J Clin Aesthet Dermatol. 2025;18(9):40–46.
by Diego Ruiz Dasilva, MD; Alondra Soto-González, MS; Nicholas K. Mollanazar, MD, MBA; Somto Ndubisi, BS; Naiem Issa, MD, PhD; James E. Song, MD; Mona Shahriari, MD; Christopher G. Bunick, MD, PhD; Peter Lio, MD; Harrison P. Nguyen, MD, MBA, MPH; Gil Yosipovitch, MD; and James Del Rosso, DO
Drs. Dasilva and Issa are with Forefront Dermatology in Virginia Beach, Virginia. Dr. Dasilva is also with the Eastern Virginia Medical School in Norfolk, Virginia. Mr. Soto-González is with Universidad Central del Caribe School of Medicine in Bayamón, Puerto Rico. Mr. Soto-González and Dr. Mollanazar are with the Department of Dermatology at Perelman School of Medicine at the University of Pennsylvania in Philadelphia, Pennsylvania. Mr. Ndubisi is with Ross University School of Medicine in Bridgetown, Saint Michael Barbados. Dr. Issa is also with Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery at the University of Miami School of Medicine in Miami, Florida and the Department of Dermatology at the George Washington University School of Medicine and Health Sciences in Washington, DC. Dr. Song is with Frontier Dermatology in Mill Creek, Washington. Drs. Shahriari and Bunick are with Central CT Dermatology Research in Cromwell, Connecticut. Dr. Shahriari is also with the Department of Dermatology and Program in Translational Biomedicine at Yale University School in New Haven, Connecticut. Dr. Lio is with Medical Dermatology Associates of Chicago in Chicago, Illinois. Dr. Nguyen is with Harrison Dermatology and Research Group in Houston, Texas, and the University of Houston College of Medicine in Houston, Texas. Dr. Yosipovitch is with the Miami Itch Center at Miller School of Medicine at University of Miami in Miami, Florida. Dr. Del Rosso is with Touro University Nevada College of Health and Human Services in Henderson, Nevada, and JDR Dermatology Research in Las Vegas, Nevada.
FUNDING: No funding was provided for this article.
DISCLOSURES: Dr. Ruiz Dasilva: AbbVie, Arcutis, Dermavant, Eli Lilly, Galderma, Janssen, LEO Pharma, Pfizer, Sanofi & Regeneron, UCB, Verrica. Dr. Naiem Issa: Abbvie, Bristol Myers Squibb, Castle Biosciences, Dermavant Sciences, DermTech, Galderma, Incyte, Jannsen, Journey, LEO Pharma, Lilly, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, SUN Pharma, Verrica Pharmaceuticals. Dr. E. James Song: AbbVie, Arcutis, Alphyn, Amgen, Apogee, Avalo, Bristol Meyers Squibb, Boehringer-Ingelheim, CorEvitas Registry, Dermavant, DermBiont, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, MoonLake, Novartis, Ortho-dermatologics, Pfizer, Sanofi & Regeneron, SUN pharma, Target Registry, TIMBER, UCB. Dr. Nicholas K Mollanazar: Boehringer Ingelheim, Janssen, Novartis, Regeneron Pharmaceuticals Inc., Sanofi, Trevi Therapeutics, Menlo Therapeutics Inc, Galderma, Leo Pharma, AbbVie, Pfizer, Beiersdorf– advisory board member; Sanofi, Regeneron Pharmaceuticals Inc, Genzyme – Investigator; Novartis, Regeneron Pharmaceuticals Inc., Sanofi, Janssen, AbbVie – consultant. Dr. Peter Lio reports being on the speaker’s bureau for AbbVie, Arcutis, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche-Posay/L’Oreal, Pfizer, Pierre-Fabre Dermatologie, Regeneron/Sanofi Genzyme, Verrica; reports consulting/advisory boards for Alphyn Biologics, AbbVie, Almirall, Amyris, Arcutis, ASLAN, Astria Therapeutics, Boston Skin Science, Bristol-Myers Squibb, Burt’s Bees, Castle Biosciences, Codex Labs, Concerto Biosci, Dermavant, Eli Lilly, Galderma, LEO Pharma, Lipidor, L’Oreal, Merck, Micreos, MyOR Diagnostics, Pelthos Therapeutics, Regeneron/Sanofi Genzyme, Sibel Health, Skinfix, Soteri Skin, Stratum Biosciences, Sun Pharma, Theraplex, Thimble Health, UCB, Unilever, Verdant Scientific, Verrica, Yobee Care. Stock options with Alphyn Labs, Codex Labs, Concerto Biosci, Soteri Skin, Stratum Biosciences, Thimble, Yobee Care, and Verdant Scientific. In addition, Dr. Lio has a patent pending for a Theraplex product with royalties paid and is a Board member and Scientific Advisory Committee Member emeritus of the National Eczema Association. Dr. Harrison Nguyen: Abbvie, Amgen, Apogee Therapeutics, Arcuitis, Boehringer Ingelheim, Bristol Myers Squibb, Castle Biosciences, Dermavant, Galderma, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, Sun Pharmaceuticals, UCB, Verrica. Somto and Alondra have no conflicts of interest. Dr Gil Yosipovitch: Abbvie, Arcutis, Almiral, Amgen, Celldex, Escient Health, Eli Lilly, Galderma, LEO Pharma, Merck, Novartis, Pfizer, Pierre Fabre, Regeneron Pharmaceuticals, Inc., Sanofi, Vifor, GSK, – advisory board member; Eli Lilly, LEO Pharma, Novartis, Pfizer, Galderma, Escient, Sanofi Regeneron, Sanofi Celldex, Kiniksa, Pfizer– grants/research funding; Regeneron Pharmaceuticals, Inc., Sanofi, Galderma – investigator. Mona Shahriari: Consultant, Speaker, and/or Investigator: AbbVie, Amgen, Apogee, Arcutis, Bristol Myers Squibb, Dermavant, Galderma, Pfizer, Incyte, Janssen, Leo Pharma, Lilly USA, Novartis, Sanofi-Genzyme, Regeneron, UCB. Christopher Bunick has served as an investigator for AbbVie, Almirall, Apogee, Daiichi Sankyo, LEO Pharma, Ortho Dermatologics, Sun Pharma, Timber, and Palvella and a consultant for AbbVie, Almirall, Amgen, Apogee, Arcutis, Botanix, Connect BioPharma, Dermavant, Eli Lilly, EPI Health/Novan, Incyte, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, Sun Pharma, Takeda, Teladoc, and UCB. The remaining authors have no conflicts of interest to disclose.
