J Clin Aesthet Dermatol 2023;16(8 Suppl 1):S4–S11
J Clin Aesthet Dermatol 2023;16(8 Suppl 1):S4–S11
Adelaide A. Hebert, MD; Neal Bhatia, MD; and James Q. Del Rosso, DO
Dr. Hebert is with UTHealth McGovern Medical School in Houston, Texas. Dr. Bhatia is with Therapeutics Clinical Research in San Diego, California. Dr. Del Rosso is with JDR Dermatology Research and Thomas Dermatology in Las Vegas, Nevada, and Clinical Research at Advanced Dermatology and Cosmetic Surgery in Maitland, Florida.
Funding: Funding for this article was provided by Verrica Pharmaceutical in West Chester, Pennsylvania.
Financial Disclosures: Dr. Hebert has received research grants (paid to UTHealth McGovern Medical School) from Verrica, Pfizer, Arcutis, GSK, Ortho Dermatologics, and Galderma, and has received honoraria from Verrica, Pfizer, Galderma, Arcutis, Vine, Almirall, Bristol Myres Squibb, Leo, Vyne, Aslan DSMB: Ortho Dermatologics, GSK, and Sanofi Regeneron. Dr. Del Rosso is a consultant/advisor and investigator for Verrica Pharmaceutical.
ABSTRACT: Molluscum contagiosum (MC) is a viral infection that affects primarily pediatric patients, sexually active young adults, and immunocompromised people of all ages. MC occurs all over the world, making up about one percent of skin disorders and appears to be increasing in prevalence. This cutaneous infection is often associated with atopic dermatitis and is typically self-limiting, although spontaneous resolution can take months to years. Many treatments exist, but only one—a drug-device product using topical cantharidin— is approved by the United States (US) Food and Drug Administration (FDA) for treatment of MC. For many years, there was a lack of an established or FDA-approved first-line treatment for MC, which might have contributed to the common “benign neglect” attitude of physicians regarding treatment of MC. Unfortunately, this noninterventional approach can increase risk of spreading infection and result in longer duration of infection, physical discomfort, and psychosocial issues due to persistence of the MC lesions. This article reviews available epidemiology data and explores treatment options and therapeutic gaps in MC management.
Molluscum contagiosum (MC) virus is an unclassified member of the Poxviridae family, encompassing Types I to IV viruses.1 This virus causes a benign, cutaneous-manifested infection that occurs only in humans. In a study of 147 patients in the United States, 96.6 percent of MC was caused by Type I virus compared to 3.4 percent caused by Type II.1 In other parts of the world, other viral types might predominate, but there appears to be no association among the virus type, the lesional morphology, and the anatomic distribution of the lesions.1
MC was first described in 1817, with a comprehensive article written in 1841 and the viral nature of MC first hypothesized in 1905.2,3 The condition often resolves over a variable time period, though study of this prevalent skin condition has been relatively limited until more recently. The absence of a cure for MC; lack of available, clear-cut, first-line treatment; and difficulty in treating multiple lesions, especially in children, all have contributed to a prevalent attitude among clinicians that withholding treatment (i.e., “benign neglect”) might be the best course of action in patients with MC. Unfortunately, this “wait and see” approach can increase the risk of spreading the infection through autoinoculation and/or transfer to others in close personal contact with the infected individual, prolong the duration, and induce negative psychosocial issues (e.g., anxiety, social isolation) related to visibility of lesions and fear of spread to others.4
Incidence and Prevalence
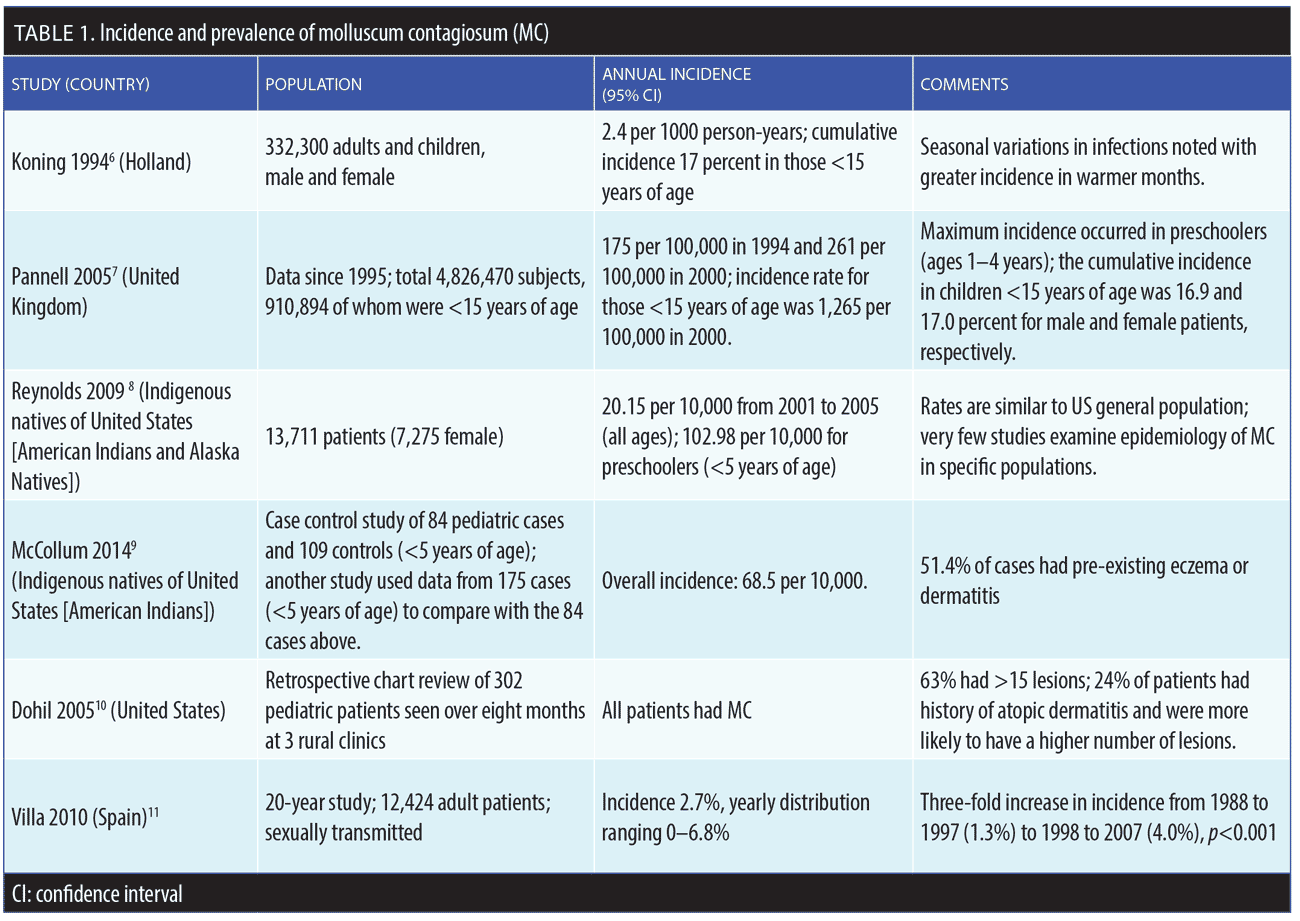
