 J Clin Aesthet Dermatol. 2020;13(7):E53–E57
J Clin Aesthet Dermatol. 2020;13(7):E53–E57
by Daniele Maggioni, PhD; Annamaria Cimicata, MS; Antonella Praticò, MS; Roberta Villa, MS; Ferdinando Bianchi, MD; Silvia Busoli Badiale, MS; Umberto Piana; and Claudio Angelinetta
All authors are with Bio Basic Europe in Milan, Italy.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Onychomycosis is a diffused fungal-associated disease affecting the nails. It induces discoloration, dystrophy, and, in the most severe cases, nail detachment. No completely effective pharmacological cures are available for onychomycosis. Available treatment options usually require a long-term treatment period and might raise safety concerns.
Objective. The aim of this preliminary clinical study is to evaluate the efficacy of Myco Clear® (MC) in improving the structural integrity and appearance of nails affected by mild onychomycosis. MC is a topical product to be applied on the nails either for onychomycosis treatment or for the prevention of fungal nail infections. Its main action is the formation of a mechanic protective barrier on the nail surface, which protects the damaged nail structure from additional external aggressions and prevents further pathogen penetration.
Methods. This monocentric, single-arm investigation enrolled 30 subjects with damaged nails on the feet. Patients were asked to apply the product once a day for 56 consecutive days; the instrumental analysis of nail roughness was obtained after seven, 14, 28, and 56 days of product application. In addition, the clinical evaluation of nail integrity and hardness were performed at baseline and after seven, 14, 28 and 56 days.
Results. The obtained data were statistically evaluated in comparison to baseline. MC treatment significantly promoted a restoration of the nail structure and an improvement in its appearance; furthermore, no adverse events were reported, thus demonstrating that MC is optimally tolerated.
Conclusion. The results of this pilot study suggest that MC is effective in improving the overall appearance and structural integrity of damaged nails affected by possible mild onychomycosis.
Keywords: Fungal infection, Myco Clear, nail, onychomycosis
Onychomycosis is a common fungal nail infection that does not spontaneously resolve and which represents 50 percent of nail diseases. It is mainly caused by dermatophytes, such as Thrichophyton rubrum and Epidermophyton, which account for most infections, or yeast (Candida albicans) which accounts for about two percent of infections.1–3
The prevalence of onychomycosis is estimated to be above 10 percent worldwide, but it varies significantly among age groups; indeed, while the prevalence in the pediatric population is around one percent, it markedly increases to 20 percent in subjects older than 60 years.4 In recent years, the incidence has been rising as a result of the population aging and increased exposure to sources of infections or predisposing factors.2 The prevalence of onychomycosis is higher among people who use inappropriate footwear, such as occlusive shoes, or participate in sports activities, especially at crowded swimming pools or gyms.5 Additional risk factors for onychomycosis are nail trauma, hyperhidrosis, and aging; furthermore, some pathologies, like diabetes mellitus, immunodeficiency disorders, or peripheral vascular insufficiency, considerably raise the risk of nail infections.4
The disease is generally asymptomatic at the beginning, when it is characterized only by a dry, hyperkeratotic nail; however, as pathology progresses, the presence of micro trauma or a promoting environment can boost the penetration of infecting agents into the nail bed. Starting from the primary site of infection, where a moderate inflammatory response can occur, the infection spreads to evolve in total-nail onychomycosis. The subsequently chronic infection is characterized by the presence of inflammatory infiltrates, while the nail becomes thickened and hyperkeratotic. Dermatophytes spreading to the entire nail plate results in distortion of the nail structure and, in the most severe cases, its complete detachment.6
The main signs of onychomycosis are discoloration and nail dystrophy, usually accompanied by a strong odor.7 The severity of the pathology is correlated with the extent and the location of the involved area and the type of the infecting agent. It is graded according to the degree of nail involvement, assessed by nail thickening, integrity, and nail surface roughness.8,9
In particular, onychomycosis can be clinically classified as distal subungual onychomycosis, superficial white onychomycosis, proximal subungual onychomycosis, candidal onychomycosis, and total dystrophic onychomycosis.10 Given its high social impact and significant impact on patient quality of life, this pathology, although is not a life-threating disease, poses a significant clinical problem, as poor response to therapy is usually observed.7 If left untreated, the infection can spread to other fingers or other body areas. Furthermore, fungal infections are highly contagious, as they transmit via direct contact or through sharing infected clothes.
