 by Jasmine C. Hollinger, MD; Kunal Angra, MD; and Rebat M. Halder, MD
by Jasmine C. Hollinger, MD; Kunal Angra, MD; and Rebat M. Halder, MD
Dr. Hollinger is with the Department of Dermatology, University of Mississippi Medical Center in Jackson, Mississippi. Drs. Angra and Halder are with the Department of Dermatology, Howard University College of Medicine in Washington, DC.
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest to relevant to the content of this article.
Abstract: Background. Hyperpigmentation disorders are commonly encountered in dermatology clinics. Botanical and natural ingredients have gained popularity as alternative depigmenting products.
Objective. We sought to review clinical studies evaluating the use of different natural products in treating hyperpigmentation so clinicians are better equipped to educate their patients. Specific ingredients reviewed include azelaic acid, aloesin, mulberry, licorice extracts, lignin peroxidase, kojic acid, niacinamide, ellagic acid, arbutin, green tea, turmeric, soy, and ascorbic acid.
Methods. Systematic searches of PubMed and SCOPUS databases were performed in March 2016 using the various ingredient names, “melasma”and “hyperpigmentation.” Two reviewers independently screened titles, leading to the selection of 30 clinical studies.
Results. Review of the literature revealed few clinical trials that evaluated the treatment of hyperpigmentation with natural ingredients. Despite the limited evidence-based research, several natural ingredients did show efficacy as depigmenting agents, including azelaic acid, soy, lignin peroxidase, ascorbic acid iontophoresis, arbutin, ellagic acid, licorice extracts, niacinamide, and mulberry.
Conclusion. The aforementioned ingredients show promise as natural treatments for patients with hyperpigmentation disorders. These agents might also provide clinicians and researchers with a way to further characterize the pathogenesis of dyschromia. However, the paucity of clinical studies is certainly a limitation. Additionally, many of the in-vivo studies are limited by the short length of the trials, and questions remain about the long-term efficacy and safety of the ingredients used in these studies. Lastly, we suggest a standardized objective scoring system be implemented in any further comparative studies.
