 J Clin Aesthet Dermatol. 2023;16(6):37–43.
J Clin Aesthet Dermatol. 2023;16(6):37–43.
by Doungkamol Siri-archawawat, MD and Weeratian Tawanwongsri, MD
Dr. Siri-Archawawat is with the Division of Neurology, Department of Internal Medicine, and School of Medicine at Walailak University in Nakhon Si Thammarat, Thailand. Dr. Tawanwongsri is with the Division of Dermatology, Department of Internal Medicine, and School of Medicine at Walailak University in Nakhon Si Thammarat, Thailand.
FUNDING: This study was funded by Walailak University in Thailand (WU-IRG-65-009).
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. The axilla is the most common site for primary hyperhidrosis (HH) affecting quality of life. No consensus on the optimal doses of botulinum toxin (BTX) has been established.
Objective. this study aimed to scrutinize the effectiveness of 25- and 50-U onabotulinumtoxinA in patients with moderate-to-intolerable primary axillary HH as well as pain scores after BTX injection.
Methods. A single-blinded, side-by-side randomized trial was conducted between January and June 2022. Participants were randomly treated with 25-unit (U) onabotulinumtoxinA in one axilla and 50-U onabotulinumtoxinA in the other. The Minor starch-iodine test and gravimetric testing, the Hyperhidrosis Disease Severity Scale (HDSS), Hyperhidrosis Quality of Life Index (HidroQoL), global self-assessment scale (GSAS), and satisfaction scores were collected and analyzed.
Results. A total of 12 participants were included in the final analysis; six (50.0%) were female. The median age was 30.3 (interquartile range: 28.7–32.3) years. No statistically significant differences were noted in the sweat rate production, hyperhidrotic area, HDSS, HidroQoL, GSAS, and satisfaction scores between 25- and 50-U BTX at any follow-up visit. No significant difference was noted in pain scores between the two groups (p=0.810).
Conclusion. Low-dose onabotulinumtoxinA is associated with similar efficacy and safety outcomes in primary axillary HH treatment as is conventional-dose onabotulinumtoxinA. No difference was noted in injection site pain between the two groups.
Keywords. Axillary hyperhidrosis, OnabotulinumtoxinA, Low-dose, Effectiveness
Hyperhidrosis (HH) is a chronic disorder characterized by excessive sweating beyond that required for thermal homeostasis.1,2 The prevalence of the disorder is 0.6 to 1.0 percent in the general population; it is more prevalent in tropical and subtropical regions.3,4 Although it does not affect life expectancy, this embarrassing condition negatively impacts daily life activities, psychosocial life domains, and quality of life.5,6 HH is classified as a primary or a secondary disorder. The vast majority (93.3%) of HH cases are due to unknown causes and are referred to as primary HH, which is characterized by a focal and symmetric distribution of excessive sweat production.7 Commonly affected sites include the axillae (65%), face (42%), and palms (40%).8 Primary HH is typically diagnosed based on medical history and physical examinations.9 Further investigation is not mandatory unless secondary comorbidities are suspected.10 The pathophysiology of primary HH has been proposed to include autonomic nervous system dysfunction, involving both sympathetic and parasympathetic systems, and aberrant central control of emotions.11
There are several treatment options for primary focal HH, including topical and systemic medications, intradermal injections, and invasive surgery.12 With a step-by-step approach, when topical therapy failures occur, intradermal injection of botulinum toxin (BTX) is considered the second-line therapy.11,13 BTX is a natural protein produced by the anaerobic gram-positive spore-forming rod Clostridium botulinum.14 It inhibits docking, presynaptic exocytosis, and cholinergic transmission to postganglionic receptors that innervate eccrine sweat glands by cleaving the soluble N-ethylmaleimide-sensitive factor attachment protein receptor.15,16 In addition to inhibiting neurotransmitter release, it reduces sweat gland responsiveness to acetylcholine.17 Its effect is temporary and reversible following the sprouting of transitory nerves and development of new synaptic junctions.18,19 Repeated BTX injections at 4- to 6-month intervals may, therefore, be needed to maintain the therapeutic effect. Among different BTX formulations, onabotulinumtoxinA is the only formulation approved by the United States Food and Drug Administration (FDA) for the management of adult patients with severe axillary primary HH.20 However, other formulations, including abobotulinumtoxinA, incobotulinumtoxinA, prabotulinumtoxinA, and rimabotulinumtoxinB, have been widely used with varying effectiveness. A consensus on the optimal doses or dilutional protocols for BTX has not been well established, and every BTX has a non-parallel dose–response curve.21 Heckmann et al22,23 reported that doses of 100 and 200 units (U) of abobotulinumtoxinA were equally effective; the lower dose was thus cost-saving.22,23 Pain is one of the most common adverse effects that is detected in patients treated with BTX.24,25 Awaida et al26 demonstrated that treatment responses were not significantly different between patients who received BTX injection using a seven-point pattern and those with a twenty-point pattern. Although the volume per injection was larger in the severe injection group, patients experienced lower pain scores. Likewise, at lower BTX concentrations, a higher injection volume results in greater diffusion, which covers a larger affected area.27 A 0.25-mL dose volume with a 2 U/0.1mL concentration affected the target area with the mean area of effect of 6.05 cm2.28 However, the enzyme units of different BTX preparations are not interchangeable.29–31 The switch of onabotulinumtoxinA and abobotulinumtoxinA was within the wide range of conversion of 1 U of onabotulinumtoxinA, equivalent to 2–5 U of abobotulinumtoxinA.11 To our knowledge, studies focusing on the effectiveness of low-dose onabotulinumtoxinA using seven-point pattern intradermal injections in patients with moderate-to-intolerable primary axillary HH are scarce. The outcomes of the intervention may also prove to be cost-saving, particularly in patients who require frequent BTX injections.