The range of therapeutic approaches to onychomycosis is wide, as both topical and systemic oral treatments are available. The primary aim of the therapeutic approach to onychomycosis is the eradication of the causative micro-organism, although successful removal of the fungus is not always enough to correct nail alterations.
Currently, the most effective and commonly used treatments are oral antimycotic agents, such as ketoconazole, itraconazole, terbinafine, and griseofulvin. However, these medications are not always completely effective. In addition, their main limitation is the long time period that is necessary to obtain a significant effect; treatment can extend to more than six months, increasing the concern for side effects, such as gastrointestinal disturbance or liver enzyme level alterations. Alternatively, locally applied drugs, such as creams or topical medication, are often not effective, as the nail is hard to penetrate.10 The nail bed, comprising highly keratinized keratinocytes at the top and by an underlying sheet of desmosome highly bound cells, is not an easily penetrable surface, especially for hydrophilic substances.11 Regardless, some topical drugs are available, such as ciclopirox olamine 8% and efinaconazole 10% nail solutions.6 Nevertheless, the efficacy of these drugs has been disappointing, as it does not exceed 10%, although they are mainly used for mild onychomycosis.3 Considering the lack of an effective and rapid cure for onychomycosis, the search for new therapeutic approaches is warranted.
The aim of this study is to evaluate the efficacy of a nail solution, Myco Clear® (MC) (Venture Life, Berkshire, United Kingdom), designed for topical application for the treatment of onychomycosis. Clinical effects were evaluated using both instrumental measurements of nail roughness and the clinical evaluation of nail integrity and hardness following long-term treatment (56 days) on patients with damaged nails. MC is a solution containing polyquaternium-7, which creates a protective barrier on the nail’s surface that prevents further fungal penetration and isolates the damaged nail structure from external aggression, promoting its restoration, together with decylene glycol and urea, which promote nail surface conditioning. In addition, the presence of acidic components, such as lactic acid, also creates an acidic environment that helps to prevent further contamination.
Methods
A monocentric study of the efficacy of Myco Clear® for patients with onychomycosis was conducted in 2017 at the Dermo-Cosmetic and Medical R&D Center of Bio Basic Europe in Milan, Italy. All subjects, enrolled in a single cohort, provided written informed consent prior to entering the study. The trial was run in accordance with the Declaration of Helsinki and with principles of Good Clinical Practices. Data were obtained, recorded, and processed in accordance with the International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH) guidelines for good clinical practice.
Healthy subjects (N=30) aged between 20 and 65 years were selected for the investigation. Following a first clinical visit, patients with damaged nails on the feet were included in the study. Subjects who were selected for inclusion were in good general health with no dermatopathies or history of atopy. Pregnant or breastfeeding women were excluded, as were any potential subject with a history of allergies to cosmetic products, sun products, or topical medical treatment.
Samples of the tested product were applied on clean and dry nails on the feet, once per day, for 56 consecutive days. Patients were instructed not to change their usual daily routine and not to use products similar to the study product throughout the entire study period. Patients were also asked to perform a two-week washout period of any decorative or curative nail products.
Instrumental evaluation of nail parameters. At the beginning of treatment and at chosen time points throughout the study period, instrumental evaluation of nail roughness and clinical evaluation of nail hardness and integrity were performed. Instrumental readings were taken at T0 (baseline) and after seven days (T7),14 days (T14), 28 days (T28), and at the end of treatment after 56 days (T56). A dermatology specialist clinically evaluated the nail integrity and hardness at the same time points.