Keywords: melasma, hyperpigmentation, natural ingredients
J Clin Aesthet Dermatol. 2018;11(2):28–37
Introduction
Disorders of hyperpigmentation such as melasma and post-inflammatory hyperpigmentation are common reasons for visits to dermatology practices. Dyschromias can occur due to alterations in the various biochemical processes that regulate melanogenesis. Such alterations might lead to an increase in melanocytes, melanosome production, melanin synthesis, or melanocyte hyperplasias, which cause more melanin deposition in the skin. Research exploring the pathophysiology of hyperpigmentation disorders has expanded greatly over the past decade, leading to the investigation and development of a number of skin lighteners. Botanical and other naturally occurring ingredients for the treatment of pigment disorders have gained increasing popularity. Despite their increasing use, many lack in-vivo and/or in-vitro studies to validate their efficacy. The objective of this review is to examine the literary evidence supporting the clinical utility of natural ingredients in the treatment of hyperpigmentation.
Methods
In March 2016, systematic searches of PubMed and SCOPUS databases were performed using “melasma,” “hyperpigmentation,” and the following ingredient names: “azelaic acid,” “aloesin,” “mulberry,” “licorice extracts,” “lignin peroxidase,” “kojic acid,” “niacinamide,” “ellagic acid,” “arbutin,” “green tea,” “turmeric”, “soy,” and “ascorbic acid.” Only clinical studies that evaluated the effect of herbal and natural supplements on pigmentation disorders were included. Two reviewers independently screened titles, leading to the selection of 30 clinical studies based on inclusion criteria.
Results
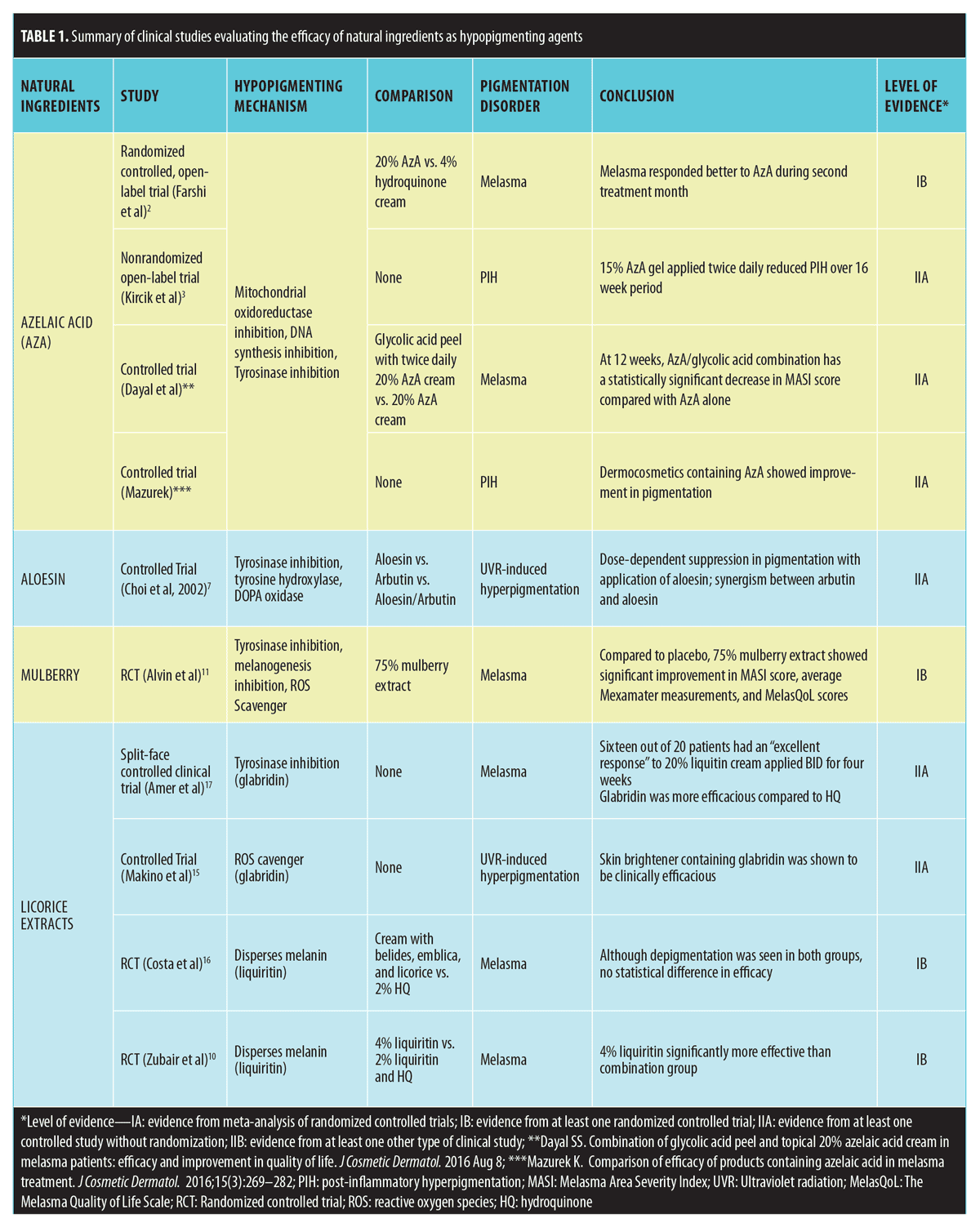
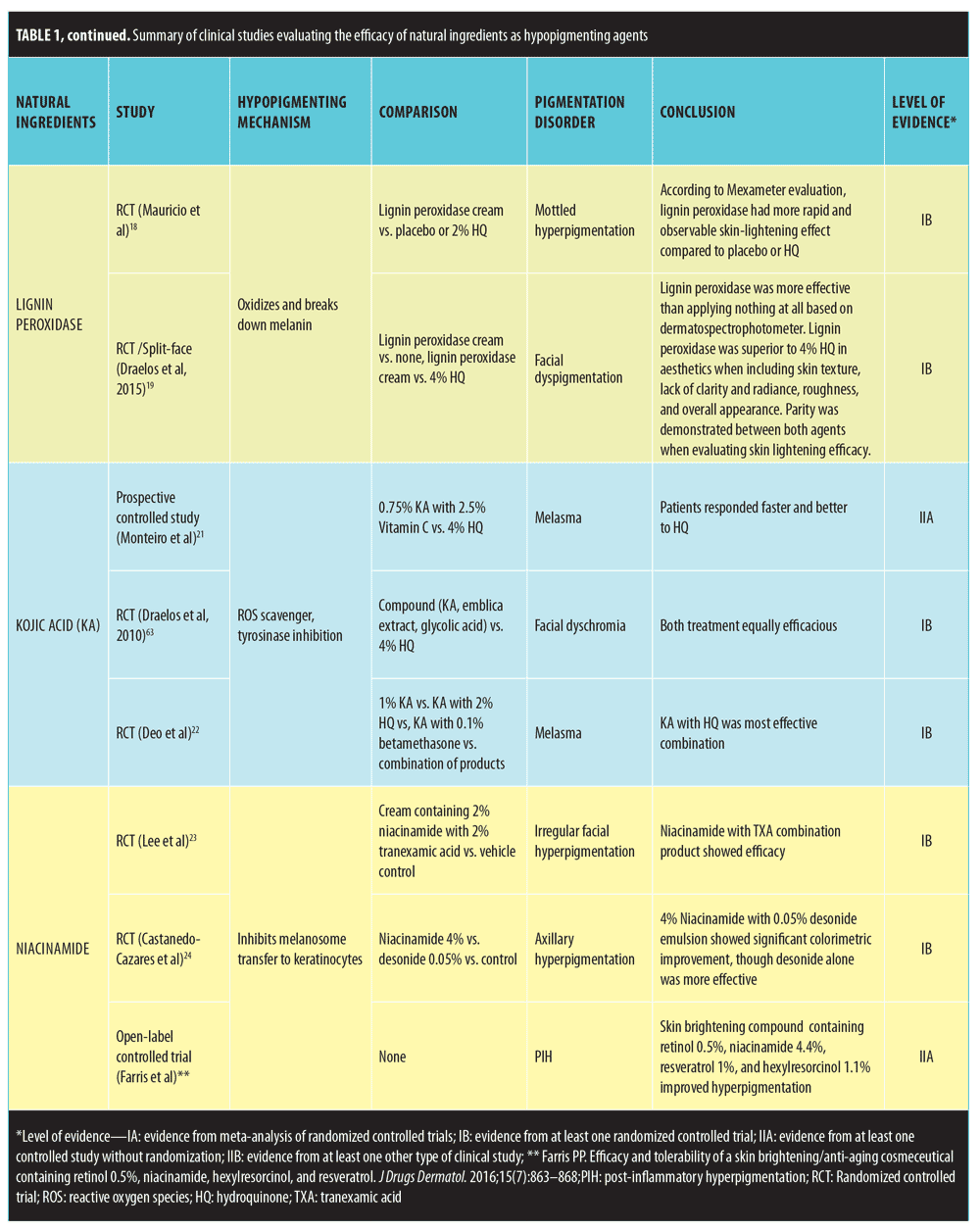
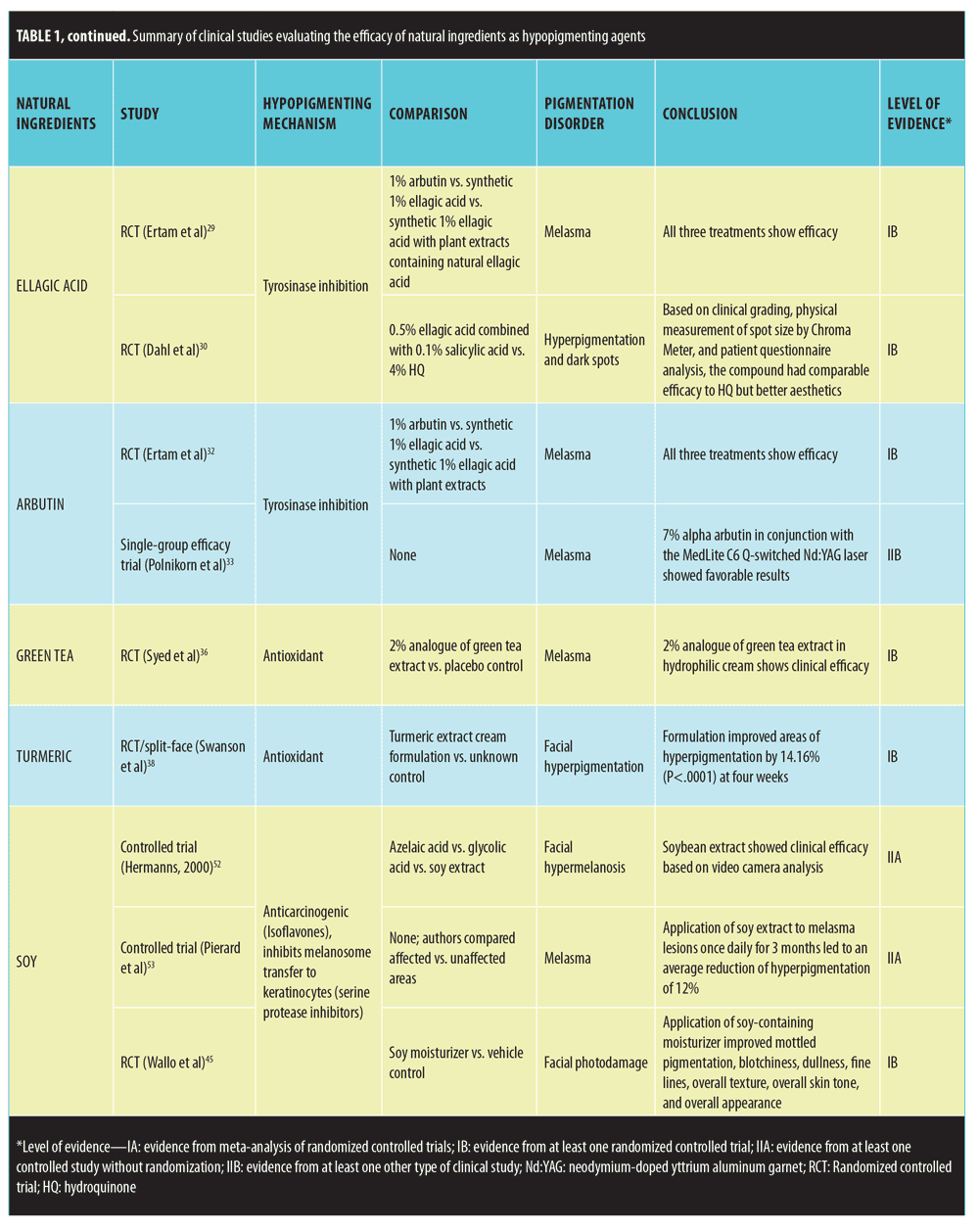
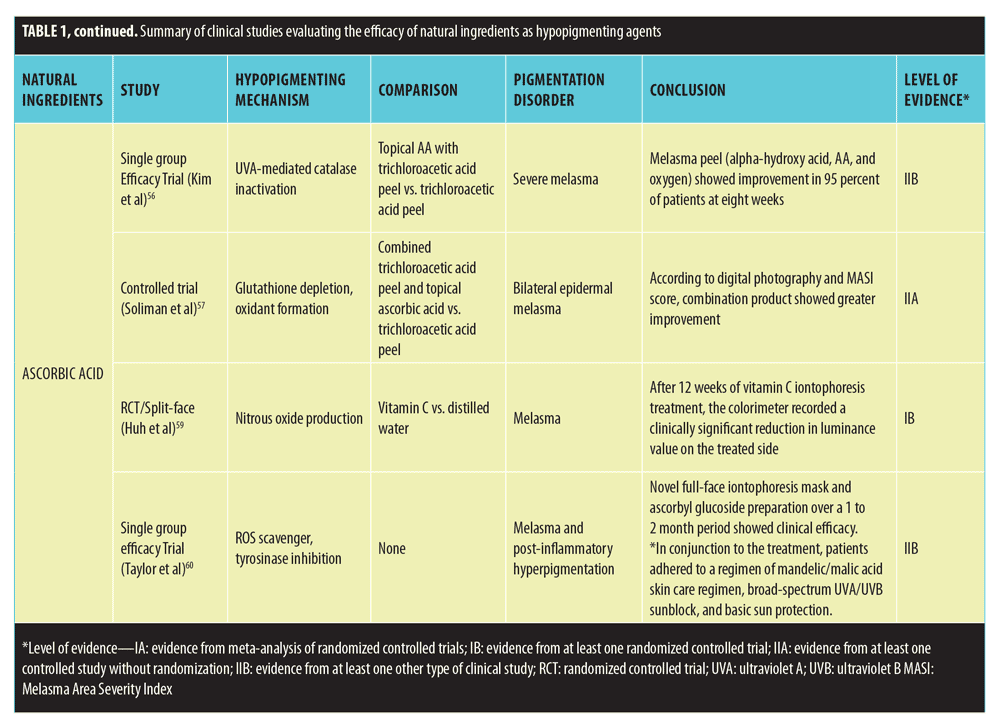