MC accounts for about one percent of all skin diagnoses worldwide, but prevalence appears to be increasing in all age groups.5–12 The underlying reasons for this increase in prevalence are not fully known. The prevalence of MC in the United States (US) general population is approximately five percent; pediatric patients are disproportionately affected by MC compared to adults, with the highest incidence of MC occurring among children under the age of 14 years.5 In real-world clinical practice, MC most commonly presents in patients 1 to 4 years of age, but older children can develop more extensive infection and require more frequent treatments.5 The prevalence rate of MC for American children might be as high as 62 percent, with a point prevalence of MC in children between the ages of 0 and 16 years of 5.1 percent and 11.5 percent.5 MC can also be transmitted to neonates through the birth canal, causing a circular formation of lesions on the scalp of the infant.5 Incidence and prevalence rates of MC are depicted in Table 1.6–11
Risk factors for MC include a compromised immune system (e.g., human immunodeficiency virus [HIV]), organ transplant, chemotherapy); frequent attendance at MC “hot spots” (e.g., day care centers, schools, pools); skin-to-skin contact with an infected individual (sexual or nonsexual); and atopic dermatitis.13–15
Patients who are immunocompromised, such as those with HIV, patients who have had organ transplants, or patients with cancer, are more likely to contract the Type II virus, which is responsible for about 60 percent of all MC infections in immunocompromised populations, with a prevalence of 5 to 18 percent.16 If the CD4 cell count is less than 100 cells/μL, then prevalence might be as high as 33 percent. Among patients with symptomatic HIV infection, the prevalence of those with comorbid MC is thought to be as high as 20 percent, with lesions most frequently appearing on the trunk and face.17,18 In patients with HIV or other immunocompromising conditions, MC tends to be persistent and is often refractory to therapy.19
Distinguishing Features and Diagnosis
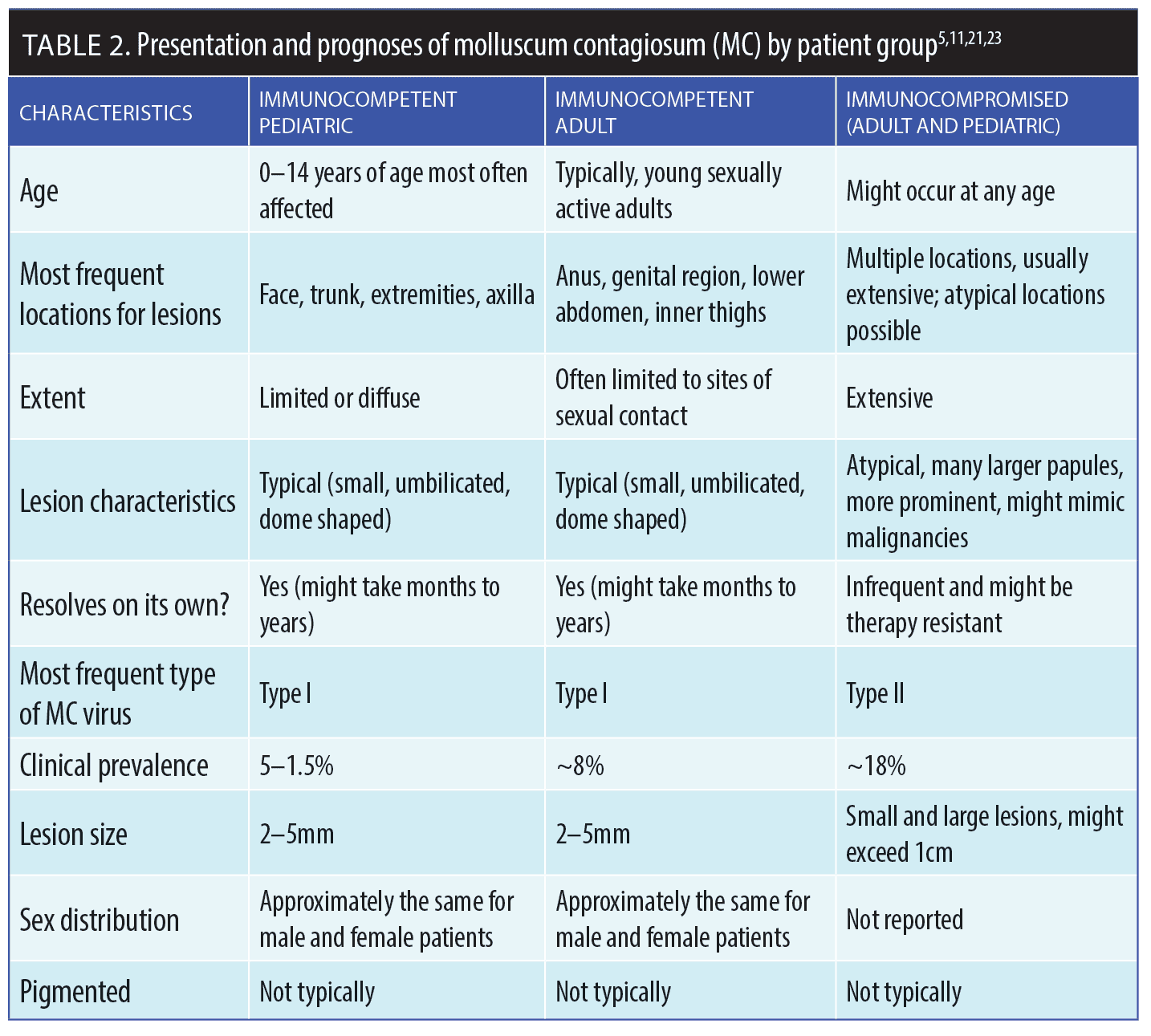
The three main populations affected by MC—pediatric patients with intact immune systems; young, immunocompetent, sexually active adult patients; and patients who are immunocompromised of all ages—have different characteristics and prognoses, and sometimes require different treatment approaches.2 The condition might be self-limiting over a period of several months to a few years in most otherwise-healthy individuals, often with development of new lesions and/or spread to others.4 The recurrence rate of MC after clearance can be as high as 35 percent (Table 2).20
In some cases of MC, the Koebner phenomenon occurs following trauma or injury, in which lesions appear on areas of skin previously unaffected by the virus.21 Minor skin trauma might be sufficient to inoculate the MC. Koebernized MC papules are often isomorphic.
MC papules are produced by a double-stranded DNA virus in humans. After invading the host, MC infects the epidermis and replicates itself in the cytoplasm of cells. The incubation period for the virus is variable and generally ranges from 2 to 6 weeks.22 Since the MC enters the body through a break in the skin, it is more prevalent in patients with atopic dermatitis, dry skin, or any interrupted structural integrity of the skin barrier.23 MC is more prevalent in hot, humid climates and among children who go swimming.3,22 Currently, it is not clear if MC transmission occurs in water, although the condition has been referred to as “water warts.” Infectious transmission seems to occur through direct contact with infected skin, by sexual or nonsexual means, or by autoinoculation. The US Centers for Disease Control and Prevention (CDC) does not offer specific guidance regarding transmission of MC in swimming pools, but many dermatologists have observed, anecdotally, that children with access to swimming pools more frequently develop MC. While there is no existing evidence that MC can be transmitted in swimming pool water, transmission is possible by sharing towels or pool equipment.23
Primary MC lesions have characteristic clinical features—they are discrete, smooth, flesh-colored, umbilicated papules 2- to 5mm in diameter that are dome shaped with a waxy surface and a central caseous plug. MC papules might present individually or in clusters (which is more common). Most lesions are asymptomatic, but some patients develop lesional or perilesional pruritus.24 The anatomic distribution of the lesions tends to be on the trunk and extremities, although the umbilicated papules can occur anywhere on the skin. Unlike verruca caused by human papilloma virus (HPV), MC rarely, if ever, manifests clinically on the palmar or plantar surfaces.
