 by Guneet Awal, MBBS, MD, and Simplepreet Kaur, MBBS, MD
by Guneet Awal, MBBS, MD, and Simplepreet Kaur, MBBS, MD
Drs. Awal and Kaur are with the Department of Dermatology, Venereology, and Leprosy and Sri Guru Ram Das Institute of Medical Sciences and Research in Amritsar, India.
J Clin Aesthet Dermatol. 2018;11(5):15–20
Funding: No funding was provided for this article.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Background. Various treatment modalities have been described in the literature for treating warts, but none thus far have demonstrated optimal results. Recently, the mumps, measles, and rubella (MMR) antigen has gained popularity because of its proven efficacy in the treatment of warts.
Aim. The goal was to evaluate the efficacy and safety of intralesional MMR antigen in the treatment of cutaneous warts.
Methods. Patients were divided into an MMR (study) group and a normal saline (control) group. Injections were administered into the single largest wart on each patient every two weeks. Follow-up was done at six weeks and 16 weeks after the last injection for any side effects and/or recurrence.
Results. Out of 150 patients, 72 received the MMR injection and 50 received normal saline injections. Twenty-eight patients did not complete the study. A statistically significant (p<0.00001) difference in results was found between the two groups: 68 percent of patients in the MMR group showed complete response compared to 10 percent in the control group. Pain during injection was the most common side effect and was seen in both groups.
Conclusion. MMR injection has shown significant results with almost negligible adverse effects. The MMR antigen vaccine has therapeutic potential as a treatment for warts with its demonstrated efficacy, safety profile, and cost-effectiveness.
Keywords: Immunotherapy, MMR vaccine, cutaneous warts
Warts are a common malady that affects people of all ages, male or female. Warts are the cutaneous manifestations of human papilloma virus (HPV) infection. HPV is a double-stranded deoxyribonucleic acid (DNA), non-enveloped virus that belongs to the Papovaviridae family and Papillomavirus genus. They show tropism for epithelial cells, hence causing muco-cutaneous manifestations.1 This virus tends to remain latent in the host cells for a long period of time, causing recurrences, and can cause benign and malignant infections. Among the resulting benign infections, cutaneous warts are the most common.2 Cutaneous warts can manifest in various forms—namely, common warts (Verruca vulgaris), plane warts (Verucca plana), plantar warts, and anogenital warts (Condyloma acuminata). To date, more than 200 subtypes of HPV have been characterized.3 Common types involved in cutaneous warts are HPV Types 1, 2, 7, 27, and 57.4,5 Extragenital cutaneous warts can be asymptomatic or painful (especially when they are present on the plantar surface) and disfiguring.6 Warts are highly contagious. They can spread from one person to another and from one site to another site in same patient. They run a chronic course and ultimately disappear after few years. Though these warts show rare malignant potential, cosmetic disfigurement will likely impel patients to seek treatment.
Various treatment modalities are available for warts. Treatment can include topical therapies, such as trichloroacetic acid, salicylic acid, podophyllotoxin, and 5-fluorouracil. Radiocautery, cryotherapy, surgical excision, and carbon dioxide laser are other methods of treatment; however, these modalities pose a high risk of scarring. Various systemic modalities have also been used, such as levamisole and zinc sulphate, but none have achieved a complete cure without recurrence. Recently, certain immunotherapeutic agents have gained popularity for the optimal cure of warts.7 Antigens such as the measles, mumps, and rubella (MMR) vaccine; Candida albicans; Bacillus Calmette–Guérin (BCG), and Mycobacterium indicus pranii are injected intralesionally, with variable responses observed. Results with MMR injection have been encouraging in past studies. In the current study, we attempted to further strengthen the evidence regarding the efficacy and safety profile of this antigen in the treatment of cutaneous warts.
Materials and Methods
This single-blind, randomized, placebo-controlled study (1:1) was conducted from January 2016 to December 2016. Patients who presented to the outpatient department of dermatology with cutaneous warts anywhere on the body other than the anogenital area were included. Before starting the study, clearance from the ethical committee of the institution was obtained. Included patients were 15 to 50 years of age with single or multiple extra-genital warts for duration of at least one month without using anti-wart treatments for the last one month. Exclusion criteria included prior hypersensitivity reaction to MMR antigen, pregnancy/lactation, presence of any active infections (e.g., herpes), tuberculosis, chronic diseases (e.g., diabetes mellitus), hypertension, immunosuppression (e.g., human immunodeficiency virus [HIV]) or if the patient was taking immunosuppressives, and patients who were nonadherent.
