 J Clin Aesthet Dermatol. 2021;14(1):27–29.
J Clin Aesthet Dermatol. 2021;14(1):27–29.
by Hassie Cooper, DO; Maheera Farsi, DO, FAAD; and Richard Miller, DO, FAOCD
All authors are with Largo Medical Center in Largo, Florida.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Primary mucosal melanomas are rare neoplasms that occur in the mouth, esophagus, nasopharynx, larynx, and anogenital mucosa. Mucosal melanomas are rare, accounting for approximately one percent of all melanomas. Of the mucosal melanomas that occur in the head and neck, oral mucosal melanomas compose approximately 25 percent. Here, we present a case of an amelanotic oral mucosal melanoma of the mucosal lip in a 77-year-old male patient with a history of non-Hodgkin’s lymphoma and multiple basal and squamous cell carcinomas. The patient presented with a pink, nonpigmented, pedunculated mass on the left superior mucosal lip. Histopathologic examination of the biopsy specimen revealed a diagnosis of a superficial spreading type of malignant melanoma with a nodular component. The patient was referred to a tertiary care center for further management. Multiple risk factors exist for developing melanoma, including immunosuppression. Lymphoproliferative disorders, such as non-Hodgkin’s lymphoma, lead to inherent immunosuppression, which can be exacerbated by chemotherapy treatments. Cases of oral mucosal melanoma have a poor prognosis due to delayed diagnosis, anatomic location, and aggressive behavior. Surgical resection is first-line therapy, with regional lymph-node dissection of the neck is recommended in most cases. Radiotherapy and targeted molecular therapy, such as c-KIT inhibitors, can also be used.
KEYWORDS: Amelanotic melanoma, mucosal melanoma, oral mucosal melanoma, c-KIT mutation
Primary mucosal melanomas are rare malignant neoplasms of melanocytes that occur in the mouth, esophagus, nasopharynx, larynx, and the mucosa of the anogenital region. Mucosal melanomas are quite rare, accounting for approximately one percent of all melanomas.1 Amelanotic melanomas, defined as melanomas that lack pigment upon visual inspection prior to biopsy,2–5 are a rare variant and comprise 2 to 20 percent of all melanomas.2,3,6,7 Herein, we present a rare and unique case of an amelanotic oral mucosal melanoma (OMM) of the mucosal lip in a 77-year-old immunocompromised male patient.