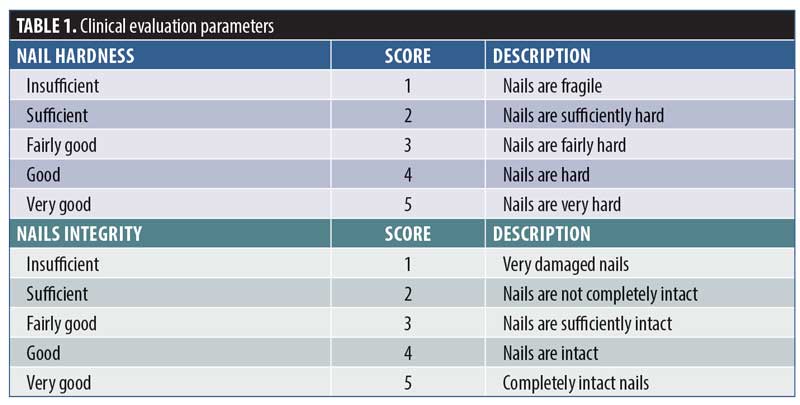
Roughness evaluation. At chosen time points, images of toenails were taken, elaborated, and evaluated using Antera 3D® (Miravex, Dublin, Ireland), a camera able to capture high-resolution, three-dimensional images, enabling the user to accurately measure texture, roughness, scars, and pigmentation. Nail roughness was determined on acquired three-dimensional images by measuring the average roughness, where the roughness was quantified from the vertical deviation of the real surface relative to the ideal composition. Large deviations indicated that the surface was rough, while small deviation values indicated a smoother surface.
Clinical evaluation. Clinical evaluation was performed through visual assessment by a specialist in dermatology. The clinical parameters were defined as insufficient, sufficient, fairly good, good, and very good and a numeric score was assigned using a five-point score system, as described in Table 1.
Patient-reported outcomes. At the end of treatment (T56), patients were asked to answer an end-of-study questionnaire, which evaluated the patient’s opinions about the product effect, with a particular focus on nail appearance (i.e., yellowing and flaking). This self-evaluation was performed according to the Visual Numeric Scale (VNS), where zero points was the minimum value and 10 points was the maximum value: judgment was considered positive when the score was above seven points.
Statistical evaluation. Statistical analysis of MC’s effect on nail roughness, nail integrity, and hardness was performed using the nonparametric Friedman test followed by the Wilcoxon signed-rank Bonferroni corrected test to compare differences in the parameters evaluated at different time points.
Results
The study included 30 subjects (mean age: 48±9 years) with at least one toenail affected by onychomycosis. The diagnosis of onychomycosis was based on clinical data. Each subject enrolled into the study was asked to apply MC once a day for 56 consecutive days.
Nail roughness instrumental analysis performed on images taken at T0, T7, T14, T28, and T56 of product application highlighted a progressive smoothing of the nail surface throughout treatment as shown in the graph of Figure 1. In particular, the most marked decrease was recorded following 56 days of MC application, with median roughness values of 10.44 (Q1 9.5 and Q3 13.6) and 9.636 (Q1 8.2 and Q3 12.9) at T0 and at T56, respectively. The decrease reached statistical significance comparison to at T0 after 14 days and persisted until the end of treatment (p<0.01).