If not treated, MC can lead to molluscum dermatitis, which allows the virus to spread more readily on the skin.15 About 10 percent of patients who contract MC will experience molluscum dermatitis.14 In patients with pre-existing eczema (typically atopic dermatitis), MC can exacerbate their condition. In patients with atopic dermatitis, widespread lesions are more common.14
MC lesions are usually easy to identify clinically and exhibit a clear pattern histologically. MC lesions characteristically contain lobules of hyalinized molluscum bodies called Henderson-Paterson bodies.25 MC differs from other forms of viral-induced skin lesions (e.g., verrucae). Papules of MC develop a “pocket” formation of molluscum bodies within a central region of umbilication, which are contained and limited within this part of the epidermis; the upper portion of the pocket represents the caseous material that is often visible and might be expressed from the central focus of umbilication.25 With verrucae, there is often lateral and horizontal subclinical involvement of infected keratinocytes beyond the clinically evident lesion, which is why many treatments for verrucae can lead to persistence or recurrence at the edge of the treated area.26 Additionally, verrucae often extend more deeply within skin than MC lesions, altering epidermal turnover with production of hyperkeratosis, which can simulate a callous.26 With MC, there is minimal epidermal change without secondary formation of a hyperkeratotic surface.29 The lesions of MC tend to be more uniform in size than verrucae, and, upon resolution, do not naturally produce visibly persistent sequelae; however, in some cases of MC, a small-pox type of scar might occur following lesion resolution or destruction of individual MC papules.25 Infection with MC appears to modulate host immunologic response in a variety of patterns as evidenced by the elicitation of molluscum dermatitis and also by the presence of spontaneous visible inflammation of individual MC papules that leads to resolution of these lesions. This latter response to some MC lesions is referred to as the BOTE sign (“beginning of the end’). The common persistence of MC may be explained at least partially by one report showing that MC, via specific gene-induced mechanisms, can inhibit activation of antigen-specific T lymphocytes, affect natural killer (NK) cell recognition, and promote evasion of cytotoxic (CD8+) T lymphocytes.27
In some cases, the morphology of MC lesions can vary, including in immunocompetent children. For example, giant MC can mimic cysts, abscesses, or condyloma. Erosive MC lesions can mimic eczema vaccinatum. Papules might also be pearly white or reddish in color.13 In certain instances, exanthem-like eruptions might form after the MC lesions are treated; these new clinical manifestations look like lesions of papular acrodermatitis of childhood (Gianotti-Crosti syndrome) or unilateral laterothoracic exanthem-like lesions.13
Impact on Quality of Life
Due its lengthy persistence, the common presence of newly emerging and multiple lesions, lack of consistently effective treatment, frequent use of destructive modalities that can cause pain, scarring and/or dyschromia, and concern regarding its transmission to others, MC can create marked psychosocial distress and have an adverse impact on the quality of life in affected patients and/or caretakers of affected children. For example, children/adolescents might be required to stay home from school and other public gatherings until the infection resolves. Additionally, patients might experience uncomfortable physical symptoms, such as pruritus or pain; and visible lesions might cause embarrassment, distress, or anxiety.13 Adults with MC can also experience diminished quality of life due to unsightly appearance, embarrassment about lesions involving the anogenital region, fear of transmission to sexual partner(s), and the adverse effects of pain and pruritus.