Following a team discussion, the following research questions (RQs) were developed: What is the effectiveness of low-dose onabotulinumtoxinA using seven-point pattern intradermal injections in patients with moderate-to-intolerable primary axillary HH? How do participants rate the level of pain in low-dose seven-point onabotulinumtoxinA injection compared with conventional-dose twenty-point onabotulinumtoxinA injection? Therefore, this study aimed to scrutinize the effectiveness of 25- and 50-U onabotulinumtoxinA in patients with moderate-to-intolerable primary axillary HH as well as pain scores after BTX injection.
Methods
Participants. This study was conducted at the Walailak University (Nakhon Si Thammarat, Thailand) between January and June 2022. All participants were screened for medical history, physical examination, and investigations, including complete blood count, thyroid function test, hemoglobin A1C, upright plain chest radiograph, and urine pregnancy test (if applicable). Adult participants, aged 18 years and older, were selected according to the following criteria: excessive sweating for at least 6-month duration without identified causes, along with at least two of the following traits: (1) bilateral and relatively symmetric distribution; (2) occurring at least once a week; (3) impaired daily activities; (4) onset before the age of 25 years; (5) positive family history of primary focal HH; or (6) cessation of sweating during sleep.32 The following were the exclusion criteria: (1) known secondary causes, including neuromuscular disease, hyperthyroidism, intake of drugs affecting muscle tone or the autonomic nervous system, pregnancy, or presence of malignancy; (2) contraindication for BTX injection, including a history of keloid scars, neuromuscular disorders, BTX allergies, body dysmorphic disorder, or amyotrophic lateralizing sclerosis myopathies; (3) concomitant therapy for HH; (4) previously treated with BTX injection within 12 months of enrolment; or (5) currently taking aspirin, nonsteroidal anti-inflammatory drugs, or vitamin E. The following were the four visits scheduled during the study period: Visit 1 (Week 0), Visit 2 (Week 2), Visit 3 (Week 4), and Visit 4 (Week 12).
Measurement of sweat production. The patient was advised to discontinue antiperspirants for a week before the evaluation. A gravimetric analysis was performed before treatment and at every subsequent visit (weeks 2, 4, and 12). Measurements were performed after 10 minutes of rest at room temperature (23–25°C). A 10-centimeter-diameter filter paper (Hyundai Micro, Seoul, Korea) was weighed on high-accuracy laboratory scales (Mettler Toledo, Columbus, OH, USA), and the weight was recorded. After placing in each axilla of the participants for one minute, the paper was reweighed. Participants with axillary sweat production rates of more than 50mg/min were selected and further investigated.
The Minor iodine-starch test was subsequently used to identify the involved areas and the residual areas of HH for the first time and during the follow-up visits (Weeks 2 and 12). A 2% iodine solution was subsequently applied to the axillae. After allowing them to dry thoroughly, we dusted the skin with a thin film of starch–corn flour (Unilever Thai Holding, Chachoengsao, Thailand). The participants waited for 15 minutes in a hot room (30°C).33 The borders of the affected areas in dark blue color were marked and photographed. The level of the Minor iodine-starch test positivity was evaluated by a blinded investigator (DS). Each axilla was divided into four quadrants that were scored 0–3 per area, providing a possible total score of 0–12.34 Additionally, this test positivity was measured using the pixel intensity threshold function in ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Study design and randomization. A prospective, single-blinded, side-by-side randomized trial was conducted. BTX (Botox, Allergan, Ireland) was diluted with 5mL sodium chloride to get a final concentration of 20U/mL. Using a randomization computer program (Microsoft Office Excel 2019), the participants were treated with 25U in one axilla and 50U in the other. Each axilla was injected intradermally in a seven-point pattern.26 The required dose for each axilla was divided equally for each injected point, spaced evenly 2 to 2.5cm apart.28 No local anesthesia was administered before or after the procedure. A 34-gauge needle was inserted with the bevel up, positioned parallel to the skin before delivering the required dose to produce a small bleb.
The sample size calculation was based on a previous study,35 with an alpha of 0.05 and a power of 80 percent. The total sample size was 12 adults. Accounting for a potential loss to follow-up of 20 percent, the sample size calculation required approximately 14 participants to be recruited for the study. This clinical trial was registered in the Thai Clinical Trials Registry (TCTR20220112005). This prospective study was approved by the Walailak Ethics Committee (WUEC-22-017-01). The ethics committee took into account and complied with the laws of Thailand, including the Personal Data Protection Act. All data files and sensitive personal information were encrypted, password-protected, and saved to a secure computer that was only accessible to the study coordinators to ensure confidentiality. Participants could access their own data by directly contacting study coordinators. No information that could link an individual to the data was revealed. After study completion, all personal data were deleted.
Questionnaire. After the procedure, pain was evaluated using a visual analog scale ranging from 0 (none) to 10 (unbearable). The Hyperhidrosis Disease Severity Scale (HDSS) was used to measure disease severity at baseline and at every subsequent visit. Severity was reported on a four-point scale ranging from 1 (mild) to 4 (intolerable). The questionnaire on the Hyperhidrosis Quality of Life Index (HidroQoL)36,37 included the following two domains: daily life activity (six questions) and psychosocial life (twelve questions). Each item was rated on a three-point scale, and the overall score was subsequently calculated. It was completed at baseline, Visit 2 (Week 2), and Visit 4 (Week 12). Moreover, participants were provided with a questionnaire that included a satisfaction scale ranging from 0 (terrible) to 10 (delighted) and a global self-assessment scale (GSAS) (0=no effect at all, 1=slight but insufficient reduction of sweating, 2=moderate but insufficient reduction of sweating, 3=marked reduction with acceptable residual sweating, and 5=sweating disappeared completely) at Visit 2 (Week 2) and Visit 4 (Week 12). Adverse effects were assessed at each follow-up.
