J Clin Aesthet Dermatol. 2025;18(10):66–72.
by Linda Stein Gold, MD; Paul Yamauchi, MD, PhD; Thomas Dirschka, MD; Athanasios Tsianakas, MD; Sunil Dhawan, MD; and Srinivas Sidgiddi, MD
Dr. Stein Gold is with the Henry Ford Health System in Detroit, Michigan. Dr. Yamauchi is with the Dermatology Institute and Skin Care Center in Santa Monica, California. Dr. Dirschka is with Centroderm GmbH in Wuppertal, Germany and the University of Witten-Herdecke in Witten, Germany. Dr. Tsianakas is with Fachklinik Bad Bentheim-Thermalsole-und Schwefelbad Bentheim GmbH in Bad Bentheim, Germany. Dr. Dhawan is with the Center for Dermatology Clinical Research, Inc. in Fremont, California. Dr. Sidgiddi is with Journey Medical Corporation in Scottsdale, Arizona.
FUNDING: Journey Medical Corporation provided funding for the trials and the investigational agents. Dr. Reddy’s Laboratories LTD provided support for the trials and the investigational medicinal products. Journey Medical Corporation also provided funding for the drafting of the manuscript.
DISCLOSURES: Dr. Stein Gold reports grants, research funding, and/or personal fees from Almirall, Journey Medical Corporation, Galderma, Ortho Dermatology, EPI Health, and Sun Pharmaceutical Industries. Dr. Yamauchi reports being an investigator, advisor, or speaker for AbbVie, Akros Pharma, Almirall, Alumis, Amgen, Anaptysbio, Apollo, Arcutis, Aristea Therapeutics, Boerhinger Ingelheim, Bristol-Myers Squibb, Connect Biopharma, Dermavant Sciences, DICE Therapeutics, Dr. Reddy’s Laboratory, Escalier, Evoimmune, Galapagos NV, Galderma, Glennmark Pharmaceuticals, GSK, Incyte, Johnson and Johnson, Journey Medical, LEO Pharma, Lilly, Medimmune, Nektar Therapeutics, Novartis, Padagis, Pfizer, Q32 Therapeutics, RAPT Therapeutics, Regeneron, Sanofi, Sun Pharmaceuticals, UCB, and Xencor. Dr. Dirschka reports being an investigator, speaker, and/or advisor for AbbVie, Aesclepion, Almirall, Beiersdorf, Biofrontera, Damae, Dr. August Wolff, Dr. Pfleger, Emblation, Galderma, GSK, Infectopharm, Intros, ISDIN, Janssen-Cilag, La Roche Posay, LEO Pharma, L‘Óreal, Louis Widmer, Mylan, Neracare, Nordberg, Novartis, Olistic, Pierre Fabre, Pfizer, Riemser, Schulze & BöhmGmbH, Scibase S.A., Smartinmedia AG, Speclipse, UCB, and Vichy. Dr. Tsianakas reports serving as an investigator in the present trial. Dr. Dhawan reports receiving research funding from AbbVie, Almirall, Arcutis, Cagebio, Cutera, Deramata, Galderma, Incyte, Journey Medical, L’Oreal, Lilly, Organon, Pfizer, Revance, Rubedolife, Sun Pharmaceuticals, and Takeda. Dr. Sidgiddi reports being an employee of Journey Medical Corporation.
Abstract: Objective: DFD-29 is a low-dose modified formulation of minocycline 40mg that has demonstrated efficacy and safety in patients with rosacea. We report the effect of DFD-29 on patient-reported assessments of disease severity and quality of life (QoL) from two clinical trials in patients with moderate-to-severe rosacea. Methods: MVOR-1 (NCT05296629) and MVOR-2 (NCT05343455) were 16-week, randomized, double-blind, active- and placebo-controlled Phase III trials that compared the impact of oral DFD-29 (EMROSI, Journey Medical Corporation), doxycycline 40mg, and placebo in adults aged 18 years or older with moderate-to-severe rosacea. Changes in QoL were exploratory endpoints that were evaluated at baseline and Weeks 2, 4, 8, 12, and 16 using the Rosacea-Specific Quality of Life (RosaQoL) questionnaire and the Dermatology Life Quality Index (DLQI). Results: Among randomized subjects, 288 completed MVOR-1 (DFD-29, n=117; doxycycline, n=98; placebo, n=73) and 296 completed MVOR-2 (DFD-29, n=115; doxycycline, n=113; placebo, n=68). In both trials, DFD-29 significantly improved QoL versus placebo (p<0.05) as assessed by RosaQoL and DLQI over 16 weeks. In MVOR-2, DFD-29 was also significantly superior to doxycycline in improving least squares mean RosaQoL scores at Week 12 (p=0.034). In MVOR-1, patients reported superior improvements in DLQI scores with DFD-29 versus doxycycline (p<0.05) at Weeks 4, 8, and 12. Limitations: RosaQoL and DLQI were exploratory endpoints. Conclusion: These data suggest that DFD-29 may be useful in improving QoL in patients with moderate-to-severe rosacea. Keywords: Rosacea, DFD-29, minocycline, doxycycline, quality of life, patient-reported outcomes, Rosacea-Specific Quality of Life (RosaQoL) questionnaire, Dermatology Life Quality Index (DLQI)
Introduction
Rosacea is a common inflammatory skin condition affecting approximately 5.5% of adults across the world.1,2 It is characterized by erythema, telangiectasia, pustules, papules, and rhinophyma, and can be accompanied by persistent burning, stinging, or pain.3 Ocular rosacea can also occur, which can result in blepharitis, conjunctivitis, keratitis, and in rare cases, corneal ulceration.4,5
The pathogenesis of rosacea is complex and multifactorial, involving genetics, immune dysregulation, microbial dysbiosis, and neurovascular dysfunction.6-9 Rosacea primarily affects the face, and since facial features can influence perceptions of attractiveness and a range of social outcomes,10-12 it can significantly increase psychosocial morbidity and reduce quality of life (QoL).13-15 Persons with rosacea also may experience dissatisfaction with their appearance and report psychosocial challenges due to the stigma associated with skin lesions.16,17 Rosacea is also associated with anxiety, depression, reduced confidence, and diminished self-esteem, which may contribute to psychological stress, ultimately exacerbating the physical symptoms of rosacea.13,18