All of the patients who fulfilled the inclusion criteria underwent clinical examination to confirm the diagnosis of wart. In suspicious cases, a biopsy for histopathological confirmation was done. Detailed history was taken to note the duration, number of warts, and the sites involved. Demographic details including age and sex were noted. Photographic documentation was done. Written consent was obtained from all of the patients.
Procedure. Patients were randomized equally into two groups using WinPepe software (ETCETERA.EXE 3.26). Using a sample size calculator tool (University of California, San Francisco, San Francisco, California, USA), keeping Type 1 error as five percent and Type 2 error as 20 percent, the sample size in each group was 71. Keeping the dropout rate in mind, the size was increased to 75 patients per group. The first group was designated as the study group wherein intralesional MMR injection was given, and the second group was designated as the control group wherein intralesional normal saline injection was given. MMR vaccine (TRESIVAC®; Serum Institute of India Pvt. Ltd., Pune, India) is available in a single dose vial of 0.5mL. Prior sensitivity testing was done using a dose of 0.1mL via injection intradermally into the volar aspect of the left forearm. The injected sites were examined after two weeks for immune response in the form of erythema or nodule formation. In sensitized patients, 0.5mL of MMR vaccine after reconstitution with distilled water was injected intradermally into their single largest wart. Injections were given every two weeks until a maximum of five injections was achieved. Similarly, 0.5mL of normal saline was injected into the single largest wart of each member of the control group. Patients were evaluated for response and any adverse effects at each visit. Follow-up was done at every visit and at six weeks and 16 weeks after the last injection. Wart recurrence was assessed at every visit.
Blinding. Single-blinding was done, where patients were not informed about their allocated groups, but dermatologists who performed the procedure and noted the responses were informed about the groups.
Data analysis. The data were analyzed statistically using GraphPad Prism 7 statistical software (GraphPad Software Inc., La Jolla, California, USA). Mean values with standard deviations (SD) were calculated for quantitative data, and nominal data were presented as percentages. Values were compared using a chi-squared test and t-test, for which a value of probability (p) was calculated. Values of p that were less than 0.05 were considered to be significant.
Results
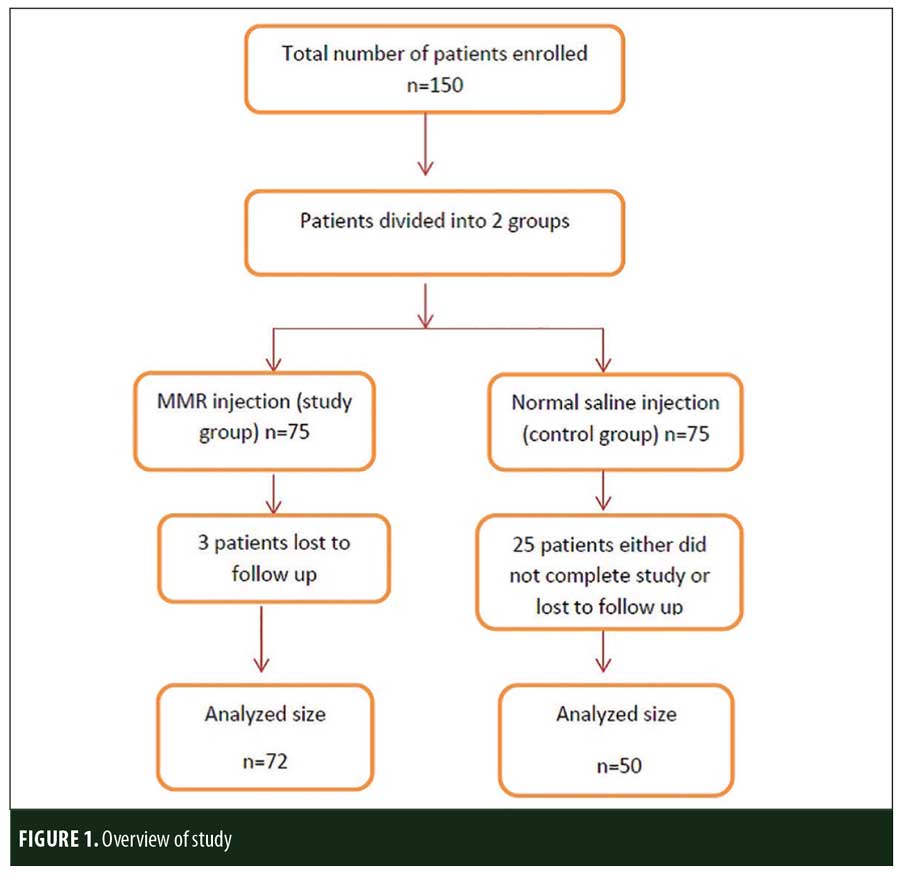