To Treat or not To Treat? That is the Question
The first step in managing MC is to recognize that this condition is a contagious viral infection. Although MC does not appear to induce any known systemic sequelae or malignancy potential, this viral disorder is associated with adverse psychosocial effects in children and can also serve as a marker for a number of comorbidities, such as atopic dermatitis.14 Many children with MC lesions are managed by pediatricians or primary care physicians who often elect to not treat MC, which may be due to the prevailing belief that the lesions will go away on their own (“benign neglect”). What is often not considered when deciding to take the noninterventional approach is that the average time to spontaneous resolution of MC is approximately 13 months, with 30 percent of the cases persisting for more than 18 months; additionally, around 40 percent of the cases will transmit the infection to other individuals in the household.4,5 The recently updated Red Book Atlas of Pediatric Infectious Diseases states that therapy for MC “may be warranted to alleviate discomfort, including itching; reduce autoinoculation; limit transmission of the virus to close contacts and reduce cosmetic concerns and prevent secondary infections.”28 This recognition that MC is highly contagious and spreads rapidly among children in contact with other children, not only through physical contact but also likely via the sharing of toys, utensils, books, clothing, and other items, is clinically relevant. While MC in a healthy child is potentially self-limiting over time, there are additional reasons to treat them beyond persistence and potential spread to others, including prevention of molluscum dermatitis and adverse psychosocial sequelae, as well as exacerbation of atopic dermatitis.13 Many cases of MC are asymptomatic, but children might be troubled by pruritus, and the subsequent scratching of the MC papules can lead to further autoinoculation.13
General Treatment Recommendations
When discussing MC lesion resolution and/or treatment approaches with patients or parents/caretakers of affected children, it is important to manage expectations by including some practical information. Patients should be informed of the potential visible sequelae some treatments may cause, such as dyschromias (i.e., erythema, hyperpigmentation, hypopigmentation), which might take months to resolve or might be permanent to some degree. In addition, pox-like or pitted scars might develop after some MC papules resolve, either after certain treatments or sometimes spontaneously. Importantly, dyschromia and scarring can develop from excoriation of the lesions by the patient if cutaneous damage extends deep enough into the dermis or if a secondary bacterial infection results at excoriated sites. Lastly, the skin color of the patient might predispose them to certain sequelae, especially dyschromias, which are more likely to develop in patients with darker or fair skin.29
Immunocompetent adults affected by MC are typically young and sexually active, with lesions often present in the suprapubic and anogenital regions.30 Anogenital lesions might also present in pediatric patients. Although this could be associated with sexual abuse in some cases, the presence of anogenital MC lesions in young children is often encountered in the absence of sexual abuse.31 The clinician must maintain an index of suspicion when assessing children with anogenital MC lesions. In a child presenting with only genital MC lesions as opposed to a generalized distribution that also includes anogenital lesions, there would be increased suspicion of sexual abuse as opposed to transmission of MC by autoinoculation.31
Educating adults with MC regarding treatment and transmission is important. In adults with MC, transmission can potentially occur not only through direct sexual contact but also via sharing personal care items (i.e., razors, brushes, combs, unwashed clothing, bar soap, epilation devices) of an infected person. Those with MC lesions in the anogenital region should refrain from sexual contact until consultation with a healthcare provider.23 Salons and other places offering hair removal services, including those used in the genital area, should sterilize equipment between clients.
Of particular importance in both adult and pediatric patients with MC is atopic dermatitis—MC and atopic dermatitis are considered to be risk factors for each other. In a retrospective, longitudinal study that included patients with MC and an age- and sex-matched case cohort of pediatric patients with atopic dermatitis,5 the rate of consultation in primary care for children (age range <14 years) with MC was 9.5 per 1,000 (95% confidence interval [CI] 9.4–9.6), which has declined by 50 percent from 2004 to 2013. The odds ratio for a child seeing a primary care physician was 1.13 (95% CI, 1.11–1.16, p<0.005) if that child had previously seen the physician for eczema.5 Patients with atopic dermatitis exhibit multiple inherent epidermal barrier dysfunctions (e.g., decrease in antimicrobial peptides, filaggrin gene mutations); thus, they are at elevated risk for contracting and spreading MC.32–34
The age of the patient and the anatomic locations of the MC lesions can influence choice of therapy. Additionally, MC might require repeated treatments, sometimes with incorporation of different approaches depending on the patient’s response to therapy and other factors. MC lesions on the eyelid, especially when involving the tarsal margin, must be treated with caution; treatment is necessary due to risk of conjunctivitis with superficial punctate keratitis.13 These patients should be referred to an ophthalmologist for specialized care.