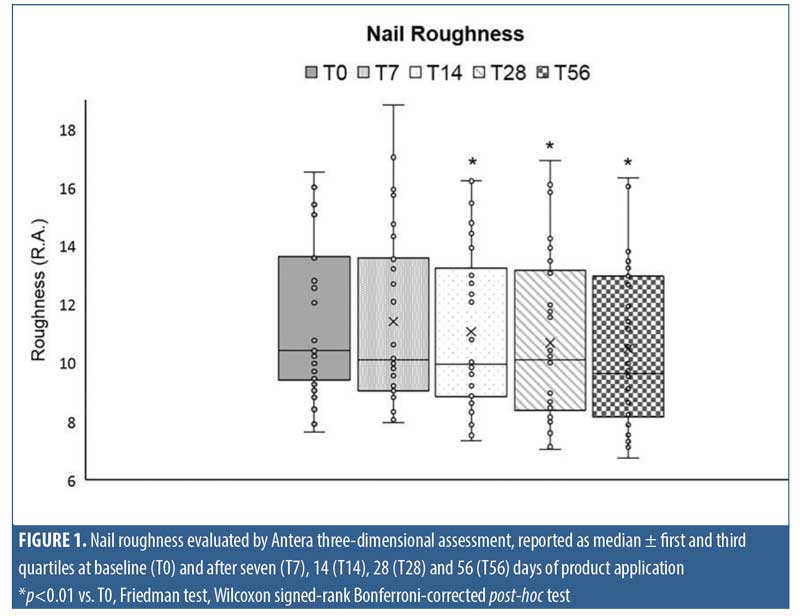
Clinical evaluation confirmed the data obtained by instrumental analysis. Indeed, as the treatment period progressed, a steady decrease in the percentage of patients having the most severe score for both nail integrity and hardness was observed.
In the case of nail hardness, as reported in Table 2, 47 percent of patients were scored as insufficient at baseline, whereas, at the end of treatment or 56 days of MC application, this percentage dropped to 10 percent. An improvement in clinical score was observed in 17 percent, 50 percent, and 67 percent of patients following after 14, 28, and 56 days of treatment with the study product, respectively. A statistical evaluation performed by nonparametric Friedman test and Wilcoxon signed-rank Bonferroni-corrected post-test highlighted a statistical significance between T28 and T0 (p<0.01) and between T56 and T0 (p<0.01).
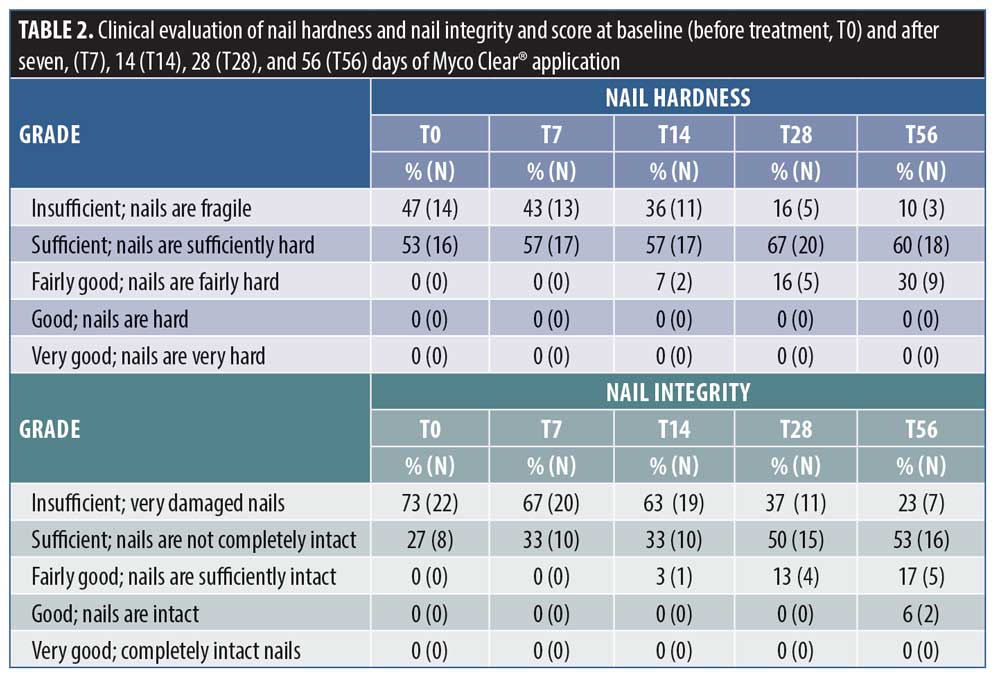
The nail integrity was scored insufficient in 73 percent of patients at T0, but this percentage decreased to 23 percent after 56 days of treatment. Furthermore, 50 percent of patients showed an improvement in nail integrity after 28 days of product application, but the percentage rose to 63 percent at the end of treatment. These variations among clinical parameters at T28 and T56 were statistically significant as compared with at T0, as determined by a Friedman rank test and Wilcoxon singed-rank post-test (p<0.01).