Studies that met inclusion criteria are summarized in Table 1.
Discussion
Azelaic acid. Azelaic acid (AzA) is a saturated 9-carbon dicarboxylic acid derived from the fungus Pityrosporum ovale and can be found in rye, wheat, and barley. Azelaic acid interferes with deoxyribonucleic acid (DNA) synthesis, inhibits mitochondrial oxidoreductase, competitively inhibits tyrosinase, and decreases free radical formation. This agent preferentially targets abnormal and highly active melanocytes with minimal effect on uninvolved skin.1,2 Most clinical trials study azelaic acid as an acne treatment. However, one recent open-label clinical trial performed over two months compared 20% azelaic acid to 4% hydroquinone cream in 29 melasma patients. Based on Melasma Area Severity Index (MASI) scores used to quantify treatment response, the authors concluded that melasma pigmentation was improved more in those using azelaic acid compared to the hydroquinone group only during the second month of treatment.2 In a 16-week, baseline-controlled study of 20 patients with Fitzpatrick Skin Types IV to VI, 15% azelaic gel applied twice daily showed a reduction in acne and post-inflammatory hyperpigmentation (PIH).3 Patients experienced a 2-point improvement according to the investigator’s global assessment score. Recently, a controlled trial performed in India studied 60 patients with epidermal melasma. Half of the participants were treated with a glycolic acid peel every three weeks and twice daily 20% AzA cream; the other half was treated with only AzA cream. The AzA/glycolic acid group showed a statistically significant decrease in MASI score compared to the AzA control group 12 weeks onwards.2 Another controlled trial performed in Poland found that dermocosmetics containing azelaic acid showed improvement in pigmentation measured with a skin colorimeter (Mexameter®, C+K Electronics, Cologne, Germany). Despite these studies, more well-designed clinical trials are still necessary. Additionally, objective methods for the quantifying pigmentation are still lacking, which presents difficulty in evaluating many natural and botanical ingredients.