Abstract: Objective: Chronic pruritus significantly impacts quality of life and remains challenging to manage, particularly in patients unresponsive to systemic therapies. Nemolizumab, a monoclonal antibody targeting the interleukin (IL)-31 receptor, has shown promise in alleviating pruritus across several dermatologic conditions. However, data on its efficacy in treatment-resistant cases, particularly in real-world settings, remain limited. This case series evaluates the efficacy and safety of nemolizumab in patients with treatment-resistant chronic pruritus. Methods: A multicenter retrospective review was conducted across private dermatology practices and large academic dermatology centers. Patient records were reviewed for demographics, comorbidities, treatment history, and clinical outcomes, including Body Surface Area (BSA), Investigator Global Assessment (IGA), and Peak Pruritus Numerical Rating Scale (PP-NRS). Adverse events were recorded. Results: Twelve patients (5 with atopic dermatitis, 2 with prurigo nodularis, 3 with chronic pruritus of unknown origin, and 2 with concomitant atopic dermatitis and prurigo nodularis) were included. All had previously failed systemic therapies. Mean baseline BSA was 45 percent (range 5–88%), with mean PP-NRS of 9 (range 6–10). After treatment, mean BSA and PP-NRS decreased to 4 percent (11-fold reduction) and 0.9 (10-fold reduction), respectively. Most (10/12) experienced significant pruritus relief within 24 to 72 hours of nemolizumab initiation. No serious adverse events were noted, including in patients with a history of malignancy or end-stage renal disease. Limitations: Small sample size, retrospective design, limited follow-up, and absence of a control group. Conclusion: Nemolizumab demonstrated rapid and substantial efficacy in treatment-resistant chronic pruritus with a favorable safety profile, even in patients with significant comorbidities. Keywords: Medical dermatology, nemolizumab, pruritus, atopic dermatitis, prurigo nodularis, biologics, inflammatory skin disease
Introduction
Chronic pruritus is a debilitating symptom associated with numerous dermatological conditions, often proving resistant to conventional therapies and profoundly impacting patients’ quality of life.1 Recent advances in understanding the pathophysiology of chronic pruritus have highlighted the critical role of interleukin-31 (IL-31) in the neuroimmune pathway of itch sensation.2–5 Signaling through the IL-31 receptor (IL-31RA), which is located on cutaneous sensory neurons and in dorsal root ganglia, immune cells, keratinocytes, and fibroblasts, contributes to a vicious itch/scratch cycle when activated.6 This insight has led to the development of nemolizumab, a recombinant, humanized, immunoglobulin G2 (IgG2) monoclonal antibody targeting the IL-31 receptor A.7 Current evidence demonstrates that nemolizumab is highly effective in improving skin lesions and itch in patients with atopic dermatitis and prurigo nodularis.8,9 However, limited data exist on its performance in the real-world setting, particularly in patients who have had an inadequate response or adverse event to other systemic therapies. Moreover, there were limited biologic-experienced patients and no patients with prior Janus kinase inhibitor (JAKi) exposure in the nemolizumab Phase 3 clinical program, which is key to guiding treatment decisions in real-world clinical practice. We highlight the versatility of nemolizumab in clinical practice to optimize chronic pruritus management.
Methods
We conducted a retrospective review of patients from multiple private practice dermatology clinics across five states and three large academic dermatology departments. Cases were identified using the patient search function and filtered by the medication “nemolizumab.” Inclusion criteria focused on patients who had failed other advanced systemic agents or experienced intolerable side effects.
Data collected included patient demographics, comorbidities, treatment duration, and prior treatments. Effectiveness outcomes, including body surface area (BSA), Investigator Global Assessment (IGA), and Peak Pruritus Numerical Rating Scale (PP-NRS), were evaluated independently.
Results
Our case series included 12 patients (Table 1) with treatment-resistant chronic pruritus who were successfully treated with nemolizumab (Table 2). The mean age of patients was 59 years (range 12–87 years), and nine were male. The primary diagnoses were atopic dermatitis (AD) (n=5), prurigo nodularis (PN) (n=2), chronic pruritus of unknown origin (CPUO) (n=3), and concomitant AD and PN (n=2). Before nemolizumab therapy, the mean BSA involvement was 45 percent (range 5–88%), and the mean Peak Pruritus NRS was 9 (range 6–10). After nemolizumab therapy, BSA and PP-NRS diminished significantly, with mean values of 4 percent (11-fold reduction) and 0.9 (10-fold reduction), respectively, among patients whose data were available (Table 3).
