 J Clin Aesthet Dermatol. 2018;11(10):24–28
J Clin Aesthet Dermatol. 2018;11(10):24–28
by Thomas J. Stephens, PhD; Michael Babcock, MD; Vivian Bucay, MD; and Vincent Gotz, MS Pharm
Drs. Stephens and Babcock are with Thomas J. Stephens and Associates, Inc. in Colorado Springs, Colorado. Dr. Bucay is with the Bucay Center for Dermatology and Aesthetics in San Antonio, Texas. Mr. Gotz is with MDRejuvena, Inc. in Carlsbad, California.
FUNDING: This study was funded by Precision Dermatology.
DISCLOSURES: Mr. Gotz reports stock options with MDRejuvena, Inc. The other authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background.The gold standard for the treatment of hyperpigmentation is hydroquinone (HQ), which has been available as a skin lightener for more than 50 years. Numerous clinical studies have proven its efficacy in various topical formulations. In the United States, HQ is available as a nonprescription product in 2% formulations and as a 4% prescription product.
Objective.This study compared the safety and efficacy of a 2% hydroquinone multi-ingredient foam with a standard 4% hydroquinone cream on photodamaged facial skin.
Methods. A 12-week, investigator-blinded, randomized trial with a split-face design was conducted in women with moderate photodamaged facial skin.
Results. Both products improved the appearance of photodamaged facial skin and were well-tolerated. No statistically significant changes were seen between treatments during the efficacy or tolerability evaluations.
Conclusion. Both treatments (2% HQ Brighten and 4% HQ) improved the appearance of photodamaged facial skin and were well-tolerated and results well-perceived by subjects over the 12-week treatment period, compared with baseline grading scores.
KEYWORDS: Arbutin, foam delivery vehicle, hydroquinone, hyperpigmentation, kojic acid, niacinamide, photodamaged facial skin
Introduction
Hyperpigmentation of the skin typically results from injury, advancing age, or specific diseases. Photodamage resulting from exposure to sunlight is the most common cause of hyperpigmentation and is likely a postinflammatory response to ultraviolet damage to the skin.1,2 Photoaging of the skin is cumulative over time and depends on the intensity and degree of sun exposure, along with the amount of skin pigment.3–5
Pigmentary disorders can be distressing for the patient and result in lower quality of life.6,7 They can also be challenging for a clinician to treat. The gold standard for the treatment of hyperpigmentation is hydroquinone (HQ),8 which has been available as a skin lightener for more than 50 years. Numerous clinical studies have proven its efficacy in various topical formulations.9–12 In the United States, HQ is available as a nonprescription product in 2% formulations and as a 4% prescription product. Higher concentrations are sometimes prescribed and compounded extemporaneously.
Despite its proven efficacy in fading uneven skin tone and lightening the skin, HQ is not without controversy. In 2006, the United States Food and Drug Administration (FDA) proposed a ban on over-the-counter HQ, based on reports of exogenous ochronosis and malignancies in animals treated with large oral doses over extended periods of time.13 In response, manuscripts documenting the safety of and need for HQ were published by specialists.14–16
The investigator-blinded, randomized study with a split-face design presented here was conducted to assess the safety and efficacy of a nonprescription 2% HQ product formulated with multiple skin brightening ingredients in a unique foam delivery vehicle in comparison with the gold standard prescription strength 4% HQ cream in women with moderate facial photodamage.
Methods
The study was conducted in Colorado Springs, Colorado, from July 2012 to October 2012. Potential subjects (healthy women 35–65 years of age with abnormal facial pigmentation) were initially screened over the phone for eligibility. Subjects meeting the criteria were scheduled for a screening visit. Prospective subjects were instructed to remove all makeup at least 20 minutes prior to the screening visit. After signing informed consent and photography release forms, subjects were evaluated for Fitzpatrick skin type classification (Types II and III qualified); for presence of mottled pigmentation/discreet pigmented lesions of the face (score of 4–6 on Griffith’s scale17 [0=none, 1–3=mild, 4–6=moderate, and 7–9=severe]) and for moderate facial photodamage (scores of 4–6, where 0=none or minimal evidence of photodamage and 9=severe photodamaged skin).