Case Report
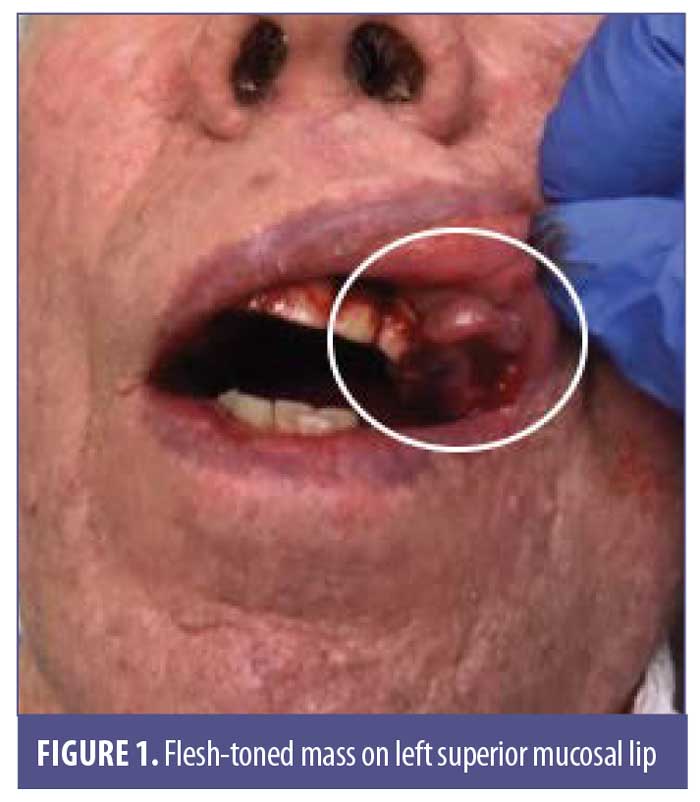
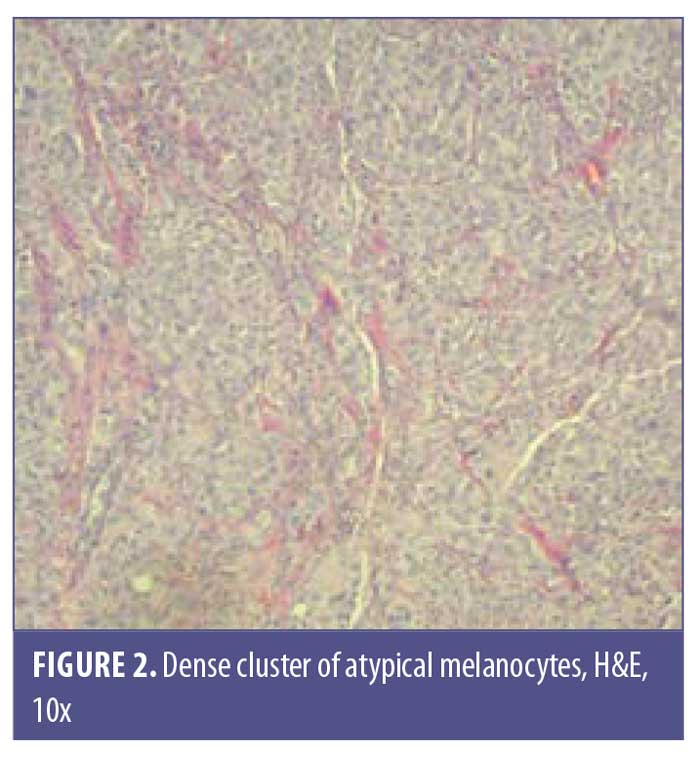
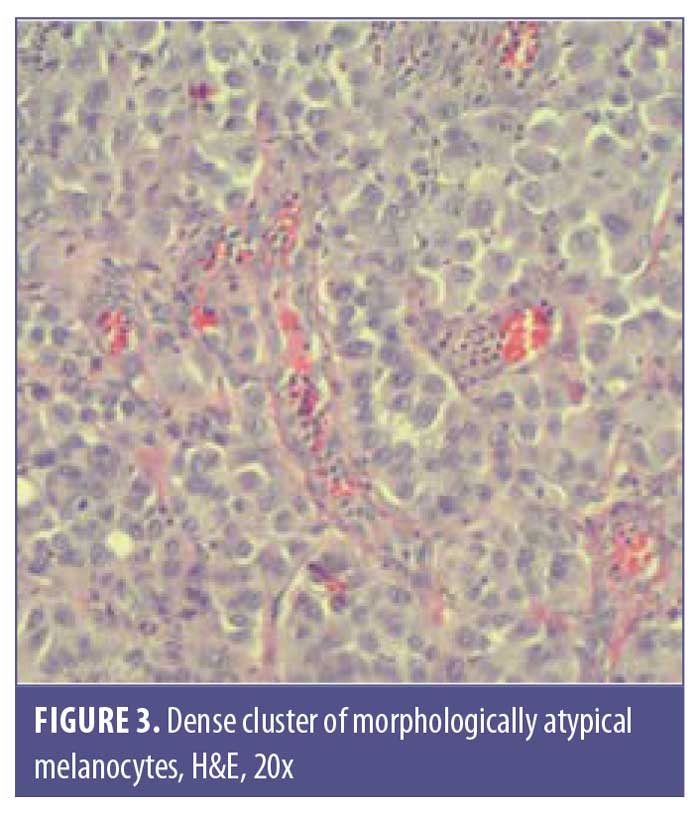
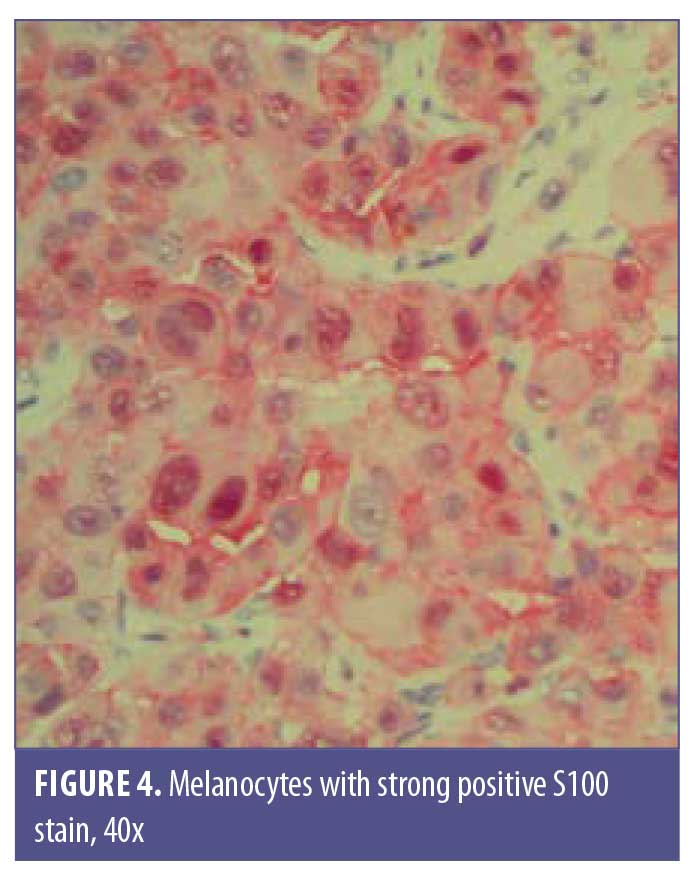
A 77-year-old man with a medical history of non-Hodgkin’s lymphoma (NHL), status posttreatment with chemotherapy, basal cell carcinoma, and squamous cell carcinoma presented to the clinic with a pink, nonpigmented, pedunculated 1.2-cm×1.0-cm mass on the left superior mucosal lip (Figure 1). The mass had been present with intermittent bleeding for several months. Shave biopsy with histopathologic examination (Figures 2 and 3) and immunohistochemical stain for S100 (Figure 4), SOX-10, and Ki-67 revealed a diagnosis of a superficial spreading type of malignant melanoma with a nodular component. The patient was referred to a local tertiary care center for further evaluation. Positron-emission tomography and computed tomography imaging together with sentinel lymph-node biopsy revealed a staging result of pT3N1M0. The patient underwent wide local excision with margins free of involvement with a plan for subsequent radiation therapy. However, the patient developed malaise, weakness, vomiting, and weight loss during his interval follow-up period. He was extensively worked up for recurrence or metastasis of his mucosal melanoma, but nothing was found. He continued to be scheduled for three-month follow-up intervals at the tertiary care center. Ultimately, he died several months later from complications related to pneumonia.
Discussion
Of the mucosal melanomas that occur in the head and neck region, OMMs comprise approximately 25 percent8 and, subsequently, about 40 percent of those OMMs are amelanotic.9 The most common sites where OMMs occur are the hard palate and maxillary gingiva, which constitute approximately 80 percent of all OMM cases.10 Less common sites include the buccal mucosa, mandibular gingiva, floor of mouth, tongue, and lips, which is where our patient’s tumor was found10