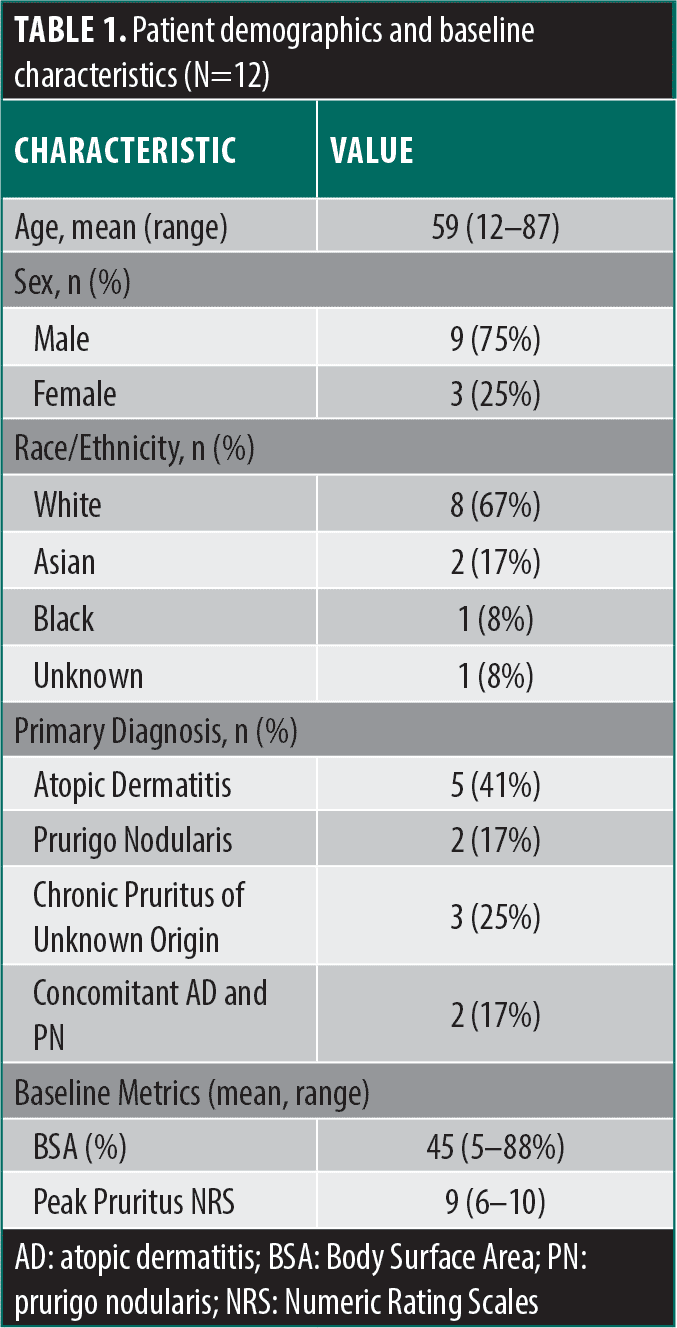
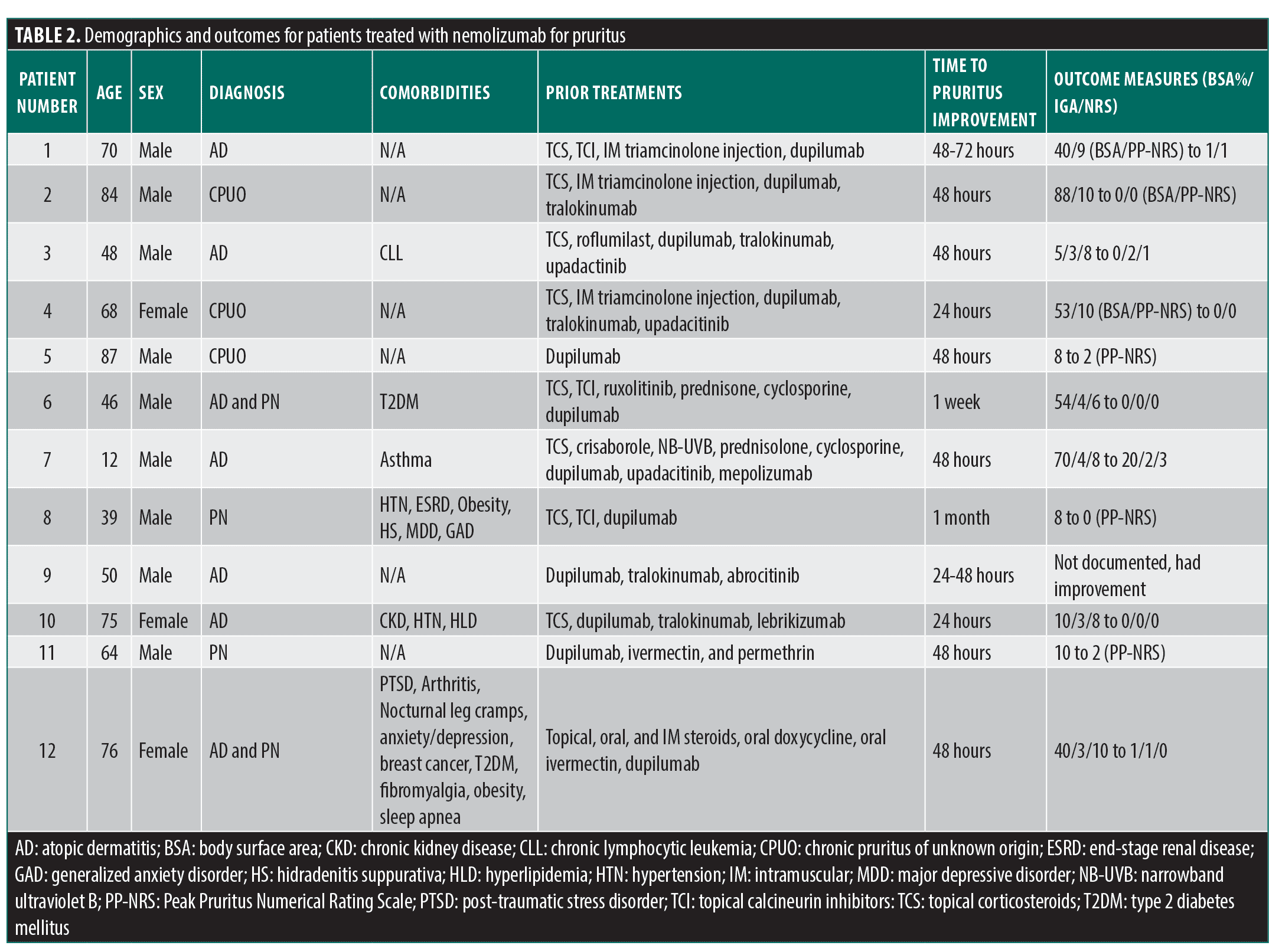
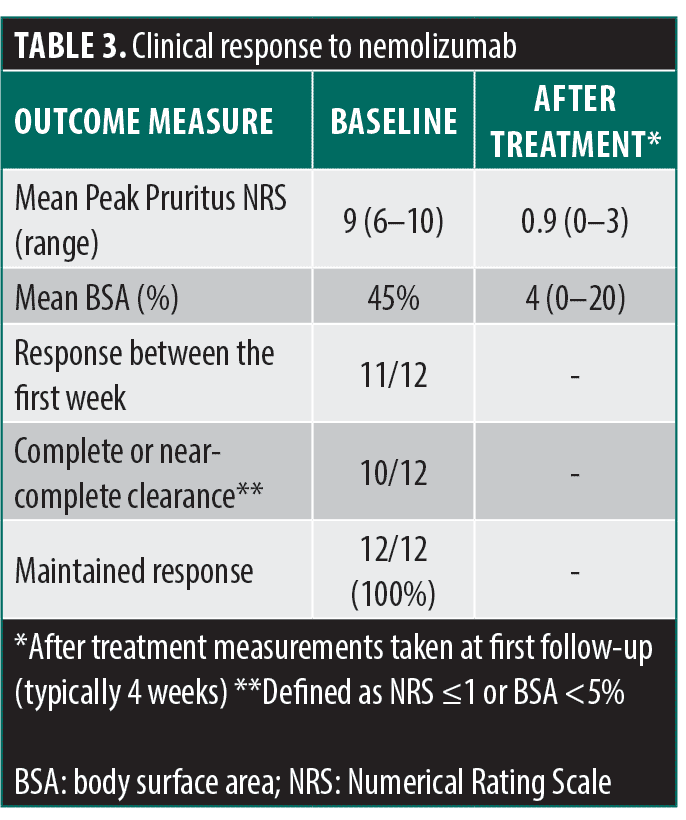
All patients had failed or were intolerant to multiple therapies prior to initiating nemolizumab. Previous biologic therapy included dupilumab (all patients, n=12), tralokinumab (n=5), lebrikizumab (n=1), and mepolizumab (n=1). JAK inhibitor experience included upadacitinib (n=3) and abrocitinib (n=1). Other systemic treatments included cyclosporine (n=2). Reasons for discontinuation included inadequate response, loss of efficacy, and adverse events (Table 4). Following the initiation of nemolizumab, most (10/12) patients experienced rapid relief from pruritus within 24 to 72 hours, with the remaining two having a response within a week to a month. The treatment was well tolerated, with no serious adverse events reported.
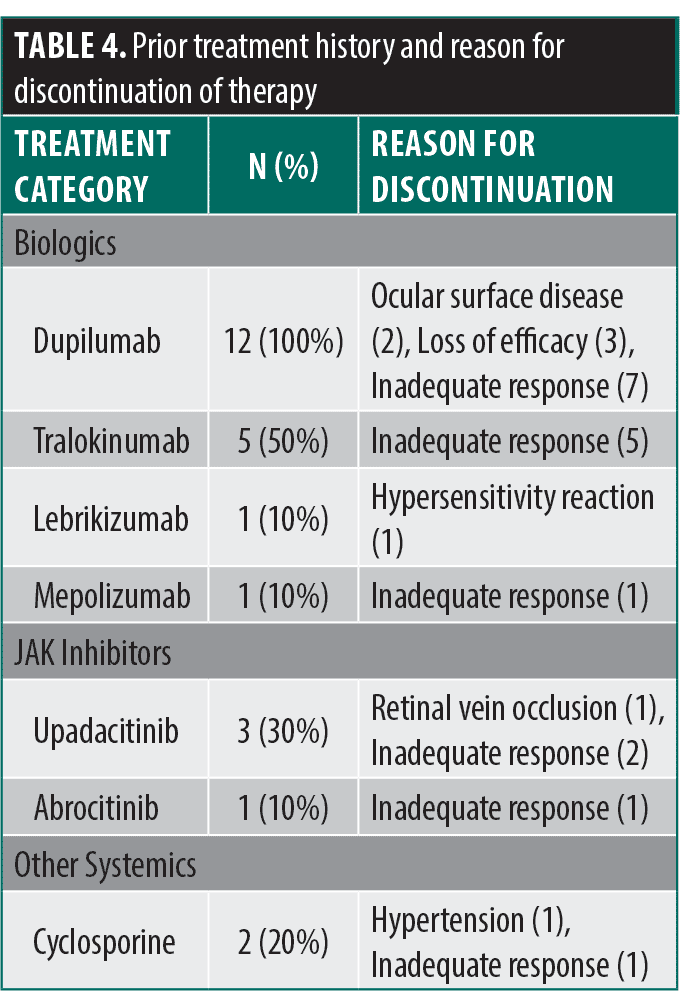
These cases were chosen to highlight nemolizumab’s success in difficult cases where patients have failed multiple therapies or experienced intolerability due to side effects. However, due to nemolizumab being available in the United States market for less than a year, we currently lack real-world data on long-term outcomes.
Prior treatment intolerance. A 70-year-old White male patient presented with a lifelong history of AD and severe pruritus with worsening disease over the past 10 years. Despite treatment with topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), and multiple rounds of intramuscular (IM) triamcinolone injection, the AD progressed, affecting 40 percent BSA and with a PP-NRS of 9/10, significantly impacting his sleep and quality of life. He was started on dupilumab, achieving disease control (BSA 2%, IGA 1, PP-NRS 3) within eight weeks. However, after two years, he developed a sharp decline in visual acuity. An ophthalmologic evaluation revealed bilateral ocular surface disease (OSD) with dry eye. Due to concerns about OSD, the patient declined to restart dupilumab or initiate an anti-IL-13 biologic. He also opted against systemic JAK inhibitors due to the need for blood monitoring and potential adverse effects (AEs). Given the absence of an association with OSD, nemolizumab was initiated. Within 48 to 72 hours of the first dose, his pruritus dramatically decreased (PP-NRS from 10 to 1), and he has not experienced any AEs.