Given the tremendous impact of rosacea on a person’s overall QoL, including patient-reported outcome assessments in clinical trials is critical to the comprehensive evaluation of patient response.19 While multiple therapeutic options are available for managing the symptoms of rosacea, the oral tetracyclines, minocycline and doxycycline, are among the most widely used systemic therapies for this disorder.20,21 The efficacy of these agents in rosacea has generally been attributed to their ability to suppress the multiple inflammatory processes contributing to the pathophysiology of the disease.22–30
Currently, a modified-release formulation of doxycycline (40mg once daily) is the only oral tetracycline approved by the United States Food and Drug Administration (FDA) for the treatment of rosacea.22 DFD-29 (minocycline HCl capsules, 40mg) is the lowest, FDA-approved dose, oral formulation of minocycline, with immediate-release (IR) minocycline (10mg) and extended-release (ER) minocycline (30mg). The approved antimicrobial dose for minocycline HCl is generally 200mg initially followed by 100mg every 12 hours,31 Importantly, current evidence suggests that the minocycline dose (40mg) contained in DFD-29 results in plasma concentrations below the threshold of antimicrobial activity.31,32
The efficacy and safety of DFD-29 has been evaluated in a randomized, double-blind, dose-ranging Phase II study and two identical randomized, double-blind, active- and placebo-controlled Phase III studies (MVOR-1 and MVOR-2) in patients with moderate-to-severe papulopustular rosacea.31,33 In these studies, DFD-29 demonstrated significantly superior efficacy compared to placebo and doxycycline 40mg in the proportions of patients with Investigator’s Global Assessment (IGA) treatment success and changes in inflammatory lesion counts and was generally well tolerated.
MVOR-1 (NCT05296629) and MVOR-2 (NCT05343455) also included patient-reported assessments as exploratory endpoints to evaluate the impact of DFD-29 on patient assessments of disease severity and QoL. The results of these patient-reported outcomes are reported here.
Methods
Study design. The designs of these clinical trials have been previously described in an earlier report.33 Briefly, MVOR-1 and MVOR-2 were identically designed, randomized, double-blind, active- and placebo-controlled Phase III trials that compared the impact of oral DFD-29, doxycycline 40mg, and placebo in adults aged 18 years or older with moderate-to-severe rosacea. Clinical sites included dermatology clinics, dermatology research clinics, and research clinics. Both studies followed Good Clinical Practice, United States (US) local laws and regulations, and the Declaration of Helsinki.34 Institutional review boards at each study center approved the protocol and consent forms, and written informed consent and photoconsent were obtained from all participants.
Participants. Participants were eligible for the studies if they were adults 18 years of age or older, had a clinical diagnosis of rosacea, had an IGA score of Grade 3 (moderate) or Grade 4 (severe), and had 15 to 60 (both inclusive) inflammatory rosacea lesions (papules and pustules) over the face. In addition, female patients were eligible if they were postmenopausal, surgically sterile, or had agreed to use a highly effective mode of contraception and those with childbearing potential had to have a negative urine pregnancy test at screening and baseline visits. Participants were excluded if they had more than two nodules or cysts at baseline, had used any topical or systemic rosacea treatment, systemic corticosteroids, immunosuppressive agents or immunomodulators in the previous 30 days, had received systemic retinoids in the previous six months, or had taken anti-inflammatory agents (eg, nonsteroidal anti-inflammatory drugs [NSAIDs]) in the two weeks preceding the baseline visit.
Study interventions. Patients were randomized in a 3:3:2 ratio to treatment with DFD-29 (now approved by US FDA as Emrosi), doxycycline 40mg modified release (using an authorized generic of Oracea [Galderma Laboratories, L.P.] marketed by Prasco Laboratories), or placebo, respectively, once daily. DFD-29 and placebo were provided by Dr. Reddy’s Laboratories Ltd, while doxycycline 40mg was procured by Journey Medical Corporation. To maintain the double-blind design, all three interventions were over-encapsulated within a second, larger capsule shell. All investigators, study staff, and sponsor staff were blinded to study treatment until study closure. Randomization was conducted via a centralized interactive web-response system (IWRS) accessible to all trial sites according to a schedule generated by a third-party statistician.
Patients were stratified by site and stratification was managed by IWRS. Patients were instructed to take the study medication at a fixed time of day once daily for 16 consecutive weeks, preferably in the morning after an overnight fast with 240mL (1 glass) of still water on an empty stomach. Substances that could potentially interfere with the absorption of minocycline, such as antacids, multivitamins, or other products containing aluminum, magnesium, and calcium, oral iron supplements, and dairy products were to be avoided within 1.5 hours before study drug administration and 3.0 hours after drug administration.