Specific Treatment Options
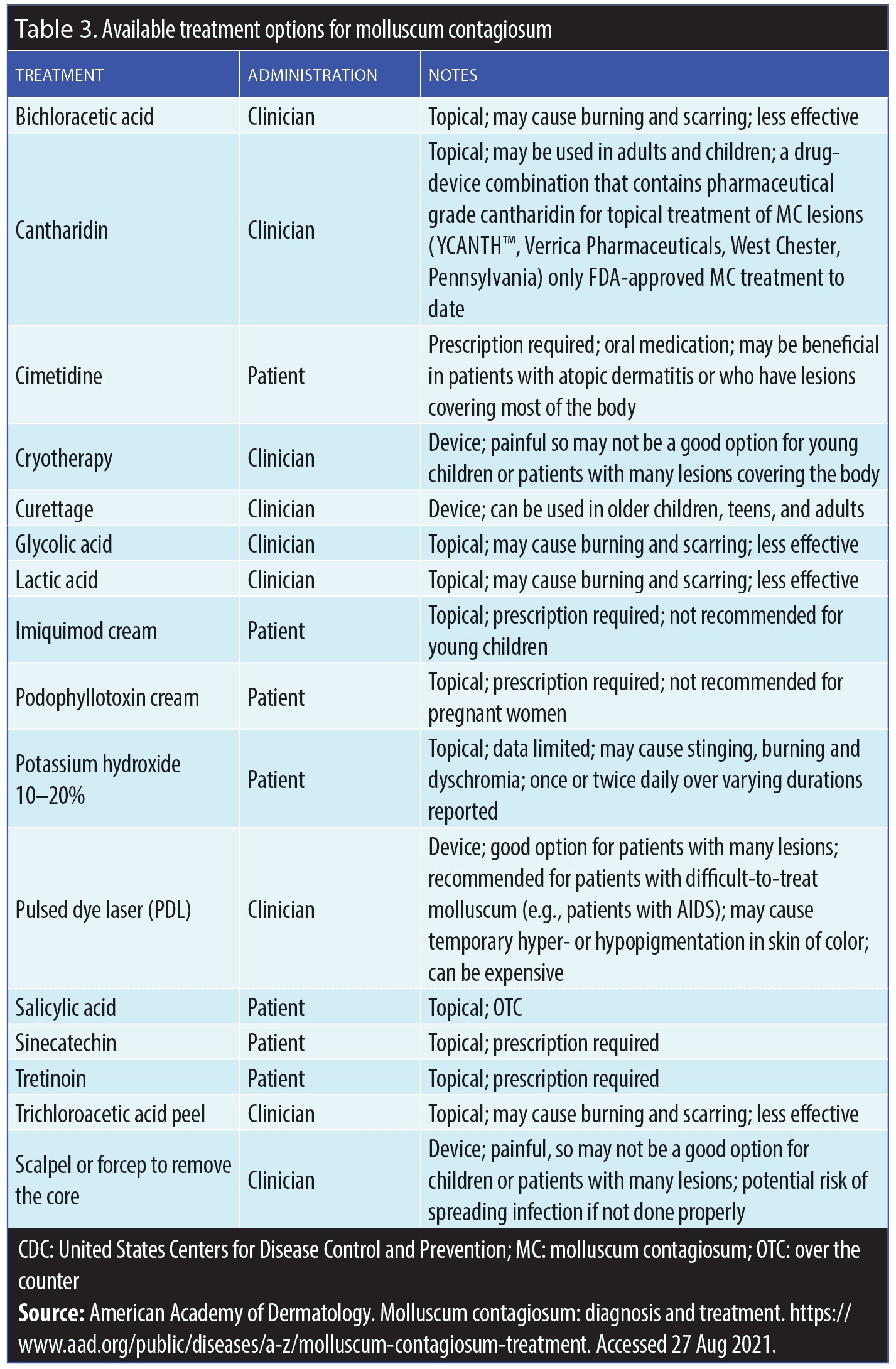
Overview. Although there is no known therapeutic cure for MC, many treatment modalities have been reported, several of which are based on case reports, open-label studies, and/or a limited number of prospective trials.19,22,35,36–51 Table 3 includes many of the potential therapies that have been used to treat MC. Some are clinician-applied approaches and others may be applied by the patient or parent/caretaker. Clinician-applied treatment approaches may include the use of topical agents and/or physical modalities, which provide localized destruction of individual lesions. Currently, only one of the available medical treatment options for MC is approved by the United States Food and Drug Administration (FDA) for MC; the rest are considered off-label therapies (Table 3).
Patient-applied therapies. Off-label treatments. Although not FDA-approved for the treatment of MC, several potential patient-applied therapies are available.19,22,35,36–51 These include topical retinoids, salicylic acid, podofilox gel or solution, and sinecatechins ointment (Table 3). The mode of action of topical retinoids when used to treat MC is not known. Catechins are the polyphenols and flavonoids found in green tea (Camellia sinensis).43 Some evidence exists for sinecatechins use in the treatment of genital warts,44 but there is less robust evidence for treating MC.46 Catechins have antioxidant, antiviral, and immune-stimulatory effects.43,46,47 Use of topical imiquimod is controversial and not generally recommended for MC due to its FDA-approved product labeling, which states specifically that it is not effective against MC in children aged 2 to 12 years.52 In a meta-analysis of 11 studies (N=1,650), imiquimod was not found to be effective for MC.35
Berdazimer gel. A therapy that has completed pivotal studies for MC and is under evaluation for FDA approval is berdazimer gel, a first-in-class nitric oxide (NO)-releasing topical treatment. This self-treatment approach incorporates the ability to activate nitric oxide upon application, which has been shown to effectively reduce MC lesions.53,54
Clinician-applied therapies. Topical cantharidin. Cantharidin is widely used to treat MC.49,50 Clinician-directed use of topical cantharidin, either as a monotherapy formulation or in combination with other additives (i.e., salicylic acid, podophyllotoxin), can be obtained from a variety of sources and is commonly utilized to treat MC in dermatology, including by pediatric dermatologists.30–51,55 The mechanism of action of cantharidin is the vesicant effect, which includes cell lysis contained in the epidermis.45 While a wooden stick is the most common applicator for non-FDA-approved topical compounded cantharidin formulations, no studies have been carried out to evaluate the best method for application with these products.
