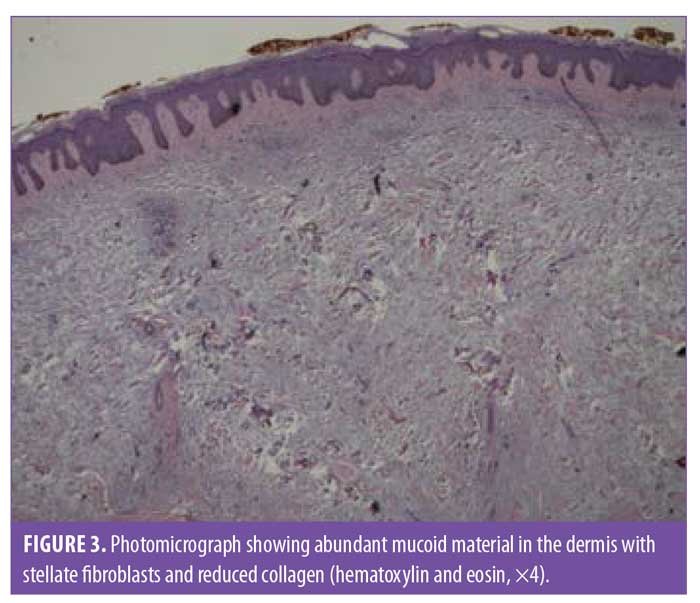
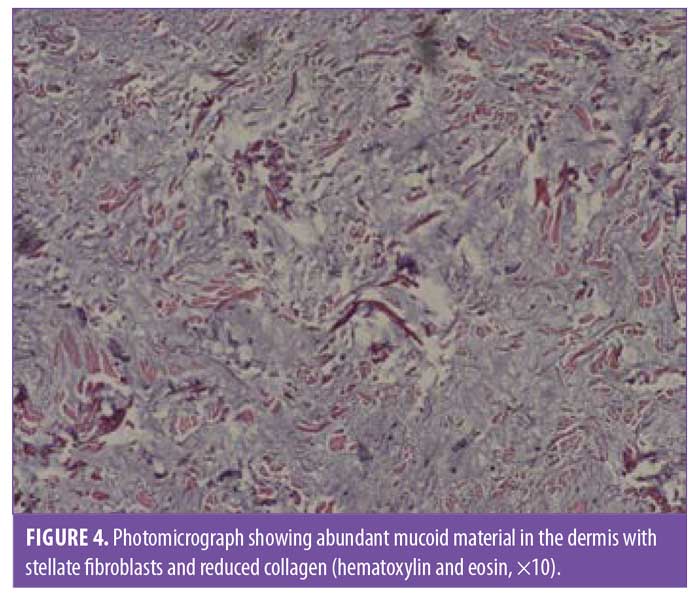
A 6-mm punch biopsy of a nodule on the right shin was performed. The pathology examination found an accumulation of abundant mucoid material in the upper half of the dermis with stellate fibroblasts and reduced collagen (Figures 3 and 4). Colloidal iron staining demonstrated increased dermal mucin throughout the dermis. These findings are diagnostic of myxedema.