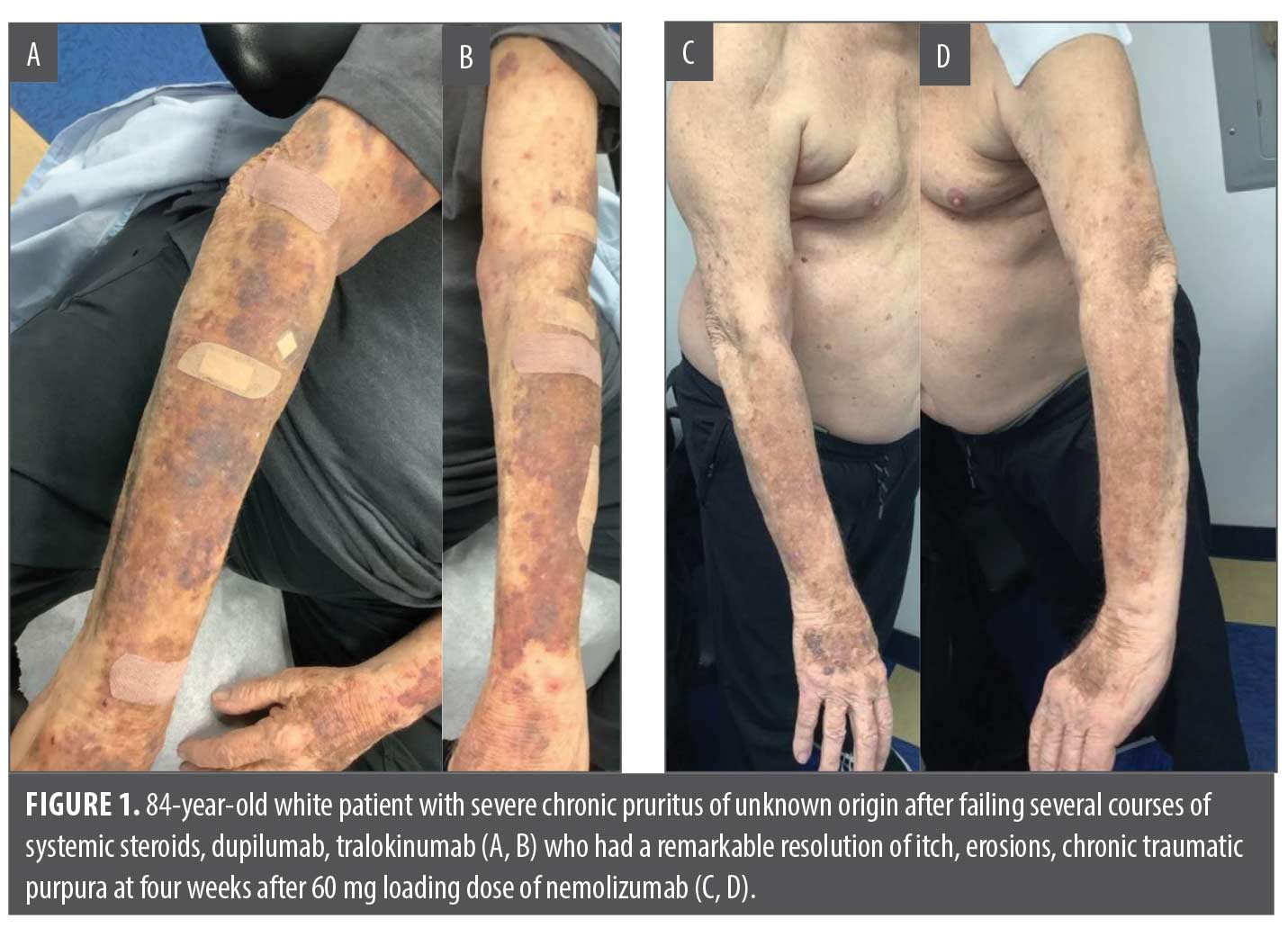
An 84-year-old White male patient with a six-year history of severe CPUO presented with progressively worsening disease, ultimately affecting 88 percent of his BSA and reaching a PP-NRS score of 10/10. After failing TCS/TCI, he required several courses of IM triamcinolone injection for transient relief. He was started on dupilumab; however, within eight weeks, he developed severe OSD before experiencing any therapeutic benefit. Due to a history of prostate cancer, he declined a trial of a JAK inhibitor. The patient reported significant distress, including hopelessness, sleep deprivation, and suicidal thoughts. He was initiated on tralokinumab, and by six months, his BSA was down to 22 percent and his PP-NRS to 6/10, with milder OSD compared to dupilumab. Nonetheless, his quality of life and sleep remained sub-optimal, prompting a switch to nemolizumab. Within 48 hours of the first dose, he reported a marked improvement in itch (PP-NRS 2). At his one-month follow-up, his skin had completely cleared, and his itch had fully resolved after a single 60mg loading dose (Figure 1); he did not report any adverse events.
A 48-year-old White male patient with itch-predominant AD and a history of untreated chronic lymphocytic leukemia presented after having an inadequate response to TCS, topical roflumilast, oral prednisone, narrowband ultraviolet B phototherapy (NB-UVB), and dupilumab. Upadacitinib 15mg daily led to complete skin clearance and itch resolution but was discontinued following the development of a central retinal vein occlusion five months into therapy. The patient was switched to tralokinumab, but his rash and itch quickly recurred and failed to show any meaningful improvement after 16 weeks of treatment. At the time of the switch to nemolizumab, the patient had 5 percent BSA, an IGA score of 3, and PP-NRS of 8. One month after starting nemolizumab 60mg, his IGA improved to 2 and his PP-NRS to 1. Despite medication adherence challenges, his PP-NRS was still a 2, even two months after his first and only dose of nemolizumab.