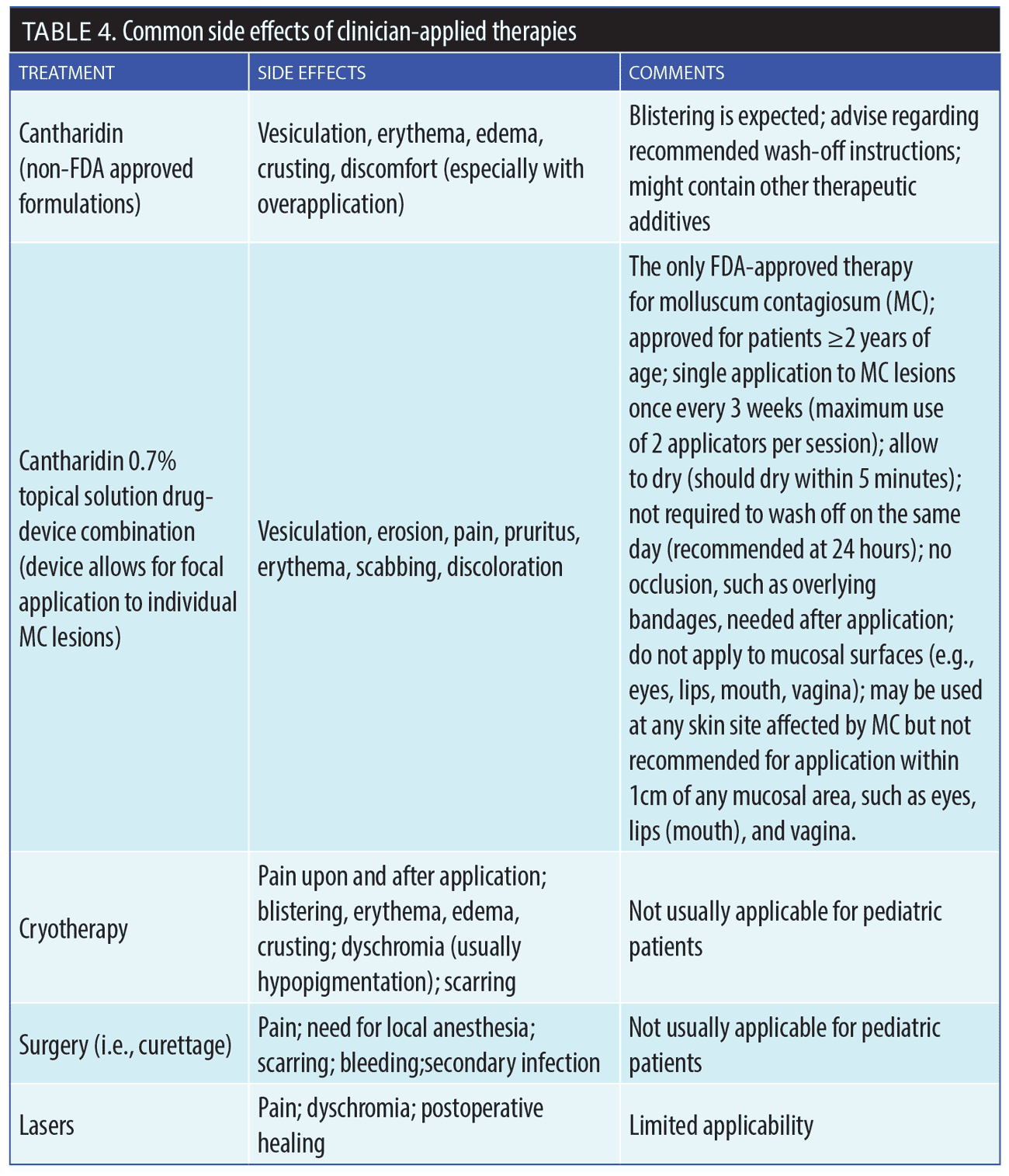
With cantharidin, certain adverse events have been reported: blistering (10–100%), discomfort or pain (7–86%), and dyschromia (2–54%).44,45 A clinical trial of 300 pediatric patients with MC who were treated with cantharidin reported that 90 percent of the participants experienced marked clearance of lesions.45 Treatment occurred over an average of 2.1 office visits per patient. Most patients (92%) developed blisters, and temporary burning, pain, erythema, and pruritus occurred in 6 to 37 percent of children treated. No secondary bacterial infections occurred, and no serious adverse events were reported. When asked about treatment, 95 percent of those patients treated with cantharidin said they would use cantharidin therapy again, if needed (Table 4).45
There have long been logistical concerns with cantharidin, despite this substance being used in many parts of the world for a number of years. Since 1962, American dermatologists could only get cantharidin through compounding pharmacies outside of the United States. Variability in raw materials and topical formulations meant products were inconsistently formulated and sometimes not readily available.
There remains a paucity of randomized, controlled, clinical trials using cantharidin, especially prior to the development of the recently and only FDA-approved drug-device combination.