Photography. Photography was performed at five selected sites at baseline and at all subsequent visits. Photographs were taken of the patient’s face to document all rosacea lesions. Neither the patient nor the investigator was permitted to refer to the photographs at any subsequent visit for the purposes of grading.
QoL assessments. Changes in QoL were exploratory endpoints that were evaluated at baseline and at Weeks 2, 4, 8, 12, and 16 using the Rosacea Quality of Life (RosaQoL) questionnaire and the Dermatology Life Quality Index (DLQI). RosaQoL is a validated, standardized rosacea-specific instrument that evaluates three constructs: symptoms, emotion, and function.35 It includes 21 questions rated using a 5-grade scale (total score range of 21 to 105), with lower scores indicating less impairment in QoL.
The DLQI is a dermatology-oriented and validated instrument that includes 10 questions categorized into six constructs: symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment.36 The potential score range of this validated instrument, which has been used in a variety of dermatological conditions, is 0 to 30, with lower scores indicating less QoL impairment.
Statistical analysis. Each study planned to enroll 320 patients (approximately 120 receiving DFD-29, 120 receiving doxycycline, and 80 receiving placebo). The primary population for the efficacy analysis was the intent-to-treat (ITT) population, which included all randomized patients. A sensitivity analysis of efficacy was also conducted in the per protocol (PP) population, which included all ITT patients who completed the Week 16 evaluation and did not have any major protocol violations.
Comparisons of changes in DLQI and RosaQoL between DFD-29, placebo, and doxycycline were exploratory analyses. There was no type I error control or missing data imputation for these analyses. Mixed model for repeated measures (MMRM) analyses were used to evaluate DLQI and RosaQoL response, using several methods. First, treatment, analysis center, visit, and treatment-by-visit interaction were included as fixed effects, with baseline of the parameter of interest as a covariate and patient as a random factor. Second, the first variance/covariance structure to converge was used following this hierarchy: (1) unstructured; (2) heterogenous first-order autoregressive; (3) first-order autoregressive; (4) heterogenous compound symmetry; and (5) compound symmetry. Lastly, least squares (LS) means and standard errors (SE) for each treatment group and difference in LS means with the corresponding SE and 95% confidence intervals (CI) were calculated.
Total RosaQoL scores were calculated as the sum of the individual ratings on each of the 21 questions. If an individual rating was missing, the total score was also missing. Total DLQI scores were calculated as the sum of each score for each of the 10 questions included in the instrument. If a score on any question was missing, the total DLQI score was considered missing. The differences between treatments in change from baseline in total RosaQoL and total DLQI scores at Weeks 2, 4, 8, 12, and 16 were evaluated using MMRM.
Results
Patient disposition. Of the 653 patients enrolled in both trials, 323 were randomized and 288 completed MVOR-1 (117 on DFD-29; 98 on doxycycline; 73 on placebo) while 330 were randomized and 296 completed MVOR-2 (115 on DFD-29; 113 on doxycycline; 68 on placebo). The disposition of patients in both trials have been described in detail in a previous report.33
Baseline characteristics. Demographic and baseline characteristics of the study patients are shown in Table 1. Demographic and baseline characteristics were generally similar between treatment groups in both studies in the ITT population, although there were more women in the DFD-29 group (82.1%) than in the doxycycline group (67.2%) in MVOR-2. Most participants were women (approximately 76%) and were White (91.3% in MVOR-1 and 95.2% in MVOR-2). Mean (SD) age was 47.2 (13.7) years in MVOR-1 and 51.6 (14.0) years in MVOR-2. All participants had moderate or severe rosacea, as indicated by their IGA scores, and had approximately 25 total inflammatory lesions at baseline. Mean (SD) RosaQoL scores at baseline ranged from 72.9 (15.0) to 73.4 (16.3) in MVOR-1 and from 73.4 (17.2) to 75.9 (14.0) in MVOR-2. Mean (SD) DLQI scores at baseline ranged from 6.2 (4.6) to 6.9 (5.2) in MVOR-1 and from 6.6 (4.9) to 6.8 (5.7) in MVOR-2.