Prior treatment failure. A 68-year-old White female patient presented with a three-year history of generalized pruritus without a significant rash. Over the years, her pruritus reached a BSA of 53 percent and PP-NRS of 10/10. Workup, including multiple skin biopsies, demonstrated “excoriation with minimal spongiosis” and unremarkable blood work (Complete blood count, comprehensive metabolic panel, serum protein electrophoresis, thyroid-stimulating hormone, and human immunodeficiency virus), leading to a diagnosis of CPUO. After failing TCS, she required several courses of IM triamcinolone injection, which culminated in hospitalization for hyperglycemia and sepsis. She was started on dupilumab, which resulted in a modest improvement of itch (PP-NRS 6/10) after four months. Given her poor quality of life, she requested to be switched to tralokinumab, but after six months, her pruritus improved only slightly (PP-NRS 5/10) over dupilumab. Desperate for further relief, she started upadacitinib 15mg daily for two weeks, which reduced her itch to a 1/10, but she declined to continue treatment due to anxiety over boxed warnings despite thorough counseling. She then chose to try nemolizumab, and within 24 hours, she reported a marked improvement in pruritus. At her one-month follow-up, she had complete resolution of itch after a single 60mg loading dose, without the need for any topical treatments. She is continuing treatment at monthly dosing.
An 87-year-old White male patient presented with a one-year history of generalized pruritus (PP-NRS of 10/10) without a significant rash. An extensive workup for pruritus was negative, leading to a diagnosis of CPUO. He was treated with dupilumab for four months with a modest improvement to PP-NRS of 8/10. He was switched to nemolizumab, and within 48 hours, he reported a remarkable improvement in pruritus (PP-NRS of 3/10). At his six-month follow-up, he reported intermittent pruritus (PP-NRS of 2/10) but is satisfied and is continuing treatment.
A 46-year-old Black male patient presented with a 30+ year history of AD and PN presented with a BSA of 54 percent, IGA of 4, and PP-NRS of 8. Two prior biopsies confirmed eczema. Despite having Type 2 diabetes mellitus (T2DM) and being obese, he was otherwise healthy. He failed numerous treatments, including TCS/TCI, topical ruxolitinib, and several oral prednisone courses, which provided temporary relief but posed risks due to his T2DM. He subsequently started cyclosporine 100mg twice daily with modest relief, but increasing hypertension led to its discontinuation. Dupilumab was then initiated, resulting in improvement in both itch and rash, but even after six months, he still reported persistent pruritus (PP-NRS 6/10) and was eager to try nemolizumab (saw online commercial). Given his weight and the combination of AD/PN, the PN dosing was used (60mg monthly). Within one week after the 60mg loading dose, he reported a dramatic reduction in pruritus to 2/10. At his one-month follow-up, he had complete clearance of skin lesions, with only residual hyperpigmentation and a PP-NRS of 1.
A 12-year-old White male patient with a lifelong history of severe AD and asthma presented after failing multiple treatments, including numerous TCS, TCI, crisaborole, and seven months of NB-UVB without improvement. He had multiple courses of prednisolone and failed three months of cyclosporine due to inadequate response. He started dupilumab, but after six months, he had an inadequate response (BSA 40%, IGA 3, PP-NRS 6). Next, he was switched to upadacitinib 15mg per day but had inadequate response after four months (BSA 30%, IGA 3, PP-NRS 5). He was started on mepolizumab for asthma with hopes of improving his AD, but it did not provide skin benefit. Finally, after several more courses of prednisolone, he turned 12 and was started on nemolizumab with a 60mg loading dose followed by 30mg monthly. At baseline, he had 70 percent BSA, an IGA score of 4, and a PP-NRS 9/10. Notably, he reported that the injection was painless, and within days, he noticed an improvement in his itch. At his two-month follow-up, his BSA had decreased to 20 percent, IGA score of two, and his PP-NRS was significantly reduced to 3/10.
A 39-year-old Asian male patient with a history of hypertension, end-stage renal disease on hemodialysis, obesity, hidradenitis suppurativa, major depressive disorder, and generalized anxiety disorder presented with PN after failing several TCS and TCI. He was started on dupilumab and completed one year with moderate improvement in itching but was dissatisfied with the results and self-discontinued therapy. After being lost to follow-up for two years, he returned to the clinic and started nemolizumab with a baseline PP-NRS of 8/10. He subsequently received a kidney transplant while on nemolizumab without complications. After approximately one month of treatment, his PP-NRS was 0/10, and he continued treatment while on anti-rejection immunosuppressants.
A 50-year-old Asian male patient with a longstanding history of AD presented after failing dupilumab, tralokinumab (minimal response), and abrocitinib (minimal response). He was initially well controlled on dupilumab, but it lost efficacy after 2 to 3 years. The patient also experienced moderate head and neck dermatitis while on dupilumab.10 He was then started on nemolizumab, and within 24 to 48 hours of receiving the loading dose, he experienced a significant improvement in itch and is now satisfied with the treatment.
A 75-year-old female patient with a lifelong history of AD, chronic kidney disease, hypertension, and hyperlipidemia presented after failing TCS/TCI. She was initially tried on dupilumab (at initiation BSA was 20%, IGA 3, PP-NRS 8), which cleared her completely after six months. However, after two years, it started to lose efficacy with a breakthrough flare occurring a few days before each injection. Off-label weekly dosing was attempted, but her symptoms persisted. The patient was then started on tralokinumab (having expressed hesitation toward JAK inhibitors due to fear of the boxed warnings). At the time of initiation, she had a BSA of 10 percent, IGA of 3, and PP-NRS of 6. She had an inadequate response and never improved beyond a BSA of 3 percent, IGA of 2, and PP-NRS of 5. She then began treatment with lebrikizumab but developed a hypersensitivity reaction after the initial loading dose, presenting as an urticarial eruption primarily on her trunk. The medication was discontinued, and her reaction was treated with a short course of prednisone, which kept her AD under control for a few weeks before symptoms recurred. At this point, she had a BSA of 10 percent, IGA of 3, and PP-NRS of 8. Given the burden of itch, nemolizumab was started. She called within a week to report that her itch was gone within 24 hours after her loading dose. At the four-week follow-up, her BSA was 0 percent, IGA was 0, and PP-NRS was 0. She has not experienced any adverse events and is relieved to have found an effective treatment.