Statistical analysis. Continuous data are presented as means and standard deviations (SDs) or medians and ranges. Categorical data are presented as frequencies and percentages. The paired t-test or Mann–Whitney Wilcoxon test was used to compare the sweat rate, sweating area evaluated by the Minor starch-iodine test, HidroQoL, and satisfaction scores after 25- and 50-U BTX injections. Pearson’s chi-square test was used to test for significant relationships between categorical variables. Two-tailed tests were considered statistically significant at a p-value of <0.05. A statistical analysis was performed using the SPSS software version 18 (SPSS Inc., Chicago, IL, USA).
Results
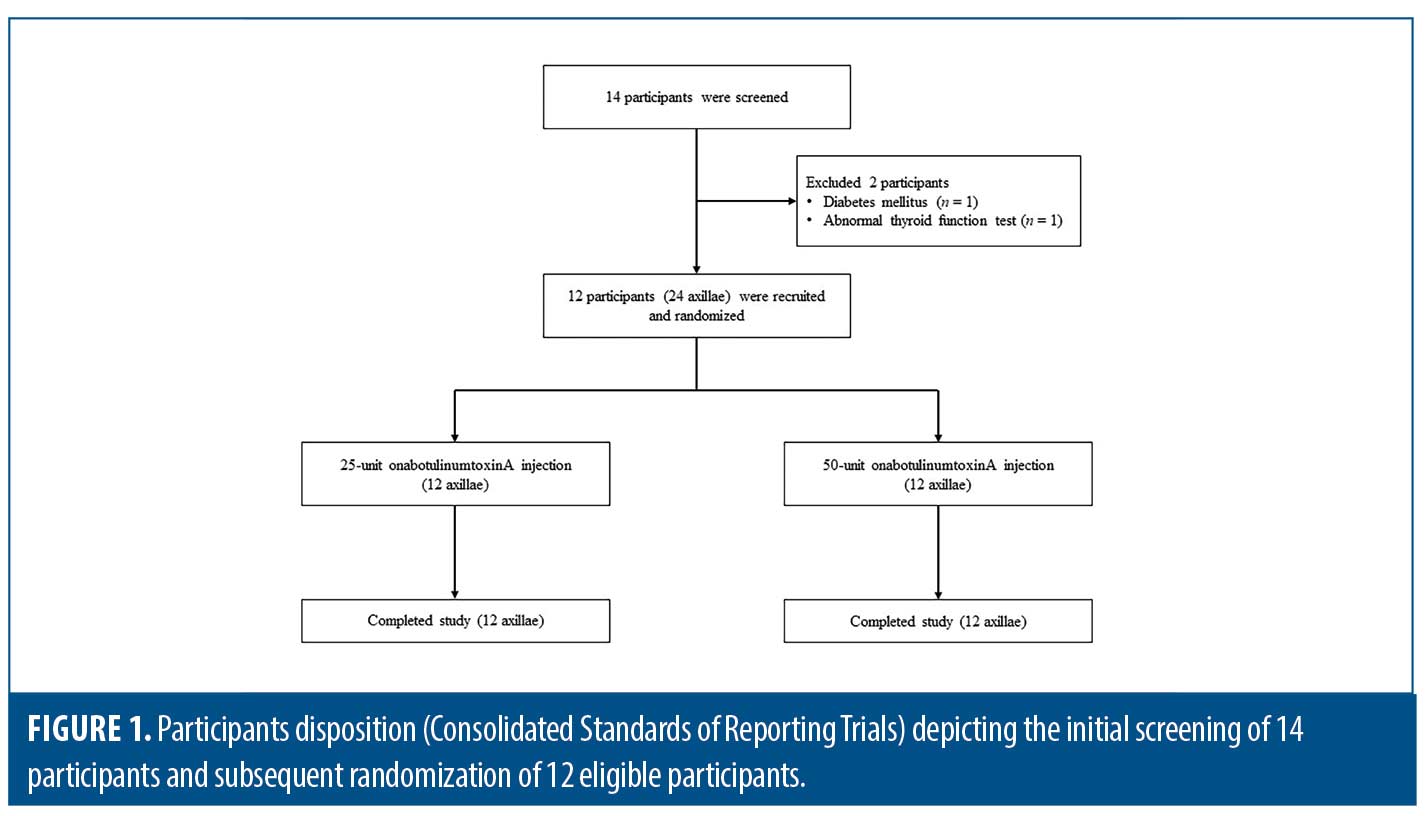
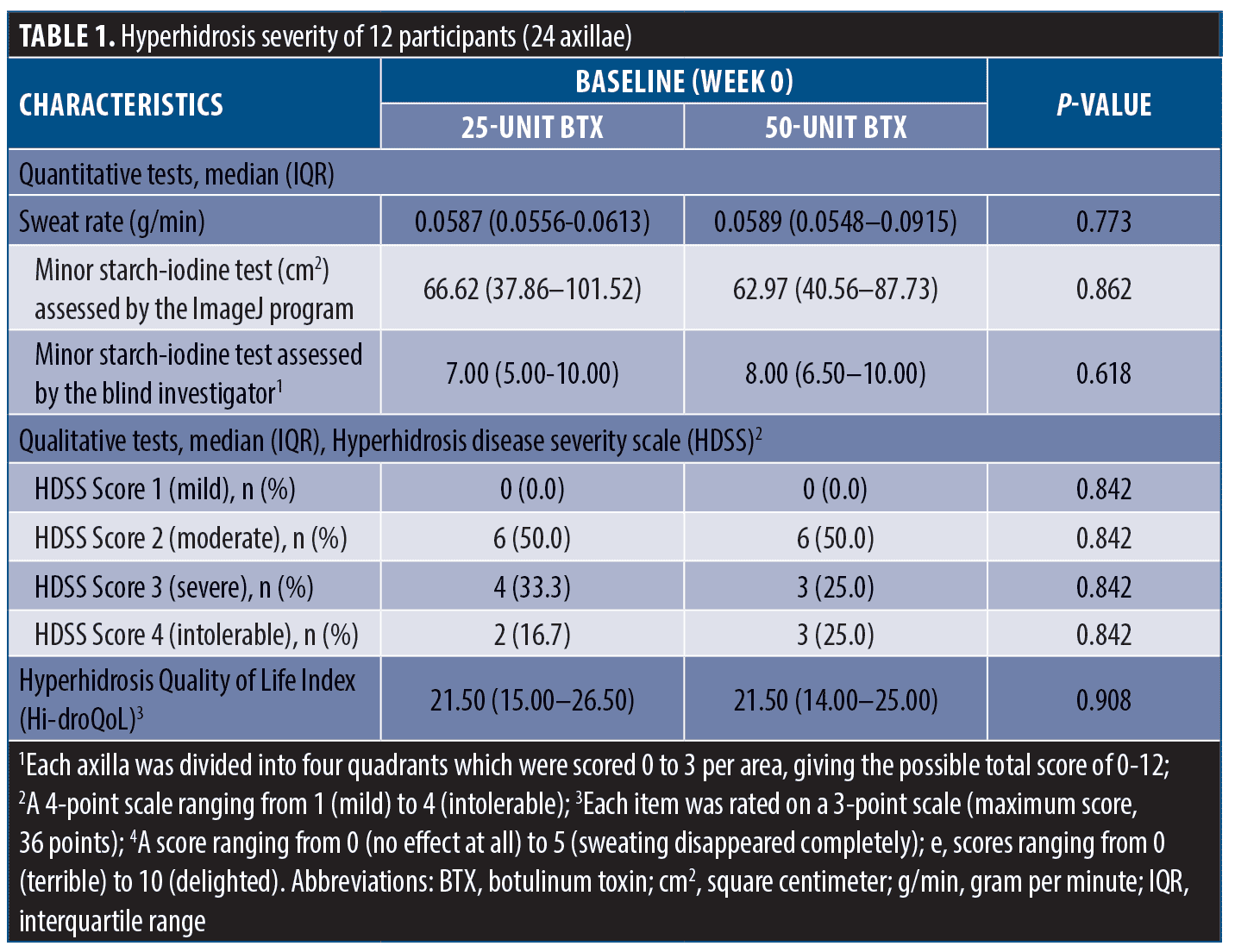
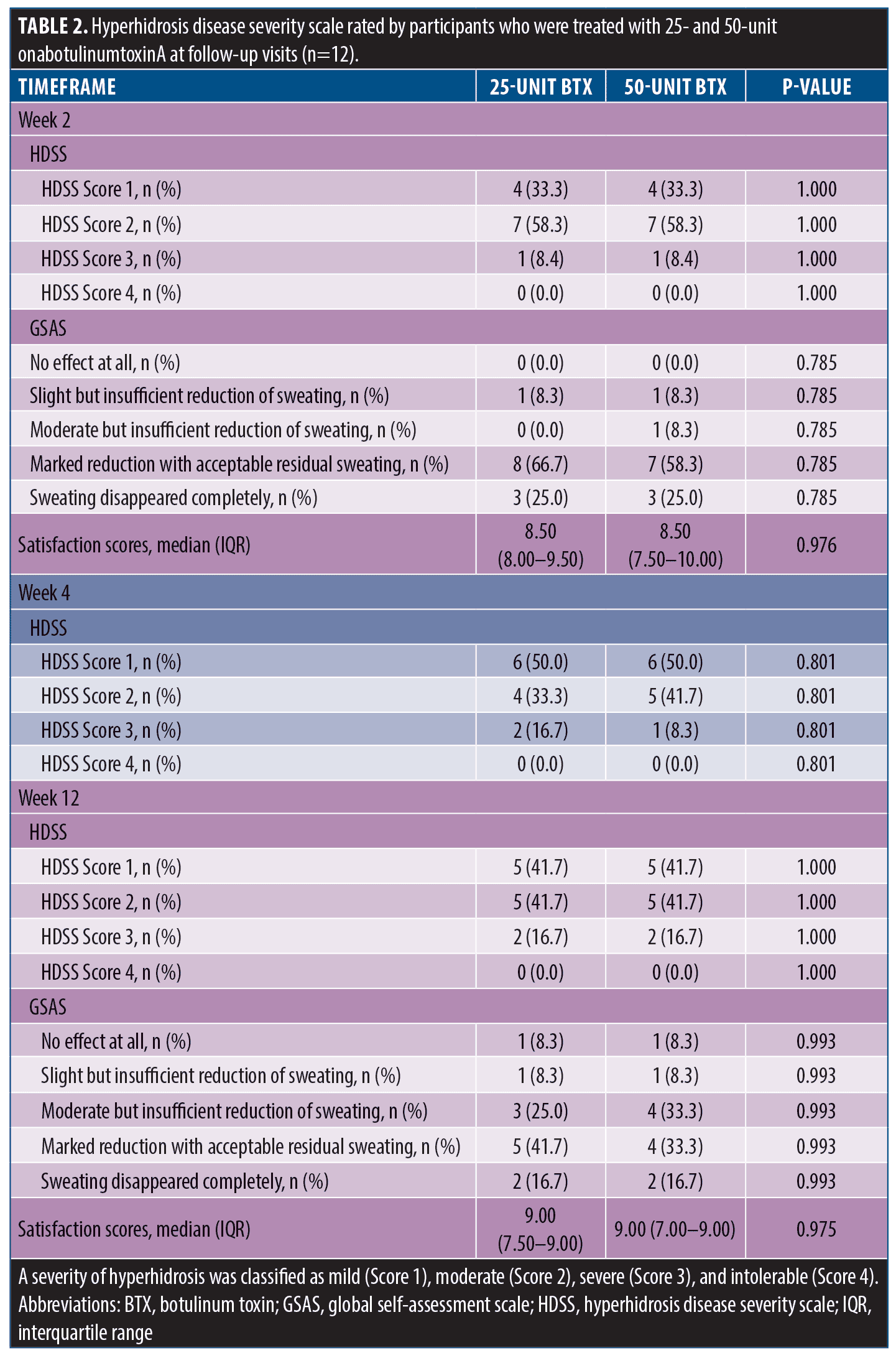
A total of 14 participants were enrolled in the study, and two were excluded owing to diabetes mellitus (n=1) and abnormal thyroid function test results (n=1). The participants’ disposition is shown in Figure 1. The remaining 12 participants were included in the statistical analysis; six (50.0%) were female. The median age and body mass index were 30.3 (interquartile range [IQR]: 28.7–32.3) years and 22.4 (IQR: 22.0–23.1) kg/m2, respectively. Participants had Fitzpatrick Skin Types III (n=10, 83.3%) and IV (n=2, 16.7%). Half of the participants had a history of BTX treatment in the facial areas within six months. The participants were randomly treated with 25U in one axilla and 50U in the other; the HH severity at baseline is detailed in Table 1. Both quantitative and qualitative parameters regarding HH severity significantly improved at Week 2, and treatment outcomes were maintained through Week 12. No statistically significant differences were noted in the sweat rate production, hyperhidrotic area evaluated using the Minor starch-iodine test and ImageJ program, hyperhidrotic area evaluated using the Minor starch-iodine test and blinded investigator, HDSS, HidroQoL, GSAS, and satisfaction scores between 25- and 50-U BTX at any follow-up visit (Figure 2 and Table 2).