Discussion
Onychomycosis is a common pathology affecting the nails, inducing alterations of nail integrity, roughness, and often, complete nail dystrophy. Given its high prevalence and significant impact on quality of life, it poses a significant clinical problem, demanding more effective therapeutic strategies.
Therapeutic approaches, mainly based on antimitotic oral or locally applied drugs, have not demonstrated complete efficacy. Although systemic treatments for onychomycosis have higher efficacy than topical products in the eradication of fungal infections, they may be contraindicated due to the the long treatment periods required and the possible interactions with other drugs and eventual side effects.12
It is noteworthy to consider that the elimination of fungal infection does not always result in a significant improvement of nail integrity and nail appearance, which is one of the most distressful symptoms of the disease in regards to patient perception. For these reasons, topical products are largely used for onychomycosis treatment. The success of topical onychomycosis treatments is hampered by the difficulty of penetrating the nail surface, which, for its intrinsic composition, is not an easily penetrable structure.11 In addition, the therapeutic effect can interfere with the nail growth rate, resulting in a significant impairment of nail homeostasis. The most recent clinical strategies often rely on a combination of different oral and topical treatments, although combinations also increase the risk of unwanted drug interactions.3 The optimal treatment strategy varies for each patient depending on the clinical form of onychomycosis, the pathogen responsible for the underlying infection, and the health conditions of the patient.13
In this preliminary study, a nail solution, Myco Clear®, containing urea, lactic acid, polyquaternium-7, and decylene glycol and facilitating the creation of a protective film of the nail surface, which promotes nail integrity recovery and prevents further infections, has been clinically evaluated.