Discussion
Pretibial myxedema or thyroid dermopathy is a condition in which there is thickening of the skin, usually in the pretibial area, due to an accumulation of acid mucopolysaccharides (e.g., glycosaminoglycans).7,8 Hyaluronic acid, the main infiltrative mucopolysaccharide, often begins in the papillary dermis and frequently advances deeper, occasionally involving the subcutis.9 PM is an uncommon extrathyroidal manifestation of Graves’ disease (GD), occurring in about 0.5 to 4.3 percent of patients, with almost all cases associated with ophthalmopathy.1,2 In addition to PM, exophthalmos and thyroid acropachy are the other two extrathyroidal manifestations of GD. PM usually occurs during the hyperthyroid state of GD.10 Nonetheless, it can rarely occur in individuals with non-thyrotoxic thyroid disease, such as Hashimoto’s disease and even in euthyroid subjects.11,12 In a retrospective study of 178 patients diagnosed with PM, only 2.8 percent were euthyroid, with 91.0 percent being hyperthyroid.13
Myxedema is an autoimmune manifestation of thyroiditis, particularly GD.14 Although the pretibial area is the most common site (93.9% of cases), areas such as the feet and toes are sometimes involved.2,14 Rarely, myxedema can present on the upper extremities, neck, shoulders, torso, and pinnae.15,16 When myxedema is present in these unusual locations, the patient often has a history of trauma to this area.17 Clinically, PM can be classified into the following five forms: nonpitting edema (43.3%), plaque (27.0%), nodular (18.5%), elephantiasic (2.8%), and unclassifiable (8.4%).13 The nonpitting type presents as flesh- or orange-colored nonpitting edema that does not show pitting edema of the ankles.2 The plaque type appears as raised plaques on a background of nonpitting edema, while the nodular form has well-circumscribed tubular or nodular lesions.2 The elephantiasic form is rare but is the most severe type and is characterized by nodular lesions and marked lymphedema.13 Lesions in the pretibial region and feet are often symmetric and tend to have prominent hair follicles resembling, in appearance and texture, an orange peel (i.e., peau d’orange) or pigskin.2,7,10,13,17
PM is rarely painful or pruritic, but localized hyperhidrosis, hyperkeratosis, hyperpigmentation, and fissuring can be present.2,7,13 PM has an unpredictable course and lesions can regress in months or years or grow slowly.18
Differential diagnoses of PM include edema, generalized myxedema, hypertrophic lichen planus, the urticarial phase of bullous pemphigoid and cutaneous mucinosis, such as lichen myxedematosus, reticular erythematosus mucinosis, alopecia mucinosa, cutaneous focal mucinosis, scleroderma myxoid cyst, and secondary mucinosis.2,18 PM is often clinically diagnosed because of its unique presentation and the fact that it is almost always associated with hyperthyroidism and ophthalmopathy. However, in the rare instances when there is no hyperthyroidism and/or ophthalmopathy present or when the clinician is uncertain, biopsy can give a definitive diagnosis.
Histopathologically, PM is characterized by an increased amount of glycosaminoglycans in the dermis with fragmented and split collagen fibers due to the massive amounts of mucin, mostly in the reticular dermis, whereas the papillary dermis is typically spared.17
The exact pathogenesis of PM is unknown. However, immunological, autoimmune, and mechanical factors contribute to the onset of PM.2 There is perhaps an interaction with a common antigen, most likely thyroid-stimulating hormone receptors, present in both the thyroid and skin. Sensitized T lymphocytes to these antigens can infiltrate dermal tissue and release cytokines, such as interleukin-1alpha and transforming growth factor-beta, which stimulate fibroblasts to secrete glycosaminoglycans.2,19 Dependent areas, such as the pretibial region and feet, are more susceptible to these lesions because there is a decrease in lymphatic fluid return, leading to an accumulation of offending cytokines in these tissues.2 Furthermore, the pretibial region and feet are susceptible to repetitive trauma, increasing their risk of developing myxedema.2
While spontaneous resolution can occur, topical corticosteroids under occlusion and intralesional corticosteroid injections are effective treatment options.15,17 There are reports that injections of octreotide, an insulin-like growth factor 1 antagonist, are beneficial in refractory PM.20,21 Furthermore, there are cases of PM successfully treated with intralesional hyaluronidase.22,23
Reviewing the literature, we identified six other published cases of biopsy-proven PM in euthyroid patients.10,12,14,17,24,25 Although a 1973 study by Lynch et al3 described 23 patients with PM and nonthyrotoxic thyroid disease, including 16 euthyroid patients, and Greer26 in 1957 discussed a similar patient, the thyroid status of these patients is uncertain due to the lack of sensitive thyrotropin assays at these times. Among the six cases, two had the nonpitting edema form,10,25 two had the plaque form,12,24 one had the nodular form,14 and one had both nodular and nonpitting edema.17 Exophthalmia was present in two of the cases,12,14 whereas acropachy was seen in only one case.14 All but one of the cases had pruritic lesions.10
Conclusion
In conclusion, our case represents an atypical presentation of nodular pretibial myxedema in a euthyroid patient with no history of GD, exophthalmos, or Hashimoto’s disease. Although serum thyroid peroxidase and thyroglobulin antibodies were positive, they were not as elevated as typically seen in PM. Furthermore, our patient’s thyroid-stimulating hormone receptor antibodies result was negative, which is unusual in PM. Although rare in patients without a history of thyroid disease, clinicians should include PM in their differential diagnosis of subcutaneous nodular lesions on the lower extremities.
References
- Kriss JP. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16(2):409–415.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6(5): 295–309.