There are multiple risk factors for developing melanoma, including but not limited to sun exposure, genetic predisposition, and immunosuppression. Lymphoproliferative disorders, such as NHL, lead to inherent immunosuppression, which can be exacerbated by chemotherapy treatments.11 Our patient’s NHL was previously treated with rituximab and bendamustine, likely exacerbating his immunocompromised state. As expected, OMMs are not related to sun exposure and have a much poorer prognosis than their cutaneous counterparts; this is likely due to an often-delayed diagnosis and their anatomic location and aggressive behavior.10 Unlike OMMs, amelanotic melanomas have historically been more commonly diagnosed on sun-exposed skin.6 Amelanotic mucosal melanomas are also associated with a delay in diagnosis and poor prognosis due to the similar risk factor of advanced histologic stage at the time of presentation9 as well as evidence demonstrating that amelanotic melanomas may grow faster than pigmented melanomas.2
Studies evaluating the genetic expression of OMMs led to complex results suggesting that OMMs may have somewhat different genetic expressions than cutaneous melanomas or mucosal melanomas in other anatomic locations. Common mutations involved in the pathogenesis of melanoma are BRAF, NRAS, and/or KIT mutations. BRAF and NRAS mutations have a low incidence in mucosal melanoma as compared with in cutaneous melanoma8,12; meanwhile, KIT mutations occur more often in mucosal melanomas than in cutaneous melanomas with up to 40 percent of mucosal melanoma cells displaying overexpression of the c-KIT protein.8 However, KIT mutations have a low incidence in head and neck melanomas compared to mucosal melanomas on other anatomic sites, such as the vulva and anorectum.12,13 This suggests that OMMs may be different from other mucosal melanomas in terms of genetic mutations and other characteristics,12 which is an important consideration when choosing appropriate systemic therapy.
Multiple stains are typically positive in OMMs, including but not limited to, S100, Mart1/melan-A, MITF, tyrosinase, and HMB45.14 All of these stains can have varying expression levels; however, HMB45 shows a higher intensity of staining in OMMs.9 Many amelanotic melanomas stain positive for S100 and vimentin, but these are not pathognomonic stains.9 Multiple stains are usually required to diagnose OMM, as none of the above-mentioned stains are 100-percent sensitive.15 Our patient was diagnosed using S100, SOX-10, and Ki-67 stains, which can also be utilized to diagnose melanoma.