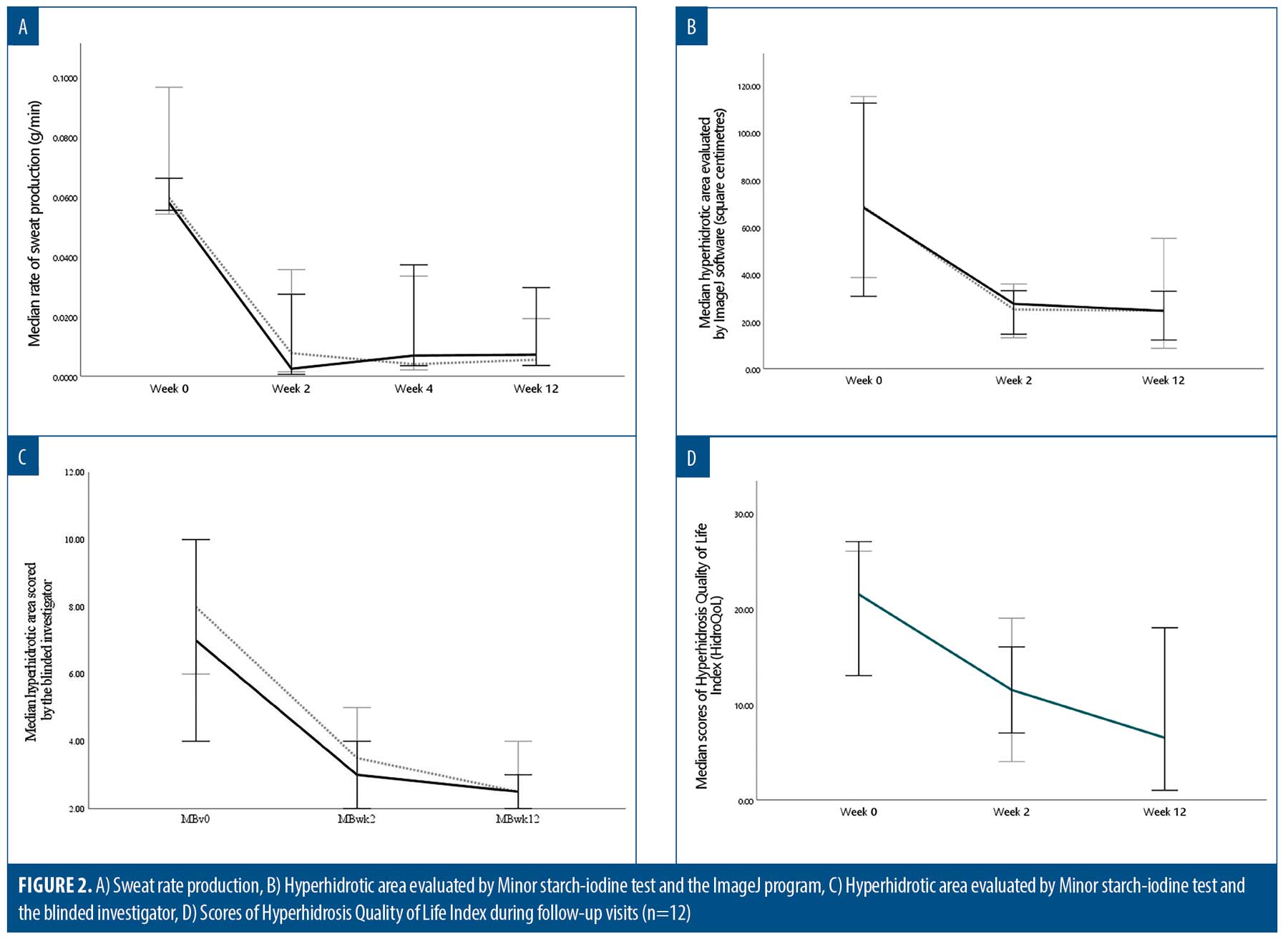
During the follow-up visits, no AE were reported in participants treated with 25- and 50-U BTX, except for injection site pain. The mean pain scores for 25- and 50-U BTX were 3.96 (SD: 1.94) and 4.67 (SD: 1.83), respectively. No significant difference was noted in pain scores between the two groups (p=0.810).