Aloesin. Aloesin is derived from the aloe vera plant and has been shown to inhibit tyrosinase, tyrosine hydroxylase, and dopa oxidase, according to in-vitro studies. Aloesin has direct inhibitory effects on melanogenesis and dose-dependent reductions in melanin content and tyrosinase activity using an in-vitro pigmented skin equivalent.4 It might even work synergistically with arbutin in vitro.5,6 Only a single in-vivo study evaluates aloesin’s efficacy as a depigmenting agent.5,6 Choi et al7 evaluated the inhibitory effect of aloesin on pigmentation induced by ultraviolet radiation (UVR) to the inner forearm. The UV-irradiated regions included a vehicle control group, aloesin treated group, arbutin treated group, and arbutin plus aloesin treated group. Aloesin and arbutin individually and in combination were applied four times daily for 15 days. The authors illustrated a dose-dependent suppression in the aloesin treatment group. This study also supported the synergism between arbutin and aloesin as cotreatment resulted in greater pigmentation suppression than either ingredient alone.7 These promising results should pave the way for further clinical studies.
Mulberry. Mulberry is an extract derived from dried mulberry leaves, Morus alba. In several East Asian countries, the leaves from mulberry trees are used to feed silkworms and have been used in traditional Chinese and Thai medicine in the treatment and prevention of diabetes.8 According to in-vitro studies, Mulberroside F, mulberry’s active component, inhibits tyrosinase activity, melanin formation in melan-cells, melanin transfer,9 and might serve as a reactive oxygen species (ROS) scavenger.6,9 To date, there has been one randomized controlled trial (RCT) investigating mulberry use in pigmentary disorders. Alvin et al11 conducted a randomized, single-blind, placebo-controlled trial investigating the safety and efficacy of 75% mulberry extract oil versus placebo in treating melasma. There was significant improvement in the MASI score, average skin colorimeter measurements, and The Melasma Quality of Life Scale (MelasQOL) scores in the treatment group.10,11
Licorice Extracts. Glabridin, extracted from the root of perennial herb Glycyrrhiza glabra linneva, is the main licorice compound.12 This ingredient has been shown to scavenge ROS, inhibit UVB-induced pigmentation and tyrosinase without affecting DNA synthesis, and possess anti-inflammatory properties. Glabridin has been shown in vitro to have a skin lightening effect 16 times greater than that of hydroquinone and might reduce UVB pigmentation.13,14 A single-center, double-blind comparison clinical study with 18 patients compared the efficacy of a hydroquinone-free skin brightener, comprising several ingredients, including glabridin, that target different pathways in melanogenesis, to 4% hydroquinone (HQ) cream in reducing ultraviolet-induced hyperpigmentation.15 The skin brightener demonstrated significant reductions in pigmentation compared to baseline and produced greater increases in L* brightness compared to HQ. In addition to assessing in-vivo data, this study used an in-vitro model called MelanoDerm™ Skin Model (MatTek Corp., to assess the ability of this product to reduce melanin production and distribution compared to controls. In the MelanoDerm Skin Model in-vitro portion, the test product resulted in greater reduction in melanin as measured by melanin content and histological staining compared to the control.15 Another single-blinded study compared a cream containing belides, emblica, and licorice applied twice daily to HQ 2% applied nightly in melasma patients of Fitzpatrick Skin Types I to IV after the patients had 60 days of exclusive use of an sun protection factor (SPF) 35 sunscreen. Although depigmentation was noted in both groups, there was no statistical difference between them in the improvement of melasma.16
Liquiritin, a flavonoid component of licorice, has multiple depigmenting properties, including dispersing melanin, reducing inflammation, and reducing UVB erythema.10 Amer et al17 conducted a double-blind, controlled, split-face study of 20 women with epidermal melasma. Subjects applied 20% liquiritin cream on one side of the face and a vehicle cream on the other side twice daily for four weeks. The majority treated with liquiritin showed an “excellent response” compared to the control group, which exhibited no response. In a RCT conducted by Zubair et al10 in epidermal melasma patients, 4% liquiritin was shown to be significantly more effective than 2% liquiritin and HQ.
Lignin peroxidase. The enzyme lignin peroxidase is derived from the tree fungus Phanerochaete chrysosporium and acts by oxidizing and breaking down melanin. In decaying trees, lignin, which is structurally similar to melanin, is broken down by lignin peroxidase, resulting in decolorization.18 Mauricio et al18 conducted a randomized, double-blind, controlled, paired, split-face, single-center study of 51 Asian female patients. Lignin peroxidase cream was applied on one side of the face and either 2% HQ or placebo was applied on the other. The primary outcome variable was reduction in the melanin index with a sin colorimeter (Mexameter). Lignin peroxidase cream had a significantly more rapid and observable skin-lightening effect than placebo and 2% HQ. A more recent randomized paired, controlled, split-face study by Draelos et al19 investigated the pigment lightening efficacy of lignin peroxidase in a cohort of women with mild-to-moderate facial dyspigmentation. In this 12 week study, Cohort 1 applied lignin peroxidase to one side of the face twice daily and nothing to the other side. Cohort 2 applied twice-daily lignin peroxidase to one half of the face and 4% HQ to the other half twice daily. Subjects were assessed at baseline and at Weeks 2, 8, and 12. Subject, investigator, and dermospectrophotometer measurements were obtained. In Cohort 1, lignin peroxidase produced skin lightening superior to the control group. In Cohort 2, lignin peroxidase produced superior results in aesthetics when compared to HQ including skin texture, lack of clarity and radiance, roughness, and overall appearance. However, parity was demonstrated between both agents when evaluating skin lightening efficacy. Lignin peroxidase does show promise as a skin lightener based on the studies available, but more studies are warranted.