FDA-approved topical cantharidin drug-in-device combination. Recently, the FDA approved a drug-in-device combination that contains pharmaceutical-grade cantharidin 0.7% for topical treatment of MC lesions (YCANTH™, Verrica Pharmaceuticals, West Chester, Pennsylvania), the only FDA-approved MC treatment to date.49,51 Cantharidin for MC has been evaluated in a thorough literature review, which reported that cantharidin appeared to be safe and effective for treating MC and verrucae.43
The recently approved, novel drug-in-device combination contains a standardized formulation of pharmaceutical-grade cantharidin 0.7% in a single-use applicator for topically applied, targeted dosing and delivery.49 This allows directed application focally to individual MC lesions using the approved single-use applicator. The drug-device combination helps clinicians avoid random application and potential excessive spillover onto normal skin of their patients, which may result in more extensive blistering and discomfort.48,49
Application using the drug-in-device combination is completed in the office setting by a healthcare professional who has been trained in the proper application technique. Each applicator contains enough solution to treat approximately 40 to 50 individual MC lesions, with each lesion to be treated only once by directed application per session. Treatment sessions are recommended every three weeks, with only active lesions to be treated, including any new lesions that may have developed since the previous office visit(s). The maximum recommended use per session is two applicators, which should allow for treatment of 80 to 100 MC lesions per session. There is no recommendations in the product’s prescribing information to wash the formulation off on the same day as treatment (though it is recommended at 24 hours post-treatment) or to apply occlusive bandages to treated lesions.56
Table 4 reviews other details on recommended use of the drug-in-device combination. Patients/caretakers should be advised that focal blistering and some discomfort post-treatment are expected, usually occurring within hours after application of the topical cantharidin.
Conclusion
MC is a prevalent condition that affects all age groups and is challenging to treat. MC can spontaneously resolve over time; thus, many physicians choose a management strategy of benign neglect, allowing MC lesions to hopefully resolve on their own. This strategy encourages the persistence and spread of MC in many affected individuals and increases the risk of psychosocial stress among affected patients and their family members. Currently, there is strong consensus that MC should be actively treated. A specific drug-device combination product containing pharmaceutical-grade cantharidin 0.7% solution for directed topical application has been FDA-approved for treatment of MC, further improving our understanding of MC management through the availability of randomized, controlled, clinical trials and providing a tested and improved method for more consistent targeted application to MC lesions. Despite MC affecting people of all ages worldwide, there is a relative paucity of epidemiologic studies and outcomes data in several patient populations around the world. Further studies are needed to address knowledge gaps related to epidemiology and therapeutic outcomes, especially in special patient populations.
Acknowledgments
The authors wish to acknowledge LeQ Medical in Angleton, Texas, for their support in preparing this manuscript.
References
- Scholz J, Rösen-Wolff A, Bugert J, et al. Epidemiology of molluscum contagiosum using genetic analysis of the viral DNA. J Med Virol. 1989;27(2):87–90.
- Paterson R. Cases and observations on the molluscum contagiosum of Bateman, with an account of the minute structure of the tumours. Edinb Med Surg J. 1841;56(148):279–288.
- Grzybowski A, Jablonska S. Fritz Juliusberg (1872-1939): his life and achievements in dermatology. Clinics Dermatol. 2010;28:467–471.
- Olsen JR et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15(2):190–195.
- Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130–136.
- Koning S, Bruijnzeels MA, van Suijlekom-Smit LW, van der Wouden JC. Molluscum contagiosum in Dutch general practice. Br J Gen Pract. 1994;44(386):417–419.
- Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. 2005;133(6):985–991.
- Reynolds MG, Holman RC, Yorita Christensen KL, Cheek JE, Damon IK. The incidence of molluscum contagiosum among American Indians and Alaska Natives. PLoS One. 2009;4(4):e5255.
- McCollum AM, Holman RC, Hughes CM, et al. Molluscum contagiosum in a pediatric American Indian population: incidence and risk factors. PLoS One. 2014;9(7):e103419.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54(1):47–54.
- Villa L, Varela JA, Otero L, et al. Molluscum contagiosum: a 20-year study in a sexually transmitted infections unit. Sex Transm Dis. 2010;37(7):423–424.
- Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. PediatrDe rmatol. 2017;34(5):504–515.
- Silverberg N. Pediatric molluscum: an update. Cutis. 2019;104(5):E1–E2.
- Hurwitz S. Viral diseases of the skin. In: Hurwitz S, Ed. Clinical Pediatric Dermatology, Second Edition. Philadelphia, PA: WB Saunders;1981:338–340.
- Treadwell PA. Eczema and infection. Peditar Infect Dis J. 2008;27:551–552.
- Chaudhary M, Kulkarni M. Molluscum contagiosum in human immunodeficiency virus infected patients. Indian J Dent Res. 2008;19(2):155–159.
- Matis WL, Triana A, Shapiro R, Eldred L, Polk BF, Hood AF. Dermatologic findings associated with human immunodeficiency virus infection. J Am Acad Dermatol. 1987;17(5 Pt 1):746–751.
- Dhar S, Jain S, Verma G, Tanwar RK. Disseminated and atypical molluscum contagiosum in an AIDS patient. Indian J Dermatol Venereol Leprol. 1996;62(5):331–332.
- van der Wouden JC, van der Sande R, van Suijlekom-Smit LW, Berger M, Butler CC, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2009(4):Cd004767.
- Bhatia A, Elston D. What is the prognosis of molluscum contagiosum. 2020. https://www.medscape.com/answers/910570-30336/what-is-the-prognosis-of-molluscum-contagiosum#:~:text=Recurrences. Accessed 26 Aug 2021.
- Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29(2):231–236.
- Meza-Romero R, Navarrete-Dechent C, Downey C. Molluscum contagiosum: an update and review of new perspectives in etiology, diagnosis, and treatment. Clin Cosmet Investig Dermatol. 2019;12:373–381.
- United States Centers for Disease Control and Prevention site. Molluscum contagiosum. 2015. https://www.cdc.gov/poxvirus/molluscum-contagiosum/index.html. Accessed 26 Aug 2021.
- Leung A, Davies H. Molluscum contagiosum–an overview. Curr Pediatr Rev. 2012;8(4):346–349.