Discussion
In our study, we aimed to assess the effectiveness of conventional and low-dose BTX using seven-point pattern injections in patients with moderate-to-intolerable primary axillary HH, as well as pain scores after injection. Both quantitative and qualitative tests were used to evaluate the severity of the HH. Quantitative sweat production tests included the Minor starch-iodine test and gravimetric testing. Quantitative methods included the HDSS, HidroQoL, GSAS, and satisfaction surveys. Quantitative evaluation revealed that BTX injection significantly alleviated HH at Week 2, and treatment effects were maintained at the 3-month follow-up. No statistically significant differences in HH severity, evaluated using quantitative methods, were noted between the groups at any follow-up time point. Using qualitative evaluation, we found that BTX injection significantly decreased HDSS and improved HidroQoL scores at Week 2, and the treatment effects were maintained at the 3-month follow-up. No statistically significant differences in HH severity, evaluated using qualitative methods, were noted between the groups at any follow-up time point. Furthermore, most participants showed at least moderate improvement with high satisfaction scores at Week 2, and the response was maintained up to Week 12. In addition, no statistically significant differences in HH severity, evaluated using qualitative methods, were noted between the groups at any follow-up time point. Participants in the 50-U BTX injection group reported higher, although non-significant, median pain scores than those in the 25-U BTX injection group. To our knowledge, this study is the first to demonstrate the effectiveness of low-dose onabotulinumtoxinA injection using a seven-point pattern on HH compared with the conventional dose onabotulinumtoxinA, which is the only FDA-approved drug for primary axillary HH.
BTX inhibits sweating via action at autonomic cholinergic nerve terminals.38,39 After BTX injection, the luminal area decreases, and denervation of the sweat glands is noted.40 Since neither a standard dose nor protocol for BTX injection for HH has been well established, lower BTX doses with acceptable effectiveness remain largely under investigation. With an optimal dose, the treatment was proposed to be cost-saving and associated with a low rate of adverse reactions. Beyond this financial issue, lower doses per treatment and overall doses were less associated with an increase in neutralizing antibodies.41 This immunogenic cause was the plausible reason for BTX treatment failure.42,43 Heckmann et al. demonstrated that abobotulinumtoxinA doses of 100- and 200U were equally effective.22, 23 Our findings are consistent with those of a previous study. Low-dose onabotulinumtoxinA injection resulted in positive quantitative outcomes and favorable qualitative outcomes. According to the differences in eccrine sweat gland densities among ethnicities and severity of disease, participants with a larger axillary area, a higher density of sweat glands, or more severe disease may need higher BTX doses or more injection sites to achieve targeted outcomes.44,45 Factors that play a role in the diffusion of BTX include injection volume, dose, concentration, and injection method.27 A 0.25mL dose volume with a 2 U/0.1mL concentration affected the target area with the mean area of effect of 6.05 cm2.28 Intradermal injections at the dermal–subcutaneous junction, approximately 2mm deep, are considered ideal for the treatment of axillary areas. Unwanted denervation and poor responses may occur when injections are administered too deeply.46 At Week 12 follow-up, low severity of axillary HH, evaluated using both quantitative and qualitative methods, despite a slight increase from the lowest point at Week 2 follow-up, was noted in our study. The therapeutic effect is temporary, and repeated treatment at regular intervals is required to maintain low HDSS scores. The duration of the treatment is variable and depends on the individual; the duration of the effect ranges from 3 to 10 months.47
There is no single best measurement to evaluate HH severity. In addition, the currently used methods showed weak to moderate correlations.36 The correlation coefficients of the HidroQoL with the HDSS and gravimetric sweat production were 0.45 and 0.08, respectively. The correlation coefficient of the HDSS with gravimetric sweat production was 0.15. In our study, we used multiple methods for a detailed analysis of HH severity. However, both the quantitative and qualitative measurements provided concordant results.
Adverse reactions after BTX injection include pain, hematomas, bruises, headache, myalgia, localized pruritus, urticaria, and compensatory sweating.24,25 We observed no adverse reactions except pain at the injection sites, the most common complaint caused by injections. The participants in the 50-U BTX injection group with a higher volume per injection site reported higher, although non-significant, median pain scores than those in the 25-U BTX injection group with a higher volume per injection site. Consistent with our findings, a previous study showed that large-volume injections were rated more painful.48 A 34-gauge needle was used to minimize pain intensity. Therefore, the participants reported low pain intensity. However, microneedle use for facial injection of BTX in reducing pain remains controversial. There were no differences in pain between BTX injection with the 30-gauge needle and 32-gauge needle.49–51 In contrast, Fouché et al52 revealed that participants in BTX injection with a 33-gauge needle reported less painful than those in BTX injection with a 29-gauge needle. Further multi-center studies focusing on pain scores after BTX injection with different needle sizes should be conducted to confirm this hypothesis. Other techniques to reduce pain include needle-free anesthesia, cryoanalgesia, vibration analgesia, pocketed microneedles, topical anesthetics, lidocaine dilution, sedation, intravenous regional anesthesia, and nerve blocks.11 We propose that an optimized combination of these techniques, along with low-volume BTX injection, fewer injection sites, and small needle size, might be able to closely approach pain-free treatment.
The strength of this study is that this is the first to ascertain the effectiveness of low-dose onabotulinumtoxinA injection using a seven-point pattern injection for HH with low pain intensities. Nevertheless, several limitations of this study deserve further consideration. First, it was neither double-blind, placebo-controlled, nor randomized. However, the efficacy of onabotulinumtoxinA injection over placebo has already been well established in a previous study.53 Second, this was a single-center study conducted in Asia, where participants may have smaller body surface areas or treatment areas than those in Western countries.54 When the hyperhidrotic area is larger, up to 100U per axilla might be required.55 Third, the neutralizing antibody titers were not tested in this study. The incidence of neutralizing antibodies is 1.8%,56 and participants with these antibodies might be resistant to BTX, resulting in poor outcomes. However, other factors resulting in BTX treatment failure should be considered, including the reconstitution process, injection techniques, dosing, and product handling.57–59 Further prospective multicenter trials and BTX antibody screening should confirm our findings.