Kojic Acid. Kojic acid (KA) is a metabolic product of the fungal species Acetobacter, Aspergillus, and Penicillium. It acts as a ROS scavenger, exhibits antioxidant properties, and inhibits tyrosinase.20 KA is used in several cosmetic skin brighteners and is also used as a food additive to prevent browning.1,12 Over the years, there have been mixed reports on the efficacy of KA. Based on earlier work, KA as a monotherapy has shown modest effectiveness, but it has been shown to be more beneficial in combination with other ingredients. KA stable derivatives increase skin penetration, which offers better skin lightening. A recent comparative study by Monteiro et al21 evaluated the efficacy of once-daily application of 4% HQ and 0.75% KA cream, which contained 0.75% KA and 2.5% vitamin C, in the treatment of melasma. Sixty patients were enrolled in this 12-week study. The authors found that at Week 4, patients responded earlier to the HQ than to the KA cream. However, at Week 12, HQ had overall superiority in lightening compared to the KA cream. Draelos et al63 performed 12-week, paired, double-blind study comparing a preparation containing KA, emblica extract, and glycolic acid to 4% HQ in 80 multiethnic patients with facial dyschromia. Interestingly, the results showed equivalent efficacy in skin lightening capabilities between the two agents. Another 12-week simple, randomized, single-center, single-blinded, parallel-group comparative study comprising 80 subjects with melasma compared the efficacy of KA 1% alone with either 2% HQ or 0.1% betamethasone valerate, and a combination of all these three agents. Patients were assessed using the MASI score. The study found that KA plus HQ was superior in depigmenting when compared with the other three groups.22 With the conflicting studies and lack of investigation exploring KA’s role as monotherapy, more clinical trials are warranted
Niacinamide. Niacinamide, an active form of vitamin B3 (niacin) found in yeast and root vegetables, is well known for its role in enzymatic reaction.12 It combats hyperpigmentation by reversibly inhibiting the transfer of melanosomes to epidermal keratinocytes. A recent eight-week, prospective, randomized, double-blind, vehicle-controlled clinical study evaluated a combination of niacinamide and tranexamic acid (TXA) as a topical moisturizer in the treatment of 42 Korean women with irregular facial hyperpigmentation. This formulation was significantly more effective in reducing the hyperpigmentation than the vehicle control.23 In a nine-week, randomized, double-blind, left-right axilla, placebo-controlled trial, the efficacy of niacinamide 4% and desonide 0.05% emulsions were compared to placebo in the treatment of axillary hyperpigmentation. Twenty-four women of Phototypes III to V were included in the study. Niacinamide and desonide both induced significant colorimetric improvement compared to placebo, but desonide showed the best depigmenting effect overall.24 A derivative of niacinamide has also shown potential as a skin lightener. Through an in-vitro study, Kim et al25 synthesized a novel derivative niacinamide, N-nicotinoyl dopamine (NND), which was shown to have high antioxidant activity and ability to decrease melanin production and induce skin lightening in a skin model. A recent open-label, single-center study investigated a compound containing 0.5% retinol, 4.4% niacinamide, 1% resveratrol, and 1.1% hexylresorcinol in a moisturizing base on 25 subjects with mild-to-moderate hyperpigmentation. This skin-brightening compound improved hyperpigmentation with statistical significance compared to baseline by Week 4. Further evaluation of niacinamide and NND should be performed in the form of clinical studies.
Ellagic acid. Ellagic acid is a polyphenol antioxidant found in trees, nuts, and fruit.27 In-vitro studies suggest that ellagic acid inhibits melanogenesis through the reduction of tyrosinase activity.28 Two randomized, controlled trials (RCT) have evaluated its skin lightening effects. The first study compared the efficacy of 1% arbutin, synthetic 1% ellagic acid, and synthetic 1% ellagic acid combined with plant extracts containing natural ellagic acid in treating 30 patients with melasma.29 All three treatment groups showed significant improvement according to a skin colorimeter (Mexameter) evaluation before and after treatment. The study was limited by the fact that the natural plant extracts were not studied independently. The second RCT compared the effects of a compound containing 0.5% ellagic acid combined with 0.1% salicylic acid to 4% hydroquinone in 54 patients with hyperpigmentation and dark spots.30 Based on clinical grading, physical measurement of spot size by chromameter, and patient questionnaire analysis, the compound had comparable efficacy to hydroquinone but better aesthetics. However, this study was limited because ellagic acid was studied in combination with salicylic acid. Since ellagic acid has not been independently studied, further studies need to be performed to support its clinical utility.
Arbutin. Arbutin is a â-D-glucopyranoside derivative of hydroquinone found in herbs such as bearberry.31 In-vitro studies have shown it to have reversible tyrosinase activity. The previously mentioned RCT that compared 1% arbutin with 1% ellagic acid in treating melasma demonstrated arbutin to be clinically efficacious as evidenced by the clinical improvement of all 10 patients in the arbutin treatment group.32 Another prospective study investigating the use of 7% alpha arbutin in conjunction with a frequency-doubled Q-switched Nd:YAG laser (MedLite C6®, Cynosure®, Westford, Massachusetts) also showed favorable results in treating melasma patients.33 However, this study was limited because arbutin was not studied independent of the laser treatment. Since there has only been a single RCT independently supporting arbutin’s clinical efficacy in treating pigmentary disorders, further studies are needed.
Green Tea. Green tea has long been studied for its antioxidant and anti-inflammatory properties.28 Green tea extracts comprise multiple polyphenolic antioxidants, of which epigallo-catechin-3-gallate (ECGC) is the main active ingredient.34,35 According to the abstract of a single RCT, green tea has shown clinical efficacy in treating melasma.36 In the study, 60 women with melasma were treated with a 2% analogue of green tea extract (ECGC) in a hydrophilic cream or placebo and were tracked using dermatologic and photographic methods. Lesions cleared in 60 percent of the experimental group versus three percent of the placebo group, a clinically significant difference. However, this study is limited because it has not yet been published in a peer-reviewed journal. Additionally, more studies are required to validate therapeutic effects of green tea extracts on pigmentary disorders. In addition to treating melasma, green tea extracts might have prophylactic properties, including inhibiting UV-induced erythema, reducing the number of sunburn cells, and protecting DNA from UV radiation in human studies.37
Turmeric. Turmeric (Curcuma longa) is a widely-used Ayurvedic herbal supplement and spice.38,39 The active ingredient of turmeric is curcumin, a hydrophobic polyphenol characterized by yellow pigment.39 Studies have shown curcumin to possess anti-inflammatory and anti-carcinogenic properties.40–42 Recently, an in-vitro study has suggested that curcumin might induce apoptosis of human melanoma cells via mitochondrial pathway and caspases activation.43 According to the abstract of a dual study RCT, application of topical turmeric extract reduced the appearance of facial hyperpigmentation and fine lines and wrinkles.38 The first study was a split-face study among Caucasian women that compared turmeric extract combined with niacinamide in cream to niacinamide alone. The combination product was significantly better at improving fine lines and wrinkles according to a group of judges. The second study was a split-face study among Chinese women that compared a turmeric extract cream formulation to an unknown control. The formulation improved areas of hyperpigmentation by 14.16 percent (p<0.0001) at four weeks according to negative cofactor-2 (NC2) image analysis. However, this RCT is limited because the study has not yet been published in a peer-reviewed journal. Further clinical studies need to be performed that evaluate the therapeutic effects of turmeric
Soy. Soybean, a legume commonly grown in East Asia, consists of many biologically active substances, including isoflavones and serine protease inhibitors.44 In-vitro studies have uncovered the anti-aging, antioxidant, pigment-reducing, photoprotective, and melanosome transfer inhibiting properties of soybean extract.45–50 Several clinical studies support the hypothesized skin-lightening role of soybean. In a controlled trial, Hermanns et al52 compared the effect of different topical hypopigmenting agents in treating facial hypermelanosis in 44 Celtic-complexioned men. Soybean extract had skin-lightening effects in the study. Another study involving Caucasian and Hispanic women found that application of soy extract to melasma lesions once daily for three months led to an average reduction of hyperpigmentation of 12 percent.53 Fourteen out of the 16 women showed some degree of improvement. Additionally, a recent double-blind, parallel-group RCT compared the efficacy of a nondenatured novel soy moisturizer to the vehicle alone in treating 65 women with moderate facial photodamage.45 Evaluation through clinical observation, self-assessment, colorimetry, and photography over a 12-week period demonstrated the soy-containing moisturizer to have a more favorable outcome in terms of improving mottled pigmentation, blotchiness, dullness, fine lines, overall texture, overall skin tone, and overall appearance than the vehicle alone. In reference to mottled hyperpigmentation, 28 out of 31 treatment-group patients experienced a certain degree of depigmentation compared to 17 out of 32 control-group patients. Promising results from multiple RCTs support the clinical use of soybean extract in treating hyperpigmentation.