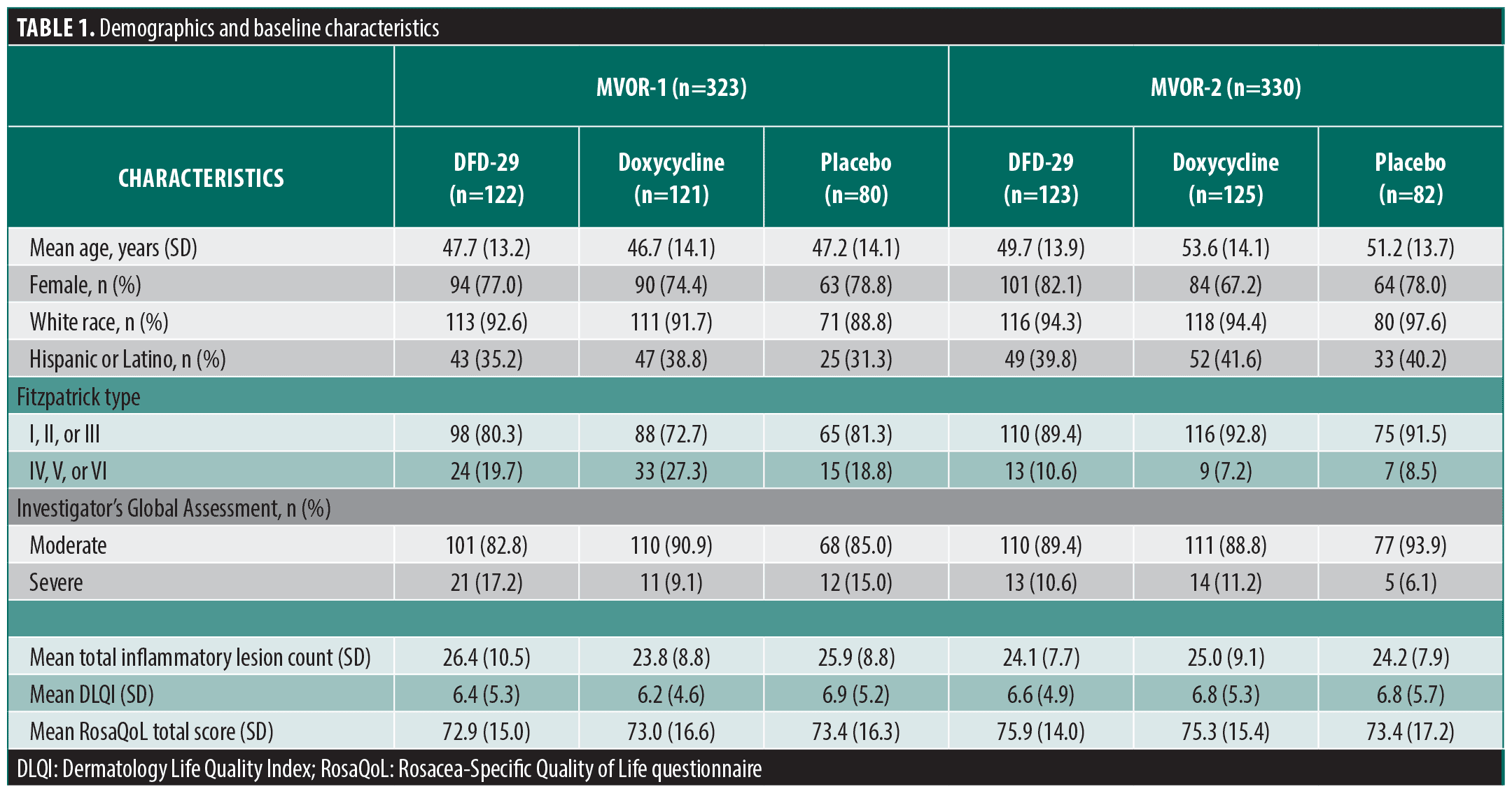
RosaQoL. In the ITT population, DFD-29 showed statistically significant superiority (p<0.05) over placebo in improvements in RosaQoL scores, starting at Week 2 in MVOR-1 and at Week 4 in MVOR-2 and continuing throughout the duration of the study (Figure 1A and 1B). An MMRM analysis of overall LS mean change (SE) in RosaQoL with treatment, analysis center, visit, and treatment-by-visit interactions as fixed factors also showed significant superiority with DFD-29 over placebo in both MVOR-1 (–12.0 [0.9] vs. 5.7 [1.2]; p<0.001) and MVOR-2 (–12.7 [0.9] vs. 6.0 [1.1]; p<0.001). At Week 16, LS mean changes (SE) in RosaQoL scores were –17.6 (1.4) with DFD-29 and –6.0 (1.7) with placebo in MVOR-1 (p<0.001) and –16.2 (1.2) with DFD-29 and –7.1 (1.5) with placebo in MVOR-2 (p<0.001).
Both DFD-29 and doxycycline improved RosaQoL scores in both trials (Figure 1C and 1D). At Week 16, there were no significant differences in LS mean improvements in RosaQOL scores between DFD-29 and doxycycline in either MVOR-1 (–17.3 [1.4] vs. –14.3 [1.5]; p=0.148) or MVOR-2 (–16.5 [1.3] vs. –14.0 [1.3]; p=0.145), but improvements with DFD-29 were numerically greater in both studies. In MVOR-2, DFD-29 was associated with a significantly superior improvement in LS mean (SE) RosaQoL scores at Week 12 (–16.1 [1.3] vs. –12.5 [1.2]; p=0.034). The MMRM analysis of overall LS mean changes (SE) in RosaQoL also revealed a significantly superior improvement with DFD-29 vs doxycycline in MVOR-2 (–13.0 [1.0] vs. –10.4 [1.0]; p=0.049) but not in MVOR-1 (–11.8 [1.1] vs. –9.8 [1.1], p=0.17).