Exclusion criteria included the following:
- Presence of melasma
- Use of hormone replacement therapies or hormones for birth control for less than 30 days prior to study initiation
- Use of nonprescription antiaging products within the previous 30 days
- Facial dermabrasion or chemical peel of the face within the previous three months
- Use of prescription facial treatments or light radiofrequency treatments within the previous six months
- Facial resurfacing or facelift in the previous 12 months
- Intense pulsed light therapy in the previous 24 months
- Preexisting dermatologic diseases of the face that could interfere with the study outcomes
Subjects applied the test materials—2% HQ brightening foam (2% HQ Brighten) (Vivatia Brighten 10; MDRejuvena, Inc., Carlsbad, California) and 4% HQ cream (OMP, Inc., Irvine, California)—on the assigned side of the face as directed according to a predetermined randomization. Subjects were instructed to apply a thin layer of the product to the assigned half of the face and neck from the middle to the outer surface in the morning and evening after cleansing. Subjects were instructed to thoroughly wash hands between applications of the two treatments. Ten minutes after applying the treatment products, a sun protection factor (SPF) 50 sunscreen was to be applied liberally to the treatment area every morning. Following baseline screening/evaluation, clinic evaluations were done at Weeks 4, 8, and 12. At each clinic visit, the subjects participated in efficacy evaluations, tolerance evaluations, digital photography, and self-assessment questionnaires.
Efficacy evaluations. The following parameters were assessed by an expert evaluator on the right and left sides of each subject’s face using Griffith’s 10-point scale:
- Overall photodamage (0=none, 9=severe)
- Mottled pigmentation (0=even skin color, 9=pronounced hyperpigmentation)
- Radiance (0=radiant, luminous, or glowing appearance; 9=sallow skin appearance)
- Visual smoothness (0= smooth, even-looking skin texture, 9=rough, uneven-looking skin texture)
- Tactile smoothness (0=smooth, even-feeling skin texture, 9=rough, uneven-feeling skin texture)
- Fine lines (0=none; 9=numerous, deep fine lines)
- Skin color (0=healthy skin color; 9=discolored appearance)
Tolerability evaluations. Local cutaneous tolerability was evaluated by assessing the signs and symptoms of objective and subjective irritation on the right and left sides of each subject’s face. Objective irritation (erythema, edema, dryness/scaling) was clinically graded by an expert evaluator using a four-point scale (0=none, 1=mild, 2=moderate, and 3=severe), whereas subjective irritation (burning, stinging, itching, dryness/tightness) was assessed by subjects on a four-point scale (0=none, 1=mild, 2=moderate, and 3=severe).
Imaging procedures. For all imaging procedures, subjects cleansed their face and removed all jewelry and makeup at least 20 minutes prior to the visit. Visible light and cross-polarized digital photographs (right, center, and left) were taken of each subject’s face to document changes in skin condition. All photography was performed using the Stephens and Associates photostation with a Nikon D200 digital SLR camera (Nikon Corporation, Tokyo, Japan).
Self-assessment questionnaires. Subjects completed a self-assessment questionnaire regarding efficacy, aesthetics, and other attributes.
Biostatistics
Wilcoxon signed-rank test with p?0.05 was utilized to evaluate mean changes from baseline for all clinical efficacy and tolerability parameters for each postbaseline visit. The null hypothesis was that the mean change from baseline would be zero. Comparisons between the treatments were made at each post-baseline time point for both efficacy and tolerability grading parameters. The null hypothesis, that there would be no difference between the two treatments, was tested using a Wilcoxon signed-rank test. For the self-assessment questionnaires completed by the subjects, a binomial test was performed. The null hypothesis here was that that the proportion of the combined designated favorable responses would be equal to the combined designated unfavorable responses for each question.
Results
Fifteen eligible women were enrolled in the study. One subject requested withdrawal and 14 subjects completed the study. Table 1 presents a summary of demographic information for the study population.