Data obtained demonstrated the efficacy of MC in improving the appearance of nails affected by onychomycosis, including roughness and decreased nail integrity and hardness. Although other clinical trials have evaluated the clinical efficacy of onychomycosis treatment in terms of nail fungal freedom, this parameter is not always correlated with a real restoration of nail structural integrity, as main deficiencies can persist even after a successful fungal infection eradication.6
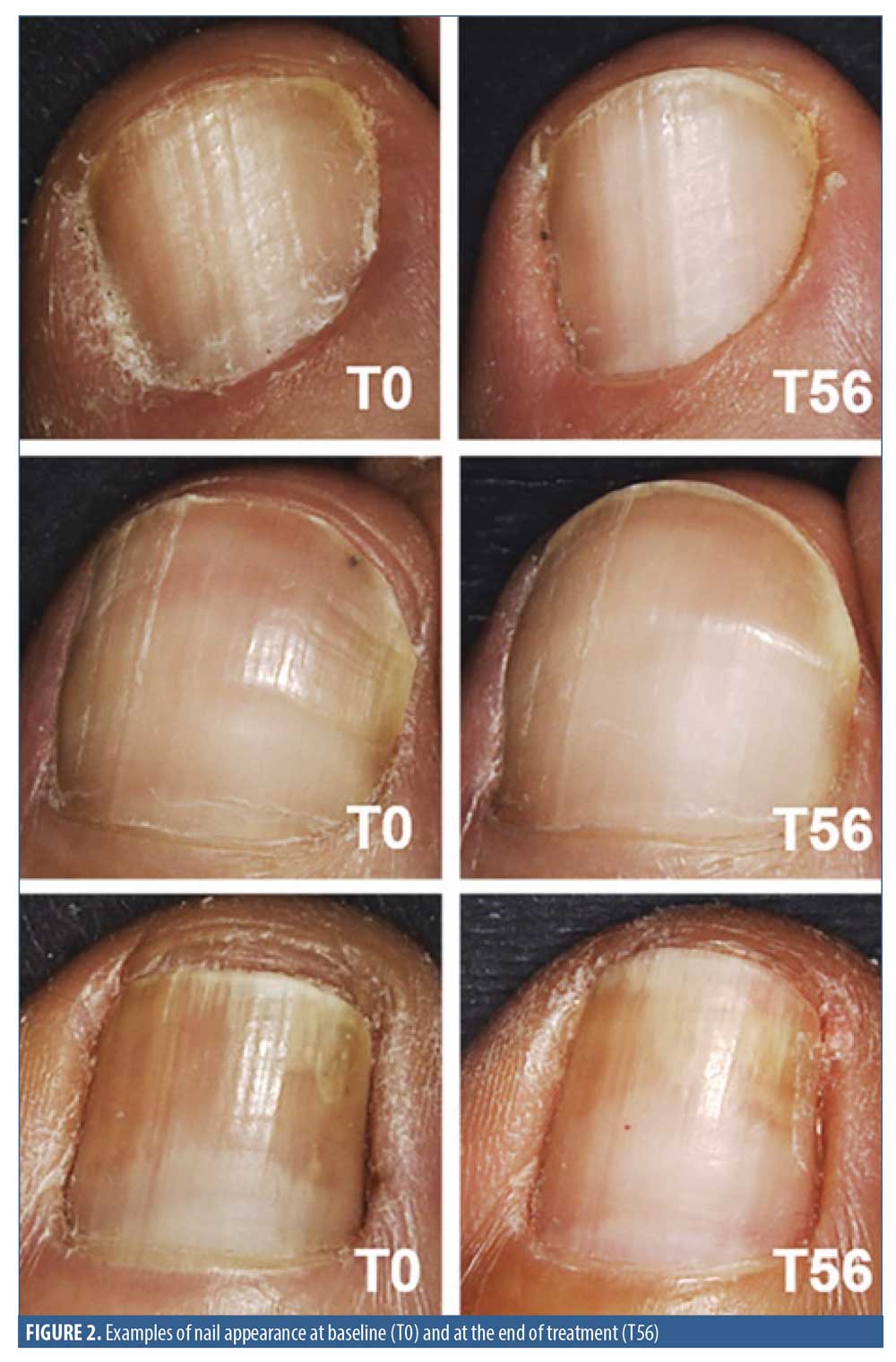
MC significantly improved the nail structure as demonstrated by the restoration of nail hardness and integrity, which was measured by clinical evaluation performed by a specialist in dermatology. At the end of the treatment, a significant decrease in nail surface roughness was instrumentally detected, thus confirming an improvement of the nail appearance following the use of the product.
The efficacy of nail lacquers containing urea has been reported by other clinical studies. In a systematic review, Dars et al7 underlined the efficacy of the use of urea alone or in combination with other compounds for onychomycosis treatment.7 The topical application of a K101-03, a product containing urea and lactic acid, was found to improve the nail appearance evaluated in terms of discoloration, thickening, and softness in patients with onychomycosis.14 Similar results were obtained also by Emtestam et al.12
The presence of acid compounds in the MC formulation induces a negative environment for pathogens as pH decreases. The MC formulation effect is prevalently tied to the presence of film forming agents that create a physical barrier on the nail, able to not only protect the nail plate, promoting its structural restoration, but also hamper further fungal infections.