Conclusion
Based on the results of our study, we conclude that 25- and 50-U onabotulinumtoxinA doses are equally effective in the treatment of HH, resulting in sweating reduction, improved quality of life, and high satisfaction. In view of its cost-effectiveness and avoidance of adverse effects, 25-U onabotulinumtoxinA should be the preferred dose for treating axillary HH. Participants tended to experience lower pain intensity with a lower volume per injection site.
References
- Atkins JL, Butler PE. Hyperhidrosis: a review of current management. Plast Reconstr Surg. 2002;110(1):222–228.
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Etiology and clinical work-up. Journal of the American Academy of Dermatology. 2019;81(3):657–666.
- Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. Journal of the American Academy of Dermatology. 2004;51(2):241–248.
- Felini R, Demarchi AR, Fistarol ED, et al. Prevalence of hyperhidrosis in the adult population of Blumenau-SC, Brazil. Anais brasileiros de dermatologia. 2009;84(4):361–366.
- Kamudoni P, Mueller B, Halford J, et al. The impact of hyperhidrosis on patients’ daily life and quality of life: a qualitative investigation. Health and quality of life outcomes. 2017;15(1):1–10.
- Solish N, Benohanian A, Jonathan WK, Hyperhidrosis CDSGoHRQoLiPA. Prospective open‐label study of botulinum toxin type A in patients with axillary hyperhidrosis: effects on functional impairment and quality of life. Dermatologic Surgery. 2005;31(4):405–413.
- Walling HW. Clinical differentiation of primary from secondary hyperhidrosis. Journal of the American Academy of Dermatology. 2011;64(4):690–695.
- Doolittle J, Walker P, Mills T, et al. Hyperhidrosis: an update on prevalence and severity in the United States. Arch Dermatol Res. 2016;308(10):743–749.
- Cohen JL, Cohen G, Solish N, et al. Diagnosis, Impact, and Management of Focal Hyperhidrosis: Treatment Review Including Botulinum Toxin Therapy. Facial Plastic Surgery Clinics of North America. 2007;15(1):17–30.
- Solish N, Wang R, Murray CA. Evaluating the patient presenting with hyperhidrosis. Thoracic surgery clinics. 2008;18(2):133–140.
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Therapeutic options. Journal of the American Academy of Dermatology. 2019;81(3):669–680.
- Stashak A-B, Brewer JD. Management of hyperhidrosis. Clin Cosmet Investig Dermatol. 2014;7:285–299.
- Hoorens I, Ongenae K. Primary focal hyperhidrosis: current treatment options and a step-by-step approach. Journal of the European Academy of Dermatology and Venereology. 2012;26(1):1–8.
- Shukla HD, Sharma S. Clostridium botulinum: a bug with beauty and weapon. Critical reviews in microbiology. 2005;31(1):11–18.
- Pantano S, Montecucco C. The blockade of the neurotransmitter release apparatus by botulinum neurotoxins. Cellular and molecular life sciences. 2014;71(5):793–811.
- Breidenbach MA, Brunger AT. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature. 2004;432(7019):925–929.
- Shibasaki M, Davis S, Cui J, et al. Botulinum toxin abolishes sweating via impaired sweat gland responsiveness to exogenous acetylcholine. British Journal of Dermatology. 2009;161(4):757–761.
- Meunier FA, Schiavo G, Molgó J. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. Journal of Physiology-Paris. 2002;96(1-2):105–113.
- Harrison AR, Berbos Z, Zaldivar RA, et al. Modulating Neuromuscular Junction Density Changes in Botulinum Toxin–Treated Orbicularis Oculi Muscle. Investigative Ophthalmology & Visual Science. 2011;52(2):982–986.
- Arora G, Kassir M, Patil A, et al. Treatment of Axillary hyperhidrosis. Journal of Cosmetic Dermatology. 2022;21(1):62–70.
- Giordano CN, Matarasso SL, Ozog DM. Injectable and topical neurotoxins in dermatology: Indications, adverse events, and controversies. Journal of the American Academy of Dermatology. 2017;76(6):1027–1042.
- Heckmann M, Ceballos-Baumann AO, Plewig G. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488–493.
- Heckmann M, Plewig G. Low-dose efficacy of botulinum toxin A for axillary hyperhidrosis: a randomized, side-by-side, open-label study. Arch Dermatol. 2005;141(10):1255–1259.
- Coté TR, Mohan AK, Polder JA, et al. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. Journal of the American Academy of Dermatology. 2005;53(3):407–415.
- Naumann M, Jankovic J. Safety of botulinum toxin type A: a systematic review and meta-analysis. Current medical research and opinion. 2004;20(7):981–990.
- Awaida CJ, Rayess YA, Jabbour SF, et al. Reduction of Injection Site Pain in the Treatment of Axillary Hyperhidrosis With Botulinum Toxin: A Randomized, Side-by-Side, Comparative Study of Two Injection Patterns. Dermatologic surgery: official publication for American Society for Dermatologic Surgery. 2021;47(1):154–157.
- Brodsky MA, Swope DM, Grimes D. Diffusion of botulinum toxins. Tremor Other Hyperkinet Mov (N Y). 2012;2.
- Hsu TS, Dover JS, Arndt KA. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch Dermatol. 2004;140(11):1351–1354.
- Rosales RL, Bigalke H, Dressler D. Pharmacology of botulinum toxin: differences between type A preparations. European Journal of Neurology. 2006;13:2–10.
- Scaglione F. Conversion ratio between Botox®, Dysport®, and Xeomin® in clinical practice. Toxins. 2016;8(3):65.