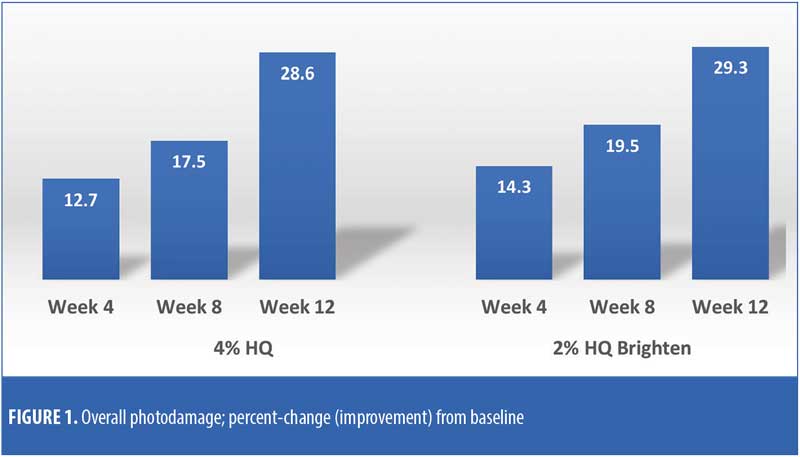
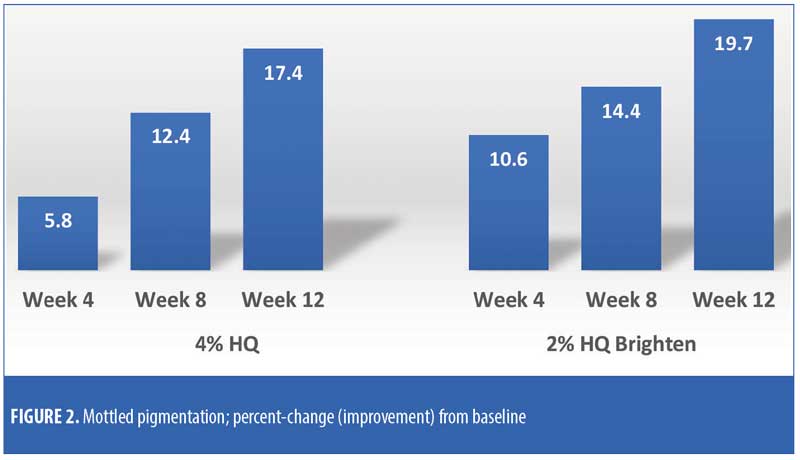
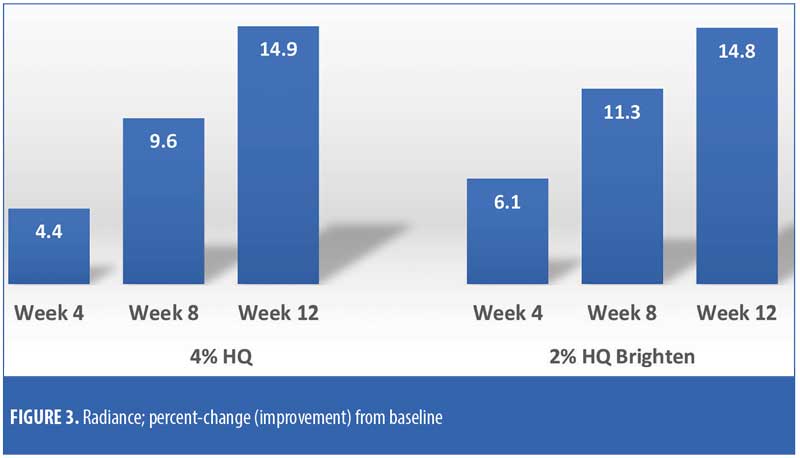
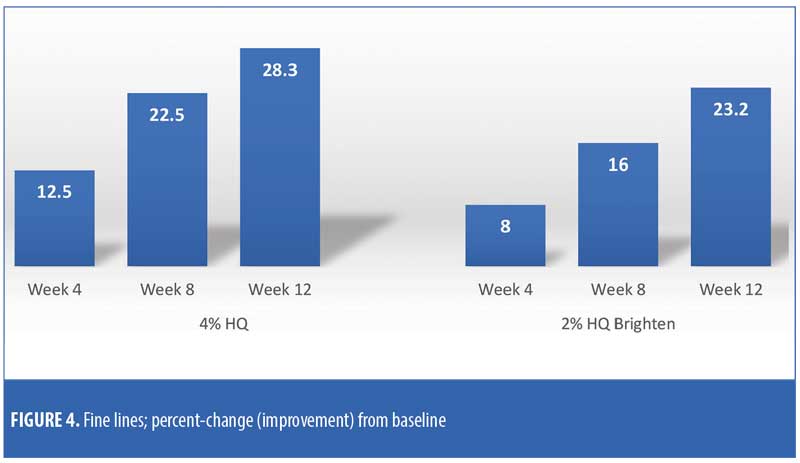
The efficacy evaluations (mean changes from baseline) were very similar between the two treatments. The changes for both treatments (2% HQ Brighten and 4% HQ) were statistically improved from baseline at Weeks 8 and 12 for the following parameters: overall photodamage, mottled pigmentation, radiance, visual smoothness, and fine lines (Figures 1–4). There were no statistical differences between the two treatments at Weeks 4, 8, or 12.