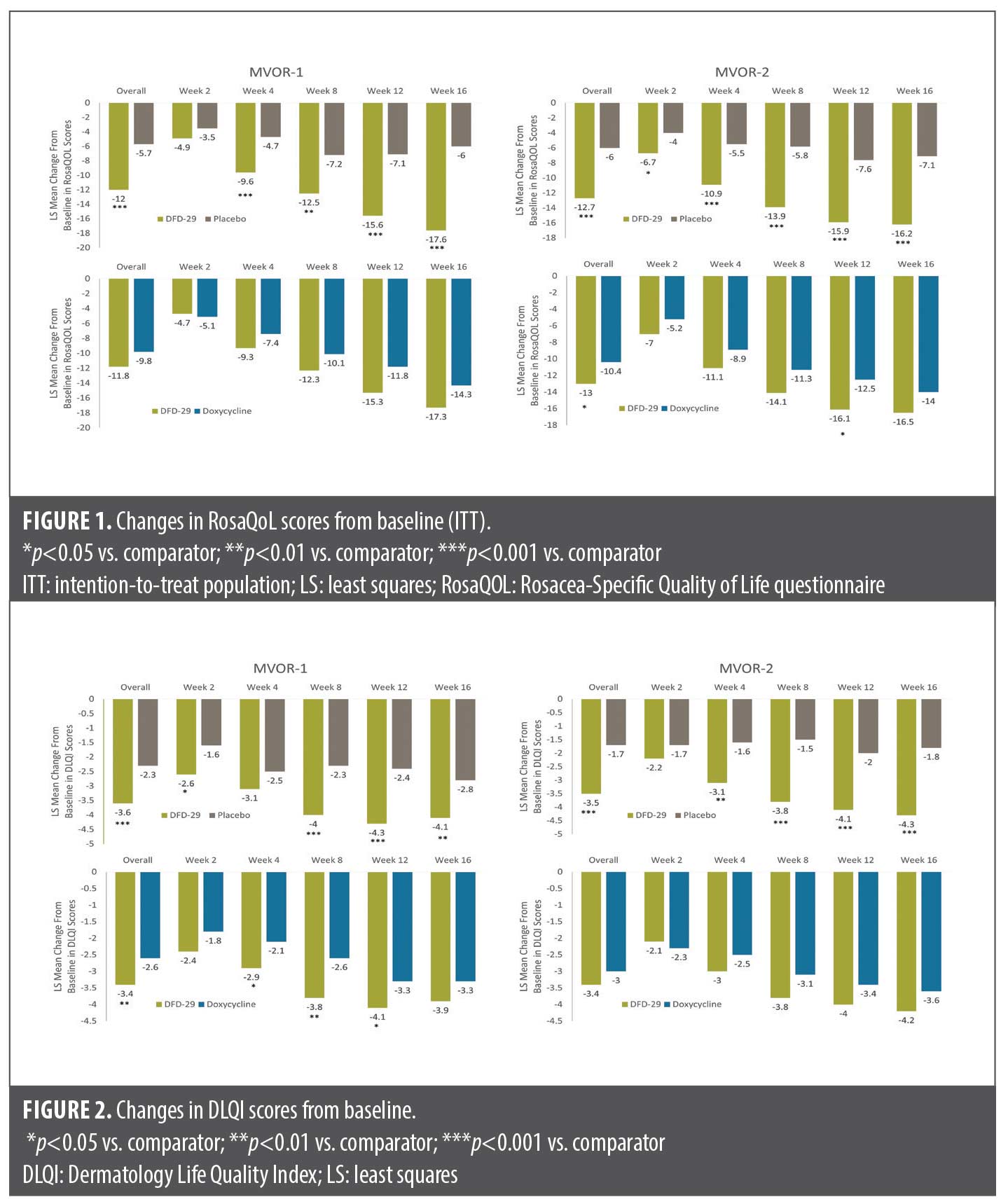
DLQI. Significantly greater improvement in DLQI scores were reported with DFD-29 vs placebo (p<0.05) as early as Week 2 in MVOR-1 and at Week 4 in MVOR-2 (Figure 2A and 2B). Improvements in DLQI were significantly greater with DFD-29 than with placebo at all subsequent timepoints except Week 4 in MVOR-1.
The MMRM analysis of overall LS mean changes (SE) in DLQI also showed superior improvements with DFD-29 vs placebo in both MVOR-1 (–3.6 [0.2] vs. –2.3 [0.3]; p<0.001) and MVOR-2 (–3.5 [0.3] vs. –1.7 [0.3]; p<0.001). At Week 16, LS mean changes (SE) in DLQI scores were –4.1 (0.3) with DFD-29 and –2.8 (0.4) in MVOR-1 (p=0.009) and –4.3 (0.3) with DFD-29 and –1.8 (0.4) with placebo in MVOR-2 (p<0.001).