Factors that impart a worse prognosis include a tumor thickness greater than 6mm, involvement of regional lymph nodes, the presence of distant metastases, a high mitotic rate, and exophytic ulcerated lesions.8 Staging of mucosal melanoma contains independent criteria from cutaneous melanoma staging. Typically, the American Joint Committee on Cancer guidelines for TNM staging of mucosal melanoma is used, beginning at stage T3, to indicate the overall poorer prognosis of mucosal variants.8,16 Because of the usual delay in diagnosis and more severe histologic stage at the time of diagnosis, the five-year survival rate of OMM is quite poor, at around 10 to 25 percent.17 Surgical resection with 1- to 2-cm margins remains the first line of therapy and regional lymph-node dissection of the neck is recommended in most cases because 50 percent of cases with no regional metastases at the time of diagnosis will go on to develop nodal disease.8 Radiotherapy and targeted molecular therapy, such as c-KIT inhibitors, may prove successful in some cases,1,8 even though OMMs remain extremely difficult to treat; this is due to the multiple interactions among several molecular and oncogenic pathways involved in the OMM pathogenesis.8,18
Conclusion
Amelanotic OMM is a very rare variant of melanoma that is historically associated with a poor prognosis due to the delay in the diagnosis as well as a later stage at the time of diagnosis. Although c-KIT mutations occur more frequently in mucosal melanomas than in cutaneous melanoma, multiple genes are likely responsible for the pathogenesis of OMMs. As a result, systemic therapies for mucosal melanoma targeting multiple genetic pathways need to be thoroughly investigated in an attempt to improve survival outcomes for this devastating disease.
References
- Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: a comprehensive review. Int J Clin Exp Pathol. 2012;5(8):739–753.
- Thomas N, Kricker A, Waxweiler W, et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol. 2014;150(12): 1306–1314.
- McClain S, Mayo K, Shada A, et al. Amelanotic melanomas presenting as red skin lesions: a diagnostic challenge with potentially lethal consequences. Int J Dermatol. 2012;51(4): 420–426.
- Huvos A, Shah J, Goldsmith H. A clinicopathologic study of amelanotic melanoma. Surg Gynecol Obstet. 1972;135(6):917–920.
- Ariel I. Amelanotic melanomas: an analysis of 77 patients. Curr Surg. 1981;38(3):151–155.
- Wee E, Wolfe R, Mclean C, Kelly J, Pan Y. Clinically amelanotic or hypomelanotic melanoma: anatomic distribution, risk factors, and survival. J Am Acad Dermatol. 2018;79(4):645–651.
- Lin M, Mar V, McLean C, et al. Diagnostic accuracy of malignant melanoma according to subtype. Australias J Dermatol. 2014;55(1): 35–42.
- Feller L, Khammissa R, Lemmer J. A review of the aetiopathogenesis and clinical and histopathological features of oral mucosal melanoma. ScientificWorldJournal. 2017;2017: 9189812.
- De Paulo L, Servato J, Rosa R, et al. Primary amelanotic mucosal melanoma of the oronasal region: report of two new cases and literature review. Oral Maxillofac Surg. 2015;19(4): 333–339.
- Uratani A, Cruz Perez D, Vargas P, et sl. Oral melanoma: review of the literature. Braz J Oral Sci. 2004;3(9):428–432.
- Kubica A, Brewer J. Melanoma in immunocompromised patients. Mayo Clin Proc. 2012;87(10):991–1003.
- Chen F, Zhang Q, Wang Y, et al. KIT, NRAS, BRAF and FMNL2 mutations in oral mucosal melanoma and a systematic review of the literature. Oncol Lett. 2018;15(6):9786–9792.
- Beadling C, Jacobson-Dunlop E, Hodi F, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res. 2008;14(21):6821–6828.
- Deyhimi P, Razavi S, Shahnaseri S, et al. Rare and extensive malignant melanoma of the oral cavity: report of two cases. J Dent (Shiraz). 2017;18(3):227–233.
- Smith M, Bhattacharyya I, Cohen D, et al. Melanoma of the oral cavity: an analysis of 46 new cases with emphasis on clinical and histopathologic characteristics. Head Neck Pathol. 2016;10(3):298–305.
- Williams M. Update from the 4th edition of the World Health Organization classification of head and neck tumours: mucosal melanomas. Head and Neck Pathol. 2017;11(1):110–117.
- Topic B, Mašic T, Radovic S, et al. Primary oral mucosal melanomas—two case reports and comprehensive literature review. Acta Clin Croat. 2017;56(2):323–330.
- Buery R, Siar C, Katase N, et al. NRAS and BRAF mutation frequency in primary oral mucosal melanoma. Oncol Rep. 26(4):783–787.