A 64-year-old White male patient presented with severe itch (PP-NRS 10/10) for the prior nine months, accompanied by widespread prurigo nodules with excoriations. His quality of life was significantly impacted by a lack of sleep. This was suspected to be postscabetic pruritus as he and his wife initially had severe itch after traveling to South America. However, he was treated and did not improve, so dupilumab was tried for six months, resulting in worsening pruritus. He stopped dupilumab and was retreated with oral ivermectin and permethrin, again with a lack of relief. At this point, he was switched to nemolizumab and after the loading dose injection, his itch was reduced to 2/10 in 72 hours. In this short period, the nodules also melted away, and he was able to get over five hours of sleep for the first time in nearly a year. He has had no AEs and continues treatment.
A 76-year-old White female patient with a diagnosis of AD and PN with past medical history of post-traumatic stress disorder (PTSD), arthritis, nocturnal leg cramps, anxiety/depression, breast cancer, T2DM, fibromyalgia, obesity and sleep apnea had previously tried topical, oral, and IM steroids, oral doxycycline (for impetiginization), and oral ivermectin (empiric therapy for scabies) for her AD and PN prior to initiation of dupilumab. Her IgE level before initiation of dupilumab was 1,769 kU/L (upper limit normal 114), and her affected BSA was 40 percent, IGA was 3, and PP-NRS was 10/10. She started and maintained dupilumab for six months using per-label dosing, without adverse events, then discontinued it due to inadequate/partial efficacy: her affected BSA at discontinuation was 20 percent, IGA was 2, and PP-NRS was 8/10. She then started nemolizumab and reported extremely rapid itch relief (PP-NRS 0/10 by Day 2) and significant skin clearance (BSA 3%) at the end of month 1 of nemolizumab. While she experienced a few nodules and eczematous patches with itch between month 1 and 3, by the end of three months of nemolizumab therapy, she reported PP-NRS 0/10, no skin pain, and had only minor residual erythema on the right lower leg (BSA 1%; IGA 1). She reported improved sleep and continues 60mg monthly dosing.
Discussion
Nemolizumab works by inhibiting IL-31-mediated signal transduction, which is critical in pruritus, inflammation, and fibrosis. Additionally, it helps preserve and repair the skin’s protective barrier function, which is often compromised due to the repetitive itch-scratch cycle.11 Numerous studies have demonstrated nemolizumab’s efficacy across various pruritic conditions from both skin/itch clearance to patient-reported outcomes.8,12–19 Nemolizumab also demonstrated faster itch relief than biologics targeting IL-4/IL-13, with a response time similar to JAK inhibitors when measured at 48 hours.20 Nemolizumab’s favorable safety profile, similar to the other targeted AD biologics, also makes it an appealing option.21, 22 Nonetheless, data on patients who have failed advanced systemic treatments and have overlapping conditions or CPUO are limited. This real-world case series begins to address this knowledge gap.
Our series highlights the teaching points from a variety of difficult cases to help dermatologists feel comfortable incorporating nemolizumab into their treatment algorithm. Many treatment options can improve the signs and symptoms of AD, often quickly. Nemolizumab can provide quick onset efficacy and demonstrates a low risk of certain AEs of special interest that are seen with other treatments, such as OSD, arthralgia, or serious infection. The 48-year-old patient described above demonstrated some patients on nemolizumab may experience sustained clinical responses even after discontinuation of therapy, a phenomenon also observed with other advanced systemic therapies for AD. However, no treatment is without potential side effects, and some patients receiving nemolizumab have experienced dermatitis flares, peripheral edema, and headaches at rates slightly higher than those observed with placebo. These potential risks should be considered when determining the best treatment approach for individual patient cases but were easy to manage in the trials.
Given the complex and heterogeneous nature of itch, no single therapy has been universally effective. Nemolizumab offers a novel mechanism that has been demonstrated to work well as an initial systemic agent, and this case series highlights that it may also be highly effective in those who have failed JAKi, IL-4, and IL-13 blockade. While many biologics do not have weight-based dosing, there are some signals that efficacy may be reduced for those with higher body mass indices.23 In such cases, when available, it may be beneficial to use weight-based dosing (such as nemolizumab in PN). However, we demonstrate that the effect can be so rapid (within hours to days after only the loading dose) that the mechanism may be equally or more important than the dosing. In AD, the possibility of q8-week dosing and the fact that it is very well tolerated with a surprisingly low rate of injection site reactions (ISR) has led to the enthusiasm surrounding nemolizumab as a novel treatment. Particularly for younger patients or those who are needle-phobic, this may be a unique and important aspect of this therapy. The excellent safety profile of nemolizumab is reinforced in this patient cohort, being used in patients with active malignancy and with ESRD through the transplant process without issue. Another interesting point is that IL-31 is highly expressed in scabetic itch. In our case of postscabetic pruritus, who failed initial treatment with dupilumab, inhibiting IL-4 and IL-13 could impair the immune response to parasites, potentially increasing the parasitic load and worsening symptoms.24 However, switching to nemolizumab, which targets IL-31, provided significant relief, suggesting that targeting this alternative pathway may be more effective in managing postscabetic pruritus.
This real-world case series evaluates the clinical utility of nemolizumab in diverse patients with chronic pruritus who have been unsuccessfully treated with advanced systemic therapies. Given its recent introduction to the therapeutic armamentarium, our data expands current knowledge on its role in clinical practice, providing insights into patient selection and treatment outcomes. By adding to the growing evidence that IL-31 inhibition is uniquely poised to bring relief to patients with chronic pruritus from a variety of etiologies, we highlight nemolizumab as a valuable option for patients who have failed or have been intolerant to other biologics and/or JAK inhibitors.25 Our observations may help inform treatment decisions and optimize the use of nemolizumab in managing refractory pruritic and inflammatory skin conditions. Lastly, it is crucial to state that post-marketing surveillance can often reveal adverse events that may not have been prominent in the pivotal trials, which is a natural limitation in attempting to compare the safety/tolerability of a newer agent to more established drugs.