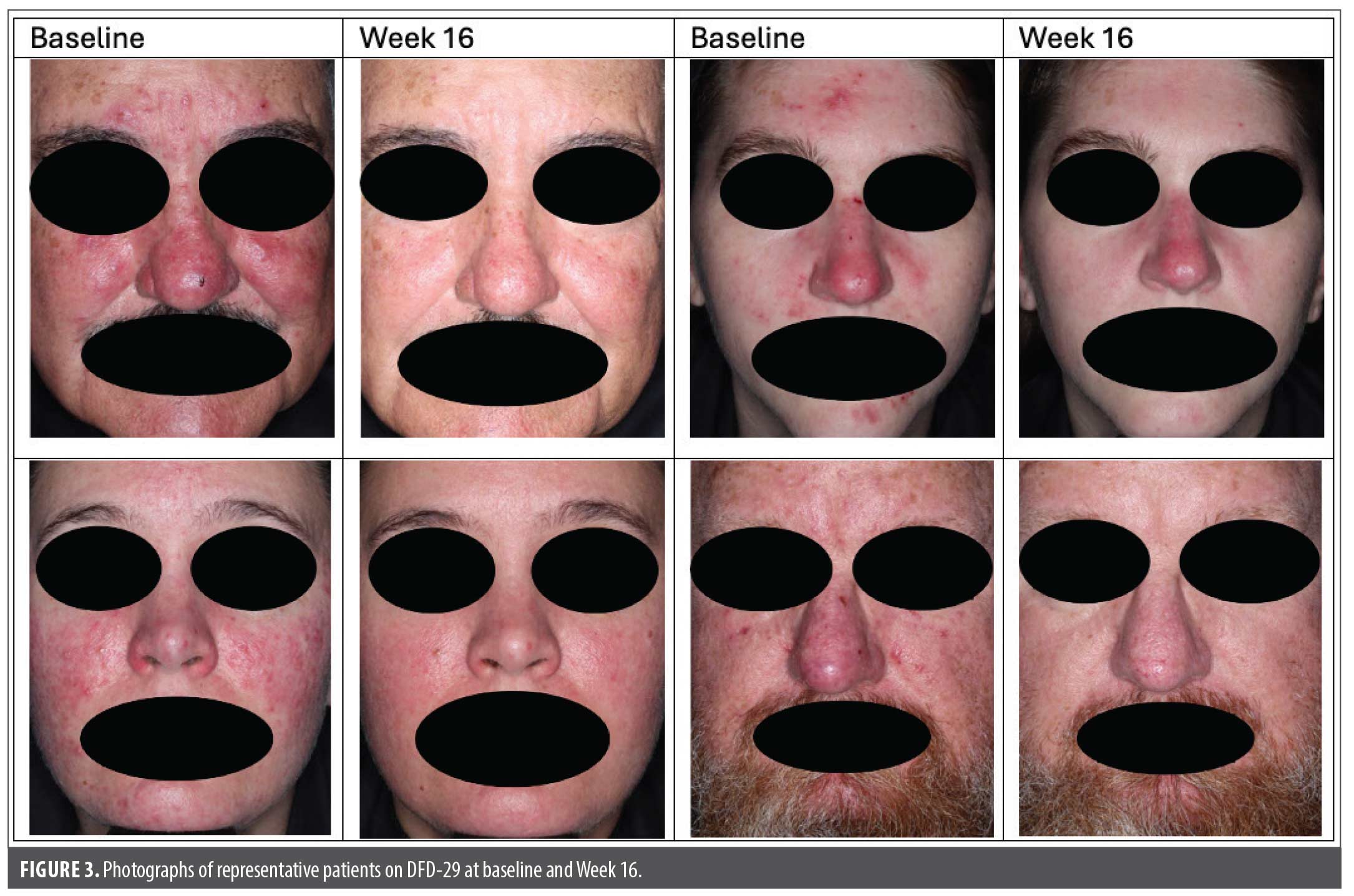
In MVOR-1, patients reported superior improvements in DLQI scores with DFD-29 vs doxycycline (p<0.05) at Weeks 4, 8, and 12, but not at Week 16 (Figures 2C and 2D). Numerically greater improvements in DLQI scores were observed with DFD-29 compared to doxycycline, but these improvements did not reach statistical significance in MVOR-2. A significant difference between DFD-29 and doxycycline in the MMRM analysis of overall LS mean changes (SE) in DLQI was noted in MVOR-1 (–3.4 [0.2] vs. –2.6 [0.2]; p=0.008) but not in MVOR-2 (–3.4 [0.3] vs. –3.0 [0.3], p=0.174). Examples of changes in the appearance of rosacea lesions in four patients on DFD-29 are shown in Figure 3.
Safety. In MVOR-1, 84 of 313 participants (26.8%) in the safety population reported at least one treatment-emergent adverse event (TEAE). In MVOR-2, 121 of 325 participants (37.2%) reported a TEAE. Study discontinuation rates due to TEAEs were 0% with DFD-29, 1.7% with doxycycline, and 1.3% with placebo in MVOR-1. In MVOR-2, rates were 1.6% with DFD-29, 0% with doxycycline, and 2.4% with placebo.
TEAEs were generally similar across groups within each trial. The most frequently reported adverse events (AE) across the two studies were nasopharyngitis and COVID-19. No significant differences among DFD-29, doxycycline, and placebo groups in vital signs or clinical laboratory tests were observed. More detailed safety results have been described in a previous report.33
Discussion
Results from MVOR-1 and MVOR-2 demonstrated that DFD-29 was significantly superior to placebo in improving QoL as assessed by RosaQoL and DLQI over 16 weeks of treatment in patients with moderate-to-severe rosacea. DFD-29 was also significantly superior to doxycycline in improving RosaQoL scores in MVOR-2 and in improving DLQI scores in MVOR-1.
The patient-reported QoL outcomes reported here are consistent with the previously reported improvements in investigator-evaluated endpoints in MVOR-1 and MVOR-2, as well as those observed in a Phase II dose-ranging study of patients with moderate-to-severe rosacea.31,33 Our results demonstrate that DFD-29 provides superior benefits in both clinical and patient-oriented outcomes and suggest an association between the positive clinical response in IGA and reductions in total inflammatory lesion counts and improved QoL.
Given the substantial impact of rosacea on QoL, it is critical to ensure that the patient’s voice is captured in clinical trials of new therapies for managing rosacea.37 RosaQoL is the most frequently used rosacea-specific tool for evaluating QoL in rosacea. This validated tool has been used in several clinical trials and has shown greater sensitivity than more generic QoL instruments.3,38,39
The DLQI is the most widely used instrument for evaluating health-related QoL in dermatology.3 It is effective in discriminating between differences in rosacea severity and has been recommended for use in assessing the burden of rosacea by the global ROSacea COnsensus (ROSCO) panel.3 In the current study, patients reported a mean DLQI score at baseline of 6.2 to 6.9, indicating that rosacea had a moderate impact on patients’ QoL at baseline.