- Karsai S, Raulin C. Botox and Dysport: is there a dose conversion ratio in dermatology and aesthetic medicine? Journal of the American Academy of Dermatology. 2010;62(2):346–347.
- Hornberger J, Grimes K, Naumann M, et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. Journal of the American Academy of Dermatology. 2004;51(2):274–286.
- Hexsel D, Rodrigues TC, Soirefmann M, et al. Recommendations for performing and evaluating the results of the minor test according to a sweating intensity visual scale. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al]. 2010;36(1):120–122.
- Skroza N, Bernardini N, La Torre G, et al. Correlation between Dermatology Life Quality Index and Minor test and differences in their levels over time in patients with axillary hyperhidrosis treated with botulinum toxin type A. Acta dermatovenerologica Croatica: ADC. 2011;19(1):16–20.
- Ibrahim O, Kakar R, Bolotin D, et al. The comparative effectiveness of suction-curettage and onabotulinumtoxin-A injections for the treatment of primary focal axillary hyperhidrosis: a randomized control trial. J Am Acad Dermatol. 2013;69(1):88–95.
- Gabes M, Jourdan C, Schramm K, et al. Hyperhidrosis Quality of Life Index (HidroQoL©): further validation and clinical application in patients with axillary hyperhidrosis using data from a phase III randomized controlled trial. Br J Dermatol. 2021;184(3):473–481.
- Kamudoni P, Mueller B, Salek MS. The development and validation of a disease-specific quality of life measure in hyperhidrosis: the Hyperhidrosis Quality of Life Index (HidroQOL©). Qual Life Res. 2015;24(4):1017–1027.
- Nawrocki S, Cha J. Botulinum toxin: Pharmacology and injectable administration for the treatment of primary hyperhidrosis. J Am Acad Dermatol. 2020;82(4):969–979.
- Dressler D, Saberi FA. Botulinum toxin: mechanisms of action. European neurology. 2005;53(1):3–9.
- Swartling C, Naver H, Pihl-Lundin I, et al. Sweat gland morphology and periglandular innervation in essential palmar hyperhidrosis before and after treatment with intradermal botulinum toxin. J Am Acad Dermatol. 2004;51(5):739–745.
- Albrecht P, Jansen A, Lee J-I, et al. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology. 2019;92(1):e48–e54.
- Bellows S, Jankovic J. Immunogenicity associated with botulinum toxin treatment. Toxins. 2019;11(9):491.
- Fabbri M, Leodori G, Fernandes RM, et al. Neutralizing antibody and botulinum toxin therapy: a systematic review and meta-analysis. Neurotoxicity research. 2016;29(1):105–117.
- Glaser DA, Hebert AA, Pariser DM, et al. Palmar and plantar hyperhidrosis: best practice recommendations and special considerations. CUTIS-NEW YORK-. 2007;79(5):18.
- Harker M. Psychological sweating: a systematic review focused on aetiology and cutaneous response. Skin pharmacology and physiology. 2013;26(2):92–100.
- Carruthers J. Botulinum Toxin: Procedures in Cosmetic Dermatology: Elsevier; 2018.
- Galadari H, Galadari I, Smit R, et al. Treatment approaches and outcomes associated with the use of abobotulinumtoxinA for the treatment of hyperhidrosis: A systematic review. Journal of the American Academy of Dermatology. 2021;85(5):1121–1129.
- Zijlstra E, Jahnke J, Fischer A, et al. Impact of Injection Speed, Volume, and Site on Pain Sensation. Journal of diabetes science and technology. 2018;12(1):163–168.
- Yomtoob DE, Dewan MA, Lee MS, et al. Comparison of pain scores with 30-gauge and 32-gauge needles for periocular botulinum toxin type a injections. Ophthalmic Plastic & Reconstructive Surgery. 2009;25(5):376–377.
- Price KM, Williams ZY, Woodward JA. Needle preference in patients receiving cosmetic botulinum toxin type A. Dermatologic surgery. 2010;36(1):109–112.
- Alam M, Geisler A, Sadhwani D, et al. Effect of needle size on pain perception in patients treated with botulinum toxin type A injections: a randomized clinical trial. JAMA dermatology. 2015;151(11):1194–1199.
- Fouché JJ, Van Loghem JAJ, Thuis J, et al. Left/right pain asymmetry with injectable cosmetic treatments for the face. Aesthetic Surgery Journal. 2017;37(6):708–714.
- Naver H, Swartling C, Aquilonius SM. Palmar and axillary hyperhidrosis treated with botulinum toxin: one-year clinical follow-up. Eur J Neurol. 2000;7(1):55–62.
- Kouno T, Katsumata N, Mukai H, et al. Standardization of the body surface area (BSA) formula to calculate the dose of anticancer agents in Japan. Japanese Journal of Clinical Oncology. 2003;33(6):309–313.
- Emer JJ, Axibal E, Marmur ES, et al. OnabotulinumtoxinA (Botox®) in dermatology. Botulinum Toxins: Cosmetic and Clinical Applications. 2017:357–368.
- Rahman E, Alhitmi HK, Mosahebi A. Immunogenicity to Botulinum Toxin Type A: A Systematic Review With Meta-Analysis Across Therapeutic Indications. Aesthetic Surgery Journal. 2021;42(1):106–120.
- Dressler D, Bigalke H. Reconstituting botulinum toxin drugs: shaking, stirring or what? Journal of Neural Transmission. 2016;123(5):523–525.
- Shtefan V, Fletcher J, Duclos OA. Causes of Botulinum Toxin Treatment Failure. Clin Cosmet Investig Dermatol. 2022;15:1045.
- Carr WW, Jain N, Sublett J. Immunogenicity of botulinum toxin formulations: potential therapeutic implications. Advances in Therapy. 2021;38(10):5046–5064.