Conclusion
Our experience with nemolizumab in patients with treatment-resistant chronic pruritus demonstrates its promising efficacy and safety profile as a powerful rescue therapeutic in addition to its original utility as a first-line biologic for these conditions. The rapid and sustained relief observed in patients with various presentations, comorbidities, and prior treatment experiences underscores its potential as an effective therapeutic option. The weight-based dosing regimen offers a potential advantage, particularly for patients with higher body mass indices. Additionally, the ability to provide long-lasting symptom control with a dosing schedule every eight weeks is unique and may improve patient adherence and overall quality of life. However, the data presented here are based on short treatment duration, and longer-term outcomes have yet to be established. Further research with larger and more diverse patient cohorts with extended treatment courses is necessary to fully explore nemolizumab’s role in the management of pruritic disorders.
References
- Butler D, Berger T, Elmariah S, et al. Chronic Pruritus: A Review. JAMA. 2024;331(24):2114–2124.
- Alvarenga J, Bieber T, Torres T. Emerging Biologic Therapies for the Treatment of Atopic Dermatitis. Drugs. 2024;84(11):1379-1394.
- Metz M. Treatments for chronic pruritus outside of the box. Exp Dermatol. 2019;28(12):1476–1481.
- Pereira M, Zeidler C, Storck M, et al. Challenges in Clinical Research and Care in Pruritus. Acta Derm Venereol. 2020; 100(2).
- Serra E, Santamaría L, Francisco Silvestre J. Nemolizumab: An Innovative Biologic Treatment to Control Interleukin 31, a Key Mediator in Atopic Dermatitis and Prurigo Nodularis. Nemolizumab: un innovador tratamiento biológico para el control de la interleuquina 31 (IL-31) clave en la dermatitis atópica y el prurigo nodular. Actas Dermosifiliogr. 2022;113(7):674–684.
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133(2):448–460.
- Keam SJ. Nemolizumab: First Approval. Drugs. 2022;82(10):1143–1150.
- Silverberg J, Wollenberg A, Reich A, et al. Nemolizumab with concomitant topical therapy in adolescents and adults with moderate-to-severe atopic dermatitis (ARCADIA 1 and ARCADIA 2): results from two replicate, double-blind, randomised controlled Phase 3 trials. Lancet. 2024;404(10451):445–460.
- Ständer S, Yosipovitch G, Legat F, et al. Efficacy and Safety of Nemolizumab in Patients With Moderate to Severe Prurigo Nodularis: The OLYMPIA 1 Randomized Clinical Phase 3 Trial. JAMA Dermatol. Published online November 27, 2024.
- Bangert C, Alkon N, Chennareddy S, et al. Dupilumab-associated head and neck dermatitis shows a pronounced type 22 immune signature mediated by oligoclonally expanded T cells. Nat Commun. 2024;15(1):2839.
- Brooks S, Yosipovitch G. A critical evaluation of nemolizumab for prurigo nodularis. Expert Rev Clin Immunol. 2024;20(6):577–587.
- Komazaki H, Sato T, Kabashima K. Mediation analysis of the relationship between pruritus and signs of eczema improvements in patients with atopic dermatitis undergoing nemolizumab intervention. Jpn J Dermatol. 2023; 133:1479–1490.
- Kabashima K, Matsumura T, Komazaki H, et al. Nemolizumab-JP01 Study Group. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N Engl J Med. 2020;383(2):141–150.
- Kabashima K, Matsumura T, Komazaki H, Kawashima M. Nemolizumab JP01 and JP02 Study Group. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: results from two phase III, long-term studies. Br J Dermatol. 2022;186(4):642–651.
- Igarashi A, Katsunuma T, Matsumura T, Komazaki H. Nemolizumab-JP04 Study Group. Efficacy and safety of nemolizumab in paediatric patients aged 6-12 years with atopic dermatitis with moderate-to-severe pruritus: results from a phase III, randomized, double-blind, placebo-controlled, multicentre study. Br J Dermatol. 2023;190(1):20–28.
- Kabashima K, Matsumura T, Komazaki H, Kawashima M. Nemolizumab-JP01 Study Group. Nemolizumab Improves Patient-Reported Symptoms of Atopic Dermatitis with Pruritus: Post Hoc Analysis of a Japanese Phase III Randomized Controlled Trial. Dermatol Ther. 2023;13(4):997–1011.
- Kabashima K, Matsumura T, Hayakawa Y, Kawashima M. Nemolizumab-JP01 Study Group. Clinically meaningful improvements in cutaneous lesions and quality of life measures in patients with atopic dermatitis with greater pruritus reductions after treatment with 60 mg nemolizumab subcutaneously every 4 weeks: subgroup analysis from a phase 3, randomized, controlled trial. J Dermatolog Treat. 2023;34(1):2177096.
- Kwatra S, Yosipovitch G, Legat F, et al. Phase 3 Trial of Nemolizumab in Patients with Prurigo Nodularis. N Engl J Med. 2023;389(17):1579–1589.
- Kim BS. Learning from nemolizumab: A promising therapy for prurigo nodularis. J Allergy Clin Immunol. 2024;153(6):1548–1549.
- Lin DH, Nguyen C, Fleischer AB Jr. Time to meaningful clinical response in reduction of itch in atopic dermatitis. J Dermatolog Treat. 2022;33(3):1568–1571.
- Saeki H, Ohya Y, Arakawa H, et al. English version of clinical practice guidelines for the management of atopic dermatitis 2024. J Dermatol. Published online December 20, 2024.
- Kabashima K, Furue M, Hanifin J, et al. Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study. J Allergy Clin Immunol. 2018;142(4):1121–1130.
- Dupuis J, Tauber M, Mahe E, et al. Efficacy and tolerance of dupilumab in patients with moderate-to-severe atopic dermatitis and obesity. J Eur Acad Dermatol Venereol. 2025;39(2):174–178.
- Hashimoto T, Satoh T, Yokozeki H. Pruritus in ordinary scabies: IL-31 from macrophages induced by overexpression of thymic stromal lymphopoietin and periostin. Allergy. 2019;74(9):1727–1737.
- Murase E, Dulai A, Marzouk S, et al. Rapid Clinical Response to Nemolizumab in Dermatologic Diseases Associated with Pruritus and Burning: A Multicenter Case Series. J Am Acad Dermatol. Forthcoming 2025.