Both the DLQI and RosaQoL have been recommended for use in evaluating QoL in patients with rosacea.3 The use of these complementary instruments in clinical trials can provide a useful assessment of both the QoL burden of rosacea and the impact of interventions on the patient experience.40
According to the European Academy of Dermatology and Venereology (EADV), the minimal clinically important difference (MCID) in DLQI can be used as a marker of clinical efficacy.3 The MCID for DLQI represents the smallest change in the DLQI score that can be considered clinically significant to a patient. The MCID also could be used to determine the minimal effectiveness of a treatment for affecting patient satisfaction with that treatment.41 For inflammatory conditions such as rosacea, a change in DLQI score of at least 4 points is considered the MCID,3,42 and a DLQI score of 0 or 1 could represent an ideal treatment goal for patients with rosacea.
A recent meta-analysis of data from 12 studies that reported changes in DLQI scores in 820 patients undergoing treatment for rosacea revealed a significant difference between pre-treatment and post-treatment scores (95% CI, –2.991 to –4.058, p<0.001), with a mean difference of 3.53 points.40 In the present analysis, mean improvements in DLQI scores were greater than the MCID with DFD-29 vs. placebo at Week 16 in MVOR-1 (–4.1) and MVOR-2 (–4.3). When comparing DLQI improvements with DFD-29 and doxycycline at Week 16, only DFD-29 achieved the DLQI MCID in MVOR-2 (–4.2) and approached the MCID in MVOR-1 (–3.9), but doxycycline did not (–3.3 in MVOR-1 and –3.6 in MVOR-2). Given the potential impact of rosacea on a patient’s QoL, they may benefit from a treatment that addresses both the physical and psychosocial impact of rosacea to maximize both clinical outcomes and patient satisfaction.
This analysis had some limitations. The two QoL endpoints, changes in RosaQoL and DLQI from baseline, were exploratory endpoints in MVOR-1 and MVOR-2. In addition, the studies enrolled smaller proportions of people with darker skin colors, which is not unexpected given its lower incidence in this population. Participants were also encouraged to minimize exposure to potential rosacea triggers during the studies, which may have contributed to a reduction in rosacea flare-ups in all three groups during the trials.
Conclusion
DFD-29 was superior to placebo in improving QoL as assessed by RosaQoL and DLQI over 16 weeks of treatment in patients with moderate-to-severe rosacea. DFD-29 was also superior to doxycycline in improving RosaQoL scores in MVOR-2 and in improving DLQI scores in MVOR-1. These data suggest that DFD-29 may be a useful option in improving QoL in patients with rosacea.
Acknowledgments
We thank the participants, investigators, and site personnel who participated in the MVOR-1 and MVOR-2 trials and made them possible. Nicole R. Cooper (Transcend Medical Communications, LLC) provided writing and graphical assistance, technical editing support, and helped manage author reviews and was compensated for work.
References
- Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282-289.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-35.
- Chernyshov PV, Finlay AY, Tomas-Aragones L, et al. Quality of life measurement in rosacea. Position statement of the European Academy of Dermatology and Venereology Task Forces on Quality of Life and Patient Oriented Outcomes and Acne, Rosacea and Hidradenitis Suppurativa. J Eur Acad Dermatol Venereol. 2023;37(5):954-964.
- Tavassoli S, Wong N, Chan E. Ocular manifestations of rosacea: a clinical review. Clin Exp Ophthalmol. 2021;49(2):104-117.
- Vassileva S, Tanev I, Drenovska K. Rosacea: the eyes have it. Clin Dermatol. 2023;41(4):528-536.
- Parkins GJ, Maan A, Dawn G. Neurogenic rosacea: an uncommon and poorly recognized entity? Clin Exp Dermatol. 2015;40(8):930-931.
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9(1):e1361574.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-26.
- Woo YR, Han YJ, Kim HS, Cho SH, Lee JD. Updates on the risk of neuropsychiatric and gastrointestinal comorbidities in rosacea and its possible relationship with the gut-brain-skin axis. Int J Mol Sci. 2020;21(22).
- Johnston VS. Mate choice decisions: the role of facial beauty. Trends Cogn Sci. 2006;10(1):9-13.
- Little AC, Jones BC, DeBruine LM. Facial attractiveness: evolutionary based research. Philos Trans R Soc Lond B Biol Sci. 2011;366(1571):1638-1659.
- Zebrowitz LA, Montepare JM. Social psychological face perception: why appearance matters. Soc Personal Psychol Compass. 2008;2(3):1497.
- Chiu CW, Tsai J, Huang YC. Health-related quality of life of patients with rosacea: a systematic review and meta-analysis of real-world data. Acta Derm Venereol. 2024;104:adv40053.
- Halioua B, Cribier B, Frey M, Tan J. Feelings of stigmatization in patients with rosacea. J Eur Acad Dermatol Venereol. 2017;31(1):163-168.
- van der Linden MM, van Rappard DC, Daams JG, Sprangers MA, Spuls PI, de Korte J. Health-related quality of life in patients with cutaneous rosacea: a systematic review. Acta Derm Venereol. 2015;95(4):395-400.