- Badri T, Gandhi G. Molluscum contagiosum. In: Badri T, Gandhi G (eds). StatPearls. Treasure Island, FL: StatPearls Publishing; 2021.
- Cubie HA. Diseases associated with human papillomavirus infection. Virology. 2013;445
(1–2):21–34. - Elasifer H, Wang ECY, Prod’homme V, et al. Downregulation of HLA-I by the molluscum contagiosum virus mc080 impacts NK-cell recognition and promotes CD8+ T-cell evasion. J Gen Virol. 2020;101:863–872.
- Davies HH, Jackson MA, Rice SG. Molluscum contagiosum. In: Baker CJ (ed). Red Book Atlas of Pediatric Infectious Diseases, 4th ed. Washington, DC: American Academy of Pediatrics, 2019;435–437.
- High WA. Cutaneous infections. In: Schwarzenberger K, Werchniak AE, Ko CJ, (eds). General Dermatology. Saunders-Elsevier, Philadephia, PA, USA, 2009;
87-88. 27Treadwell PA. Eczema and infection. Peditar Infect Dis J. 2008;27:551–552 - Achdiat P, Rowawi R, Fatmasari D. A case series: experience of using 20% potassium hydroxide solution to treat adult sexually transmitted molluscum contagiosum. Clin Cosmet Investig Dermatol. 2020:13 671–676.
- Bargman H. Is genital molluscum contagiosum a cutaneous manifestation of sexual abuse in children? J Am Acad Dermatol. 1986;14(5 Pt 1):847–849.
- Williams TS, Callen JP, Owen LG. Vulvar disorders in the prepubertal female. Pediatr Ann. 1986;15(8):588–589, 592–601, 604–605.
- Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4(9):22–42.
- Levin J, Friedlander SF, Del Rosso JQ. Atopic dermatitis and the stratum corneum: part 1: the role of filaggrin in the stratum corneum barrier and atopic skin. J Clin Aesthet Dermatol. 2013;6(10):
16–22. - Seo SH, Chin HW, Jeong DW, Sung HW. An open, randomized, comparative clinical and histological study of imiquimod 5% cream versus 10% potassium hydroxide solution in the treatment of molluscum contagiosum. Ann Dermatol. 2010;22(2):156–162.
- 3M Health Care Limited. Highlights of prescribing information. Bristol, TN: Aldara (Imiquimod); 1997.
- Rivera A, Tyring SK. Therapy of cutaneous human papillomavirus infections. Dermatol Ther. 2004;17(6):441–448.
- Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea–a review. J Am Coll Nutr. 2006;25(2):
79–99. - Yuan J, Ni G, Wang T, et al. Genital warts treatment: beyond imiquimod. Hum Vaccin Immunother. 2018;14(7):1815–1819.
- Padilla España L, Mota-Burgos A, Martinez-Amo JL, et al. Recalcitrant molluscum contagiosum successfully treated with sinecatechins. Dermatol Ther. 2016;29(4):217–218.
- Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78(18):2073–2080.
- Hu ML. Dietary polyphenols as antioxidants and anticancer agents: more questions than answers. Chang Gung Med J. 2011;34(5):449–460.
- Torbeck R, Pan M, DeMoll E, Levitt J. Cantharidin: a comprehensive review of the clinical literature. Dermatol Online J. 2014;20(6).
- Vakharia PP, Chopra R, Silverberg NB, Silverberg JI. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19(6):791–803.
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43(3):503–507.
- Moye V, Cathcart S, Burkhart CN, Morrell DS. Beetle juice: a guide for the use of cantharidin in the treatment of molluscum contagiosum. Dermatol Ther. 2013;26(6):445–451.
- Del Rosso JQ, Kircik L. Topical Cantharidin in the management of molluscum contagiosum: preliminary assessment of an ether-free, pharmaceutical-grade formulation. J Clin Aesthet Dermatol. 2019;12(2):27–30.
- 52Achdiat P, Rowawi R, Fatmasari D. A case series: experience of using 20% potassium hydroxide solution to treat adult sexually transmitted molluscum contagiosum. Clinical Cosmet Investig Dermatol. 2020:13 671–676.
- 53Guzman AK, Schairer DO, Garelik JL, Cohen SR. Safety and efficacy of topical cantharidin for the treatment of pediatric molluscum contagiosum: a prospective, randomized, double-blind, placebo-controlled pilot trial. Int J Dermatol. 2018;57(8):1001–1006.
- 54Jahnke MN, Hwang S, Griffith JL, Shwayder T. Cantharidin for treatment of facial molluscum contagiosum: a retrospective review. J Am Acad Dermatol. 2018;78(1):198–200.
- 55Javed A, Coulson I. Molluscum contagiosum. In: Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, (eds). Treatment of Skin Disease, fourth Edition. Philadelphia, PA: Elsevier-Saunders;2014:460–463.
- 26Schaffer JV, Berger EM. Molluscum contagiosum. JAMA Dermatol. 2016;152(9):1072.
- 58Browning JC, Enloe CE, Cartwright M, et al. Efficacy and safety of topical nitric oxide-releasing berdazimer gel in patients with molluscum contagiosum: a phase 3 randomized clinical trial. JAMA Dermatol. 2022;158(8):871-878.
- Cartwright M, Enloe CE, Stripling S, et al. Pharmacokinetic profile, safety, and tolerability of topical berdazimer gel, 10.3% in patients with molluscum contagiosum. J Drugs Dermatol. 2022 Oct 1;21(10):1104-1110. doi: 10.36849/JDD.6938.
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26(4):405–408.
- YCANTHTM (cantharidin) topical solution, Prescribing information, Copyright © 2023 Verrica Pharmaceuticals Inc., Verrica Pharmaceuticals Inc. West Chester, Initial U.S. Approval: 2023.