Treatment with either product did not produce a statistically significant increase (worsening) in clinical grading scores for erythema, edema, dryness/scaling, burning, stinging, itching, and dryness/tightness at any assessment visit when postbaseline clinical grading scores were compared to baseline clinical grading scores.
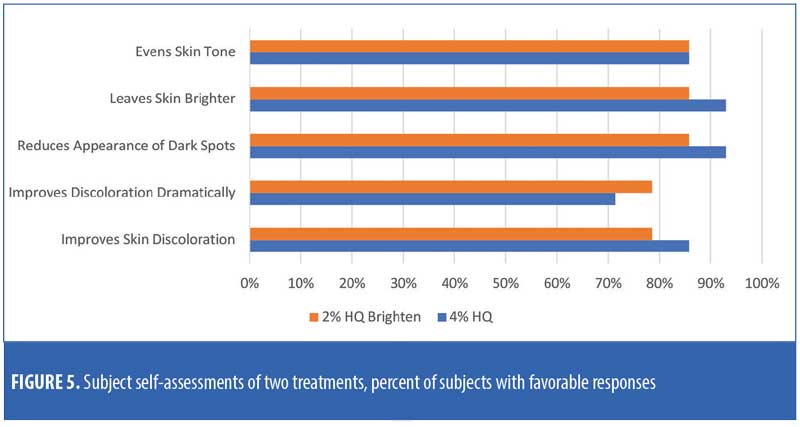
There were no major consistent differences in subject-reported efficacy responses between the two treatments (Figure 5).
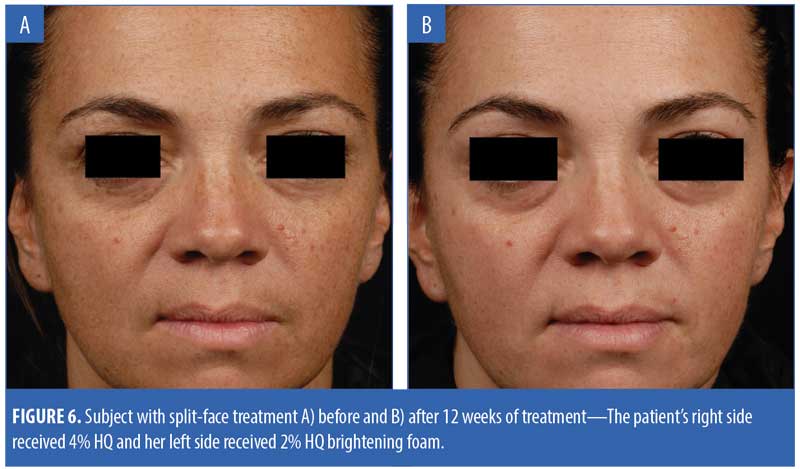
Figure 6 presents the images of a subject who received split-face treatment of the test products.
Discussion
Skin lightening (or whitening) is a common cosmetic practice of lightening the skin tone or evening the complexion by reducing the melanin content within the skin. It is used to treat specific skin conditions (e.g., melasma) or for culture-specific beauty preferences.
This study, which compared a nonprescription, multi-ingredient skin lightener (2% HQ Brighten) against the gold standard 4% HQ, showed similar efficacy and tolerability profiles for both products. In addition to 2% HQ, the Brighten formulation contains nine other ingredients shown to have skin-brightening properties, with varying mechanisms of action (including arbutin, niacinamide, kojic acid, licorice root extract, and ascorbic acid)18,19 in therapeutic concentrations. In addition to multiple ingredients targeting different segments of the melanogenesis pathway, the formulation is in a lipophilic (oil-in-water emulsion) foam.
Notably, only a single product was employed in this study for treatment purposes. The addition of a retinoid (retinol or tretinoin) would likely have led to enhanced efficacy.20–22
One concern with HQ use is exogenous ochronosis. This blue-black or gray-blue cutaneous discoloration is rare, but is associated with prolonged usage, higher concentrations, and application over larger areas of the body.23
There seems to be a rationale for the use of a lower concentration of topical HQ to treat disorders of hyperpigmentation if a product had similar effects as those of 4% HQ.