Ascorbic acid. Ascorbic acid (AA; vitamin C) is an acidic, hydrophilic antioxidant most commonly found in citrus fruit and serves as a cofactor for several human enzymatic processes.54 AA plays a notable role in wound healing, catecholamine synthesis, tyrosine degradation, bile acid synthesis, iron absorption, neurotransmitter synthesis, and immune system function.54 According to in-vitro and in-vivo studies, AA might have antimelanogenic properties and, as a result, might be beneficial in treating hyperpigmentation.55 With regard to clinical studies, Kim et al56 investigated a superficial chemical peel (Theraderm®, Therapon Skin Health, LP, Springdale, Arizona), which consists of alpha-hydroxy acid, AA, and oxygen in treating 25 Korean patients with severe melasma. According to photographic assessment during eight weeks of treatment, 96 percent of patients showed improvement in hyperpigmentation. Another clinical study compared the effect of combined topical AA and trichloroacetic acid peel versus trichloroacetic acid peel alone in treating 30 women with bilateral epidermal melasma.57 Evaluation by digital photography and MASI demonstrated that 87 percent of patients using the combination therapy versus 67 percent of patients using trichloroacetic acid peel alone showed improvement or maintained improvement in their melasma. Unfortunately, all of these studies are limited by the fact that AA was not studied independently.
Fortunately, a few studies have investigated vitamin C iontophoresis as a possible treatment for hyperpigmented lesions as it allows for greater AA penetration. Huh et al59 also utilized vitamin C iontophoresis to treat 29 women with melasma. In this double-blind, placebo-controlled RCT, vitamin C solution was applied to one half of the face and distilled water (control) was applied to the other half. After 12 weeks of iontophoresis treatment, the colorimeter recorded a clinically significant reduction in luminance value on the treated side compared to the control side. Similarly, a controlled study was performed by Taylor et al60 that involved treating 35 patients with melasma or post-inflammatory hyperpigmentation with a novel full-face iontophoresis mask and ascorbyl glucoside preparation over a 1- to 2-month period. In conjunction with the treatment, patients adhered to a regimen of mandelic/malic acid skin care regimen, broad-spectrum UVA/UVB sunblock, and basic sun protection. A group of four independent graders determined that there was a mean 73-percent improvement in abnormal pigmentation, greater than 25-percent improvement in 32 patients, and greater than 50-percent improvement in 22 patients. Both these studies support the role of vitamin C iontophoresis in treating melasma.
Summary
AzA’s depigmentation mechanism involves inhibition of mitochondrial oxidoreductase, DNA synthesis, and tyrosinase activity. Two RCTs showed that AzA can be used to treat melasma and PIH.2,3 Aloesin inhibits tyrosinase, tyrosine hydroxylase, and DOPA oxidase.4 In a single RCT, Choi et al7 found that aloesin is effective at treating UVR-induced pigmentation both independently as well as synergistically with arbutin. Mulberry is an ROS scavenger with tyrosinase and other melanogenesis inhibitory properties.8 Alvin et al11 showed in a RCT that 7% mulberry extract is beneficial in treating melasma. Licorice extract contains liquiritin, which disperses melanin, and glabridin, an ROS scavenger and tyrosinase inhibitor. Multiple RCTs show that licorice extract components have clinical efficacy in treating melasma and UVR-induced pigmentation.14–16 Lignin peroxidase, which oxidizes and breaks down melanin, can successfully treat mottled hyperpigmentation and facial dyspigmentation according to two RCTs.17,18 Mauricio et al18 revealed that lignin peroxidase had more rapid and observable skin-lightening effect compared to HQ or placebo Kojic acid, an ROS scavenger and tyrosinase inhibitor, shows clinical efficacy in treating facial dyschromia and melasma.20 In a RCT performed by Monteiro et al,21 kojic acid was found to work better and faster than HQ for treating melasma. Niacinamide, which inhibits the transfer of melanosomes to keratinocytes, has shown clinical efficacy in treating facial and axillary hyperpigmentation in two separate RCTs.23–25 Ellagic acid is a tyrosinase inhibitor that can successfully treat melasma, as well as hyperpigmentation and dark spots.27–30 Arbutin, which is also a tyrosinase inhibitor, has been successful in treating melasma according to two RCTs.31,32 Green tea and turmeric have been studied for their antioxidant properties.34–37 Interestingly, green tea and turmeric are clinically efficacious in treating melasma and facial hyperpigmentation, respectively. Soy, an anticarcinogen, inhibits melanosome transfer to keratinocytes. Multiple RCTS have shown soy to have promising results in treating facial hypermelanosis, melasma, and facial photodamage.45–53 Lastly, ascorbic acid’s depigmenting mechanism might involve UVA-mediated catalase inactivation, glutathione depletion, oxidant formation, and nitrous oxide production. Ascorbic acid has been shown to successfully treat severe melasma, bilateral epidermal melasma, melasma, and PIH.54–63
Conclusion
The number of patients that visit dermatologists with pigmentary disorders is significant.61,62 Patients are often overwhelmed with numerous over-the-counter skin lightening agents, many without clinical evidence of efficacy. Botanical and natural ingredients have become popular as depigmenting products and provide an alternative to the current gold standard, hydroquinone. However, evidence-based studies on many of these agents is still lacking. Much of the data that exist for these agents consist primarily of in-vitro studies and a handful of clinical trials. Also, many of the in-vivo studies are limited by the short length of the trials, leaving questions regarding long-term efficacy and safety. Despite the need for more long-term, well-designed, randomized, controlled studies, several botanical and natural ingredients do show inital promise in treating disorders of hyperpigmentation based on the results of clinical trials. These ingredients are AA, soy, lignin peroxidase, ascorbic acid iontophoresis, arbutin, ellagic acid, licorice extracts, niacinamide, and mulberry. In addition to showing promise in treating hyperpigmentation, these agents also provide greater insight into the pathogenesis of dyschromias, thus enhancing our understanding of the many complexities of pigment disorders.