- Lynch PJ, Maize JC, Sisson JC. Pretibial myxedema and nonthyrotoxic thyroid disease. Arch Dermatol. 1973;107(1):107–111.
- Chang TC, Wu SL, Hsiao YL, et. al. TSH and TSH receptor antibody-binding sites in fibroblasts of pretibial myxedema are related to the extracellular domain of entire TSH receptor. Clin Immunol Immunopathol. 1994;71:113–120.
- Morris JC, Hay ID, Nelson RE, et al. Clinical utility of thyrotropin-receptor antibody assays: comparison of radioreceptor and bioassay methods. Mayo Clin Proc. 1988;63 (7):707–17.
- Antonelli A, Navarranne A, Palla R, et al. Pretibial myxedema and high-dose intravenous immunoglobulin treatment. Thyroid. 1994;4(4):399–408.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). Review of 150 cases. Medicine (Baltimore). 1994;73(1):1–7.
- Omohundro C, Dijkstra JW, Camisa C, Bergfeld WF. Early onset pretibial myxedema in the absence of ophthalmopathy: a morphologic evolution. Cutis. 1996;58(3):211–214.
- Georgala S, Katoulis AC, Georgala C, et al. Pretibial myxedema as the initial manifestation of Graves’ disease. J Eur Acad Dermatol Venereol. 2002;16(4):380–383.
- Anagnostis P, Artzouchaltzi A, Grekou A, et al. Pretibial myxedema in a euthyroid patient. Hormones (Athens). 2018;17(1):133–135.
- Cannavo SP, Borgia F, Vaccaro M, et al. Pretibial myxoedema associated with Hashimoto’s thyroiditis. J Eur Acad Dermatol Venereol. 2002;16(6):625–627.
- Nair PA, Mishra A, Chaudhary A. Pretibial myxedema associated with euthyroid Hashimoto’s thyroiditis: a case report. J Clin Diagn Res. 2014;8(6):YD01–YD02.
- Schwartz KM, Fatourechi V, Ahmed DD, Pond GR. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87(2): 438–446.
- Senel E, Gulec AT. Euthyroid pretibial myxedema and EMO syndrome. Acta Dermatovenerol Alp Pannonica Adriat. 2009;18(1):21–23.
- Noppakun N, Bancheun K, Chandraprasert S. Unusual locations of localized myxedema in Graves’ disease. Report of three cases. Arch Dermatol. 1986;122(1):85–88.
- Verma S, Rongioletti F, Braun-Falco M, Ruzicka T. Preradial myxedema in a euthyroid male: a distinct rarity. Dermatol Online J. 2013;19(4):9.
- Buljan-Cvijanovic M, Neal JM, Zemtsov A. Euthyroid pretibial myxedema. Endocr Pract. 1998;4(6):375–377.
- del-Rio E, Velez A, Sanchez Yus E. Persistent asymptomatic nodules on the legs. Nodular pretibial myxedema. Arch Dermatol. 1993;129(3):365–366, 368–369.
- Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis, and dermatologic manifestations. J Am Acad Dermatol. 2003;48(5):641–659; quiz 60–62.
- Shinohara M, Hamasaki Y, Katayama I. Refractory pretibial myxoedema with response to intralesional insulin-like growth factor 1 antagonist (octreotide): downregulation of hyaluronic acid production by the lesional fibroblasts. Br J Dermatol. 2000;143(5):1083–1086.
- Felton J, Derrick EK, Price ML. Successful combined surgical and octreotide treatment of severe pretibial myxoedema reviewed after 9 years. Br J Dermatol. 2003;148(4):825–826.
- Hoesly PM, Tolaymat LM, Sluzevich JC, Keeling JH. Pretibial myxedema successfully treated with intralesional hyaluronidase. JAAD Case Rep. 2018;4(9):874–876.
- Sorenson E, Shive M, Holland V, Chuang G. Intralesional hyaluronidase for the treatment of pretibial myxedema. J Am Acad Dermatol. 2018;79(3).
- Srebrnik A, Ophir J, Brenner S. Euthyroid pretibial myxedema. Int J Dermatol. 1992;31(6):431–432.
- Chen JJ, Ladenson PW. Euthyroid pretibial myxedema. Am J Med. 1987;82(2):318–320.
- Greer MA. Exophthalmos and localized pretibial myxedema in a euthyroid patient; studies with triiodothyronine. J Clin Endocrinol Metab. 1957;17(12):1466–1471.