Limitations. Although this was a small trial, it utilized a split-face design to directly compare the two treatments side-by-side.
Conclusions
Both treatments (2% HQ Brighten and 4% HQ) improved the appearance of photodamaged facial skin and were well-tolerated by subjects over the 12-week treatment period as compared with baseline grading scores. Analysis of the change from baseline treatment comparison scores for efficacy and tolerability evaluations revealed no statistically significant differences between baseline and after 12 weeks of treatment. According to the findings of the self-assessment questionnaires, both treatments were well-perceived by subjects.
References
- Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79(6):547–568.
- Ortonne JP, Bissett DL. Latest insights into skin hyperpigmentation. J Invest Dermatol Symp Proc. 2008;13(1):10–14.
- Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989;21(3 Pt 2):610–613.
- Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470.
- Taylor CR, Stern RS, Leyden JJ, Gilchrest BA. Photoaging/photodamage and photoprotection. J Am Acad Dermatol. 1990;22(1):1–15.
- Taylor A, Pawaskar M, Taylor SL, et al. Prevalence of pigmentary disorders and their impact on quality of life: a prospective cohort study. J Cosmet Dermatol. 2008;7(3):164–168.
- Balkrishnan R, Kelly AP, McMichael A, Torok H. Improved quality of life with effective treartment of facial melasma: the pigment trial. J Drugs Dermatol. 2004;3(4):377–381.
- Draelos ZD. Skin lightening preparations and the hydroquinone controversy. Dermatol Ther. 2007;20(5):308–313.
- Grimes PE. An efficacy study of 3 commercially available hydroquinone 4% treatments for melasma. Cutis. 2007;80(6):497–502.
- Grimes PE. A microsponge formulation of hydroquinone 4% and retinol 0.15% in the treatment of melasma and postinflammatory hyperpigmentation. Cutis. 2004;74(6):362–368.
- Haddad AL, Matos LF, Brunstein F, et al. A clinical, prospective, randomized double-blind trial comparing skin whitening complex with hydroquinone vs placebo in the treatment of melasma. Int J Dermatol. 2003;42(2):153–156.
- Torokk HM, Jones T, Rich P, et al. Hydroquinone 4%, tretinoin 0.05%, fluocinolone acetonide 0.01%: a safe and efficacious 12- month treatment for melasma. Cutis. 2005;75(1): 57–62.
- Department of Health and Human Services. Food and Drug Administration. Skin bleaching drug products for over-the-counter human use; proposed rule. Federal Register. 2006;71: 51146–51154.
- Levitt J. The safety of hydroquinone: a dermatologist’s response to the 2006 Federal Register. J Am Acad Dermatol. 2007;57(5): 854–872.
- Norlund JJ, Grimes PE, Ortonne JP. The safety of hydroquinone. J Eur Acad Dermatol Venereol. 2006;20(7):781–787.
- O’Donoghue JL. Hydroquinone and its analogues in dermatology—a risk-benefit viewpoint. J Cosmet Dermatol. 2006;5(3):196–203.
- Griffiths CE, Wang TS, Hamilton TA, et al. A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992;128(3):347–351.
- Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31(7 Pt 2):886–889.
- Gillbro JM, Olsson MJ. The melanogenesis and mechanisms of skin-lightening agents—existing and new approaches. Int J Cosmet Sci. 2011;33(3):210–221.
- Kang HY, Valerio L, Bahadoran P, Ortonne JP. The role of topical retinoids in the treatment of pigmentary disorders: an evidence-based review. Am J Clin Dermatol. 2009;10(4):
251–260. - Rendon M, Dryer L. Investigator-blinded, single-center study to evaluate the efficacy and tolerability of a 4% hydroquinone skin care system plus 0.02% tretinoin cream in mild-to-moderate melasma and photodamage. J Drugs Dermatol. 2016;15(4):466–475.
- Rendon MI, Barkovic S. Clinical evaluation of a 4% hydroquinone + 1% retinol treatment regimen for improving melasma and photodamage in Fitzpatrick skin types III–IV. J Drugs Dermatol. 2016;15(11):1435–1441.
- Bhattar PA, Zawar VP, Godse KV, et al. Exogenous ochronosis. Indian J Dermatol. 2015;60(6): 537–543.