No adverse effects were recorded throughout the treatment, highlighting the optimal tolerability of the test product, even after long-term application. This is noteworthy, as safety concerns have been raised in regards to the use of oral antifungal drugs, especially for long-term treatment and in patients taking other drugs. For this reason, the observed tolerability and safety of MC makes it a suitable candidate for use in long-term application to prevent infection recurrence.
The majority of patients (63%) reported the product was effective in improving the overall nail appearance. Eighty-three percent of patients reported that nail color (i.e., yellowing) had improved by the end of treatment. Similarly, the 80 percent of patients also perceived improved nail flaking by the end of the treatment period. The favorable patient self-evaluation following MC application is noticeable, as patient-perceived nail appearance is one of the primary factors affecting the quality of life patients with onychomycosis.
Limitations. This study has some limitations, first of which is the lack of a control or placebo group. Further studies should confirm data obtained in this pilot trial by introducing a comparison group and a double-blinded study. Another minor limitation of this study is that it was conducted in a single center in Italy. Additionally, all subjects were Caucasian; however, previous studies have demonstrated that onychomycosis treatment is only minimally affected by ethnicity.14
Conclusion
This preliminary clinical study demonstrated that MC is effective in improving the overall structural integrity and appearance of nails with possible mild onychomycosis.
Acknowledgment
We thank Mrs. Greta Grandi for the language revision.
References
- Lipner SR, Scher RK. Onychomycosis: treatment and prevention of recurrence. J Am Acad Dermatol. 2019;80(4):853–867.
- Papinbi M, Piraccini BM, Difonzo E, Brunoro A. Epidemiology of onychomycosis in Italy: prevalence data and risk factor identification. Mycoses. 2015;58(11):659–664.
- Del Rosso JQ. The role of topical antifungal therapy for onychomycosis and the emergence of newer agents. J Clin Aesthetic Dermatol. 2014;7(7):10–18.
- Lipner SR, Scher RK. Onychomycosis: Clinical overview and diagnosis. J Am Acad Dermatol. 2019;80(4):835–851.
- Hanna S, Andriessen A, Beecker J, et al. Clinical insights about onychomycosis and its treatment: a consensus. J Drugs Dermatol. 2018;17(3)253–262.
- Bodman MA, Krishnamurthy K. Onychomycosis. [Updated 2020 May 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. https://www.ncbi.nlm.nih.gov/books/NBK441853/. Accessed July 20, 2020.
- Dars S, Banwell HA, Matricciani L. The use of urea for the treatment of onychomycosis: a systematic review. J Foot Ankle Res. 2019;12:22.
- Christenson JK, Peterson GM, Naunton M, et al. Challenges and opportunities in the management of onychomycosis. J Fungi (Basel). 2018;4(3):87.
- Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: onychomycosis severity index. Arch Dermatol. 2011;147(11):1277–1282.
- Shirwaikar AA, Thomas T, Shirwaikar A, et al. Treatment of onychomycosis: an update. Indian J Pharm Sci. 2008;70(6):710–714.
- Cutrín-Gómez E, Anguiano-Igea S, Delgado-Charro MB, et al. Effect of penetration enhancers on drug nail permeability from cyclodextrin/poloxamer-soluble polypseudorotaxane-based nail lacquers. Pharmaceutics. 2018;10(4):273.
- Emtestam L, Kaaman T, Rensfeldt K. Treatment of distal subungual onychomycosis with a topical preparation of urea, propylene glycol and lactic acid: results of a 24-week, double-blind, placebo-controlled study. Mycoses. 2012;55(6):532–540.
- Drago L, Micali G, Papini M, et al. Management of mycoses in daily practice. G Ital di Dermatologia e Venereol. 2017;152(6):642–650.
- Piraccini BM, Starace M, Toft A. Early visible improvements during K101-03 treatment: an open-label multicenter clinical investigation in patients with onychomycosis and/or nail psoriasis. Dermatology. 2017;233(2-3)178–183.