References
- Vashi NA, Kundu RV. Facial hyperpigmentation: causes and treatment. Br J Dermatol. 2013;169 Suppl 3:41–56.
- Farshi S. Comparative study of therapeutic effects of 20% azelaic acid and hydroquinone 4% cream in the treatment of melasma. J Cosmet Dermatol. 2011;10(4):282–287.
- Kircik LH. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of post-inflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10(6):586–90.
- Wang Z, Li X, Yang Z, et al. Effects of aloesin on melanogenesis in pigmented skin equivalents. Int J Cosmet Sci. 2008;30(2):121–130.
- Gillbro JM, Olsson MJ. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int J Cosmet Sci. 2011;33(3):210–221.
- Zhu W, Gao J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J Investig Dermatol Symp Proc. 2008;13(1): 20–24.
- Choi S, Lee SK, Kim JE, et al. Aloesin inhibits hyperpigmentation induced by UV radiation. Clin Exp Dermatol. 2002;27(6):513–515.
- Nattapong S, Omboon L. A new source of whitening agent from a thai mulberry plant and its betulinic acid quantitation. Nat Prod Res. 2008;22(9):727–734.
- Singh SK, Baker R, Wibawa JI, et al. The effects of sophora angustifolia and other natural plant extracts on melanogenesis and melanin transfer in human skin cells. Exp Dermatol. 2013;22(1):67–69.
- Zubair S, Mujtaba G. Comparison of efficacy of topical 2% liquiritin, topical 4% liquiritin and topical 4% hydroquinone in the management of melasma. J Pakistan Assoc Dermatologist 2009;19:158–163.
- Alvin G, Catambay N, Vergara A, Jamora MJ. A comparative study of the safety and efficacy of 75% mulberry (morus alba) extract oil versus placebo as a topical treatment for melasma: a randomized, single-blind, placebo-controlled trial. J Drugs Dermatol. 2011 Sep;10(9):1025–1031.
- Leyden JJ, Shergill B, Micali G, et al. Natural options for the management of hyperpigmentation. J Eur Acad Dermatol Venereol. 2011;25(10):1140–1145.
- Rendon MI, Gaviria JI. Review of skin-lightening agents. Dermatol Surg. 2005;31(7 Pt 2):886,9; discussion 889.
- Yokota T, Nishio H, Kubota Y, Mizoguchi M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res.1998;11(6):355–361.
- Makino ET, Mehta RC, Banga A, et al. Evaluation of a hydroquinone-free skin brightening product using in-vitro inhibition of melanogenesis and clinical reduction of ultraviolet-induced hyperpigmentation. J Drugs Dermatol. 2013;12(3):s16–20.
- Costa A, Moises TA, Cordero T, Alves CR, Marmirori J. Association of emblica, licorice and belides as an alternative to hydroquinone in the clinical treatment of melasma. An Bras Dermatol. 2010;85(5):613–620.
- Amer M, Metwalli M. Topical liquiritin improves melasma. Int J Dermatol. 2000;39(4):299–301.
- Mauricio T, Karmon Y, Khaiat A. A randomized and placebo-controlled study to compare the skin-lightening efficacy and safety of lignin peroxidase cream vs. 2% hydroquinone cream. J Cosmet Dermatol. 2011;10(4):253–259.
- Draelos ZD. A split-face evaluation of a novel pigment-lightening agent compared with no treatment and hydroquinone. J Am Acad Dermatol. 2015;72(1):105–107.
- Lajis AF, Hamid M, Ariff AB. Depigmenting effect of kojic acid esters in hyperpigmented B16F1 melanoma cells. J Biomed Biotechnol. 2012;2012:952452.
- Monteiro RC, Kishore BN, Bhat RM, et al. A comparative study of the efficacy of 4% hydroquinone vs 0.75% kojic acid cream in the treatment of facial melasma. Indian J Dermatol. 2013;58(2):157,5154.108070.
- Deo KS, Dash KN, Sharma YK, et al. Kojic acid vis-a-vis its combinations with hydroquinone and betamethasone valerate in melasma: a randomized, single blind, comparative study of efficacy and safety. Indian J Dermatol. 2013;58(4):281–285.
- Lee do H, Oh IY, Koo KT, et al. Reduction in facial hyperpigmentation after treatment with a combination of topical niacinamide and tranexamic acid: a randomized, double-blind, vehicle-controlled trial. Skin Res Technol. 2014;20(2):208–212.
- Castanedo-Cazares JP, Larraga-Pinones G, Ehnis-Perez A, et al. Topical niacinamide 4% and desonide 0.05% for treatment of axillary hyperpigmentation: a randomized, double-blind, placebo-controlled study. Clin Cosmet Investig Dermatol. 2013;6:29–36.
- Kim B, Kim JE, Lee SM, et al. N-nicotinoyl dopamine, a novel niacinamide derivative, retains high antioxidant activity and inhibits skin pigmentation. Exp Dermatol. 2011;20(11): 950–952.
- Herndon JH, Makino ET, Stephens TJ, Mehta RC. Hydroquinone-free skin brightener system for the treatment of moderate-to-severe facial hyperpigmentation. J Clin Aesthet Dermatol. 2014;7(5):27–31.