- Dai R, Lin B, Zhang X, Lou Y, Xu S. Depression and anxiety in rosacea patients: a systematic review and meta-analysis. Dermatol Ther (Heidelb). 2021;11(6):2089-2105.
- Vinding GR, Knudsen KM, Ellervik C, Olesen AB, Jemec GB. Self-reported skin morbidities and health-related quality of life: a population-based nested case-control study. Dermatology. 2014;228(3):261-268.
- Chang HC, Huang YC, Lien YJ, Chang YS. Association of rosacea with depression and anxiety: a systematic review and meta-analysis. J Affect Disord. 2022;299:239-245.
- Perez-Chada L, Taliercio VL, Gottlieb AB, et al. Achieving consensus on patient-reported outcome measures in clinical practice for inflammatory skin disorders. J Am Acad Dermatol. 2023;88(1):86-93.
- Del Rosso JQ, Tanghetti E, Webster G, Stein Gold L, Thiboutot D, Gallo RL. Update on the management of rosacea from the American Acne & Rosacea Society (AARS). J Clin Aesthet Dermatol. 2019;12(6):17-24.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: a status report on topical agents. Cutis. 2013;92(6):277-284.
- Oracea (doxycyline) prescribing information. Galderma Laboratories, LP; 2022.
- Akamatsu H, Asada M, Komura J, Asada Y, Niwa Y. Effect of doxycycline on the generation of reactive oxygen species: a possible mechanism of action of acne therapy with doxycycline. Acta Derm Venereol. 1992;72(3):178-179.
- Cazalis J, Bodet C, Gagnon G, Grenier D. Doxycycline reduces lipopolysaccharide-induced inflammatory mediator secretion in macrophage and ex vivo human whole blood models. J Periodontol. 2008;79(9):1762-1768.
- Griffin MO, Ceballos G, Villarreal FJ. Tetracycline compounds with non-antimicrobial organ protective properties: possible mechanisms of action. Pharmacol Res. 2011;63(2):102-107.
- Hoyt JC, Ballering J, Numanami H, Hayden JM, Robbins RA. Doxycycline modulates nitric oxide production in murine lung epithelial cells. J Immunol. 2006;176(1):567-572.
- Ishikawa C, Tsuda T, Konishi H, Nakagawa N, Yamanishi K. Tetracyclines modulate protease-activated receptor 2-mediated proinflammatory reactions in epidermal keratinocytes. Antimicrob Agents Chemother. 2009;53(5):1760-1765.
- Pruzanski W, Stefanski E, Vadas P, McNamara TF, Ramamurthy N, Golub LM. Chemically modified non-antimicrobial tetracyclines inhibit activity of phospholipases A2. J Rheumatol. 1998;25(9):1807-1812.
- Ryan ME, Usman A, Ramamurthy NS, Golub LM, Greenwald RA. Excessive matrix metalloproteinase activity in diabetes: inhibition by tetracycline analogues with zinc reactivity. Curr Med Chem. 2001;8(3):305-316.
- Ueyama Y, Misaki M, Ishihara Y, Matsumura T. Effects of antibiotics on human polymorphonuclear leukocyte chemotaxis in vitro. Br J Oral Maxillofac Surg. 1994;32(2):96-99.
- Tsianakas A, Pieber T, Baldwin H, et al. Minocycline extended-release comparison with doxycycline for the treatment of rosacea: a randomized, head-to-head, Clinical Trial. J Clin Aesthet Dermatol. 2021;14(12):16-23.
- Bhatia N SS. Impact of DFD-29, a low-dose oral minocycline, on skin, gastrointestinal tract, and vaginal microflora in healthy adults: a randomized, double-blind, placebo-controlled trial. Paper presented at: American Academy of Dermatology Annual Meeting; March 8-12, 2024, 2024; San Diego, CA.
- Bhatia N DRJ, Gold LS, Lain E, Draelos ZD, Sidgiddi S. Efficacy, safety, and tolerability of oral DFD-29, a low-dose formulation of minocycline, for the treatment of rosacea: two Phase III randomized clinical trials. JAMA Dermatol. 2024;in press.
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194.
- Nicholson K, Abramova L, Chren MM, Yeung J, Chon SY, Chen SC. A pilot quality-of-life instrument for acne rosacea. J Am Acad Dermatol. 2007;57(2):213-221.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210-216.
- Ly S, Miller J, Tong L, Blake L, Mostaghimi A, Barbieri JS. Use of patient-reported outcomes in acne vulgaris and rosacea clinical trials from 2011 to 2021: a systematic review. JAMA Dermatol. 2022;158(12):1419-1428.
- Harper J, Del Rosso JQ, Ferrusi IL. Cross-sectional survey of the burden of illness of rosacea by erythema severity. J Drugs Dermatol. 2018;17(2):150-158.
- Tyring S, Solomon JA, Staedtler G, Lott JP, Nkulikiyinka R, Shakery K. Patient-reported outcomes of azelaic acid foam 15% for patients with papulopustular rosacea: secondary efficacy results from a randomized, controlled, double-blind, Phase III trial. Cutis. 2016;98(4):269-275.
- Yang F, Zhang Q, Song D, Liu X, Wang L, Jiang X. A cross-sectional study on the relationship between rosacea severity and quality of life or psychological state. Clin Cosmet Investig Dermatol. 2022;15:2807-2816.
- Copay AG, Subach BR, Glassman SD, Polly DW, Jr., Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
- Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230(1):27-33.