- Lei Z. Use of methanolysis for the determination of total ellagic and gallic acid contents of wood and food products. J Agric Food Chem. 2001;49(3):1165–1168.
- Shimogaki H, Tanaka Y, Tamai H, Masuda M. In-vitro and in-vivo evaluation of ellagic acid on melanogenesis inhibition. Int J Cosmet Sci. 2000;22(4):291–303.
- Ertam I, Mutlu B, Unal I, et al. Efficiency of ellagic acid and arbutin in melasma: a randomized, prospective, open-label study. J Dermatol. 2008;35(9):570–574.
- Dahl A, Yatskayer M, Raab S, Oresajo C. Tolerance and efficacy of a product containing ellagic and salicylic acids in reducing hyperpigmentation and dark spots in comparison with 4% hydroquinone. J Drugs Dermatol. 2013;12(1):52–58.
- Chakraborty AK, Funasaka Y, Komoto M, Ichihashi M. Effect of arbutin on melanogenic proteins in human melanocytes. Pigment Cell Res. 1998;11(4):206–212.
- Ertam I, Mutlu B, Unal I, Alper S, et al. Efficiency of ellagic acid and arbutin in melasma: a randomized, prospective, open-label study. J Dermatol. 2008;35(9):570–574.
- Polnikorn N. Treatment of refractory melasma with the MedLite C6 Q-switched nd:YAG laser and alpha arbutin: a prospective study. J Cosmet Laser Ther. 2010;12(3):126; 126–131.
- Katiyar SK. Green tea and skin. Arch Dermatol (1960). 2000;136(8):989–994.
- Farris P. Idebenone, green tea, and coffeeberry® extract: new and innovative antioxidants novel antioxidants. Dermatol Ther. 2007;20(5):322–329.
- Syed T, Aly R, Ahmad SA, et al. Management of melasma with 2% analogue of green tea extract in a hydrophilic cream: a placebo-controlled, double-blind study. J Am Acad Dermatol. 2009;60(3 Suppl 1):AB160.
- Elmets CA. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol. 2001;44(3):425–432.
- Swanson, C, Deng, D, Robinson, L, Raleigh, P. Topical turmeric extract in a moisturizing cream formula reduces the appearance of facial spots and fine lines and wrinkles on human facial skin. J Am Acad Dermatol. 2010;62(3 Suppl1):AB19.
- Arct J, Ratz-Lyko A, Mieloch M, Witulska M. Evaluation of skin colouring properties of curcuma longa extract. Indian J Pharm Sci. 2014;76(4): 374–378.
- Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer -I. curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000;3(2):84–93.
- Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer. II. curcumin inhibits tyrosine kinase activity of epidermal growth factor receptor and depletes the protein. Mol Urol. 2000;4(1):1–6.
- Dorai T, Cao Y, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. 2001;47(4):293–303.
- Jiang AJ, Jiang G, Li LT, Zheng JN. Curcumin induces apoptosis through mitochondrial pathway and caspases activation in human melanoma cells. Mol Biol Rep. 2014:42(1);267–275.
- Fritz H. Soy, red clover, and isoflavones and breast cancer: A systematic review. PloS One. 2013;8(11):e81968.
- Wallo W. Efficacy of a soy moisturizer in photoaging: a double-blind, vehicle-controlled, 12-week study. J Drugs Dermatol. 2007;6(9): 917–922.
- Gopaul R, Knaggs HE, Lephart ED. Biochemical investigation and gene analysis of equol: a plant and soy-derived isoflavonoid with antiaging and antioxidant properties with potential human skin applications. Biofactors. 2012;38(1):44–52.
- Mahmoud AM, Yang W, Bosland MC. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J Steroid Biochem Mol Biol. 2014;140:116–132.
- Callender VD. Postinflammatory hyperpigmentation etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12(2):87–99.
- Chen N. Nondenatured soy extracts reduce UVB-induced skin damage multiple mechanisms. Photochem Photobiol. 2008;84(6):1551–1559.
- Huang M, Xie J, Lin CB, et al. Inhibitory effect of topical applications of nondenatured soymilk on the formation and growth of UVB-induced skin tumors. Oncol Res. 2004;14(7–8):387–397.
- Hermanns JF, Petit L, Piérard-Franchimont C, et al. Assessment of topical hypopigmenting agents on solar lentigines of asian women. Dermatology. 2002;204(4):281–286.
- Hermanns JF, Petit L, Martalo O, et al. Unraveling the patterns of subclinical pheomelanin-enriched facial hyperpigmentation: effect of depigmenting agents. Dermatology. 2000;201(2):118–122.
- Pierard G, Graf R, Gonzalez R, Cauwenbergh W. Effects of soy on hyperpigmentation in Caucasian and Hispanic populations. 59th annual meeting of the American Academy of Dermatology; March 2–7, 2001; Washington, DC.
- Moores J. Vitamin C: A wound healing perspective. Br J Community Nurs. 2013;Suppl:S6, S8–S11.
- Panich U. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch Pharm Res. 2011;34(5):811–820.
- Kim W. Efficacy and safety of a new superficial chemical peel using alpha-hydroxy acid, vitamin C and oxygen for melasma. J Cosmet Laser Ther. 2013;15(1):21–24.
- Soliman MM, Ramadan SA, Bassiouny DA, Abdelmalek M. Combined trichloroacetic acid peel and topical ascorbic acid versus trichloroacetic acid peel alone in the treatment of melasma: a comparative study. J Cosmet Dermatol. 2007;6(2):89–94.
- Khemis A, Cabou J, Dubois J, Ortonne J. A randomized controlled study to evaluate the depigmenting activity of l-ascorbic acid plus phytic acid – serum vs. placebo on solar lentigines. J Cosmet Dermatol. 2011;10(4):266–272.
- Huh C, Seo K, Park J, et al. A randomized, double-blind, placebo-controlled trial of vitamin C iontophoresis in melasma. Dermatology. 2003;206(4):316-320.
- Taylor MB, Yanaki JS, Draper DO, et al. Successful short-term and long-term treatment of melasma and postinflammatory hyperpigmentation using vitamin c with a full-face iontophoresis mask and a mandelic/malic acid skin care regimen. J Drugs Dermatol. 2013;12(1):45–50.
- Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32(4):388, 390.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80(5):387–94.
- Draelos ZD, Yatskayer M, Bhushan P, et al. Evaluation of a kojic acid, emblica extract, and glycolic acid formulation compared with hydroquinone 4% for skin lightening. 2010;86(3):153–158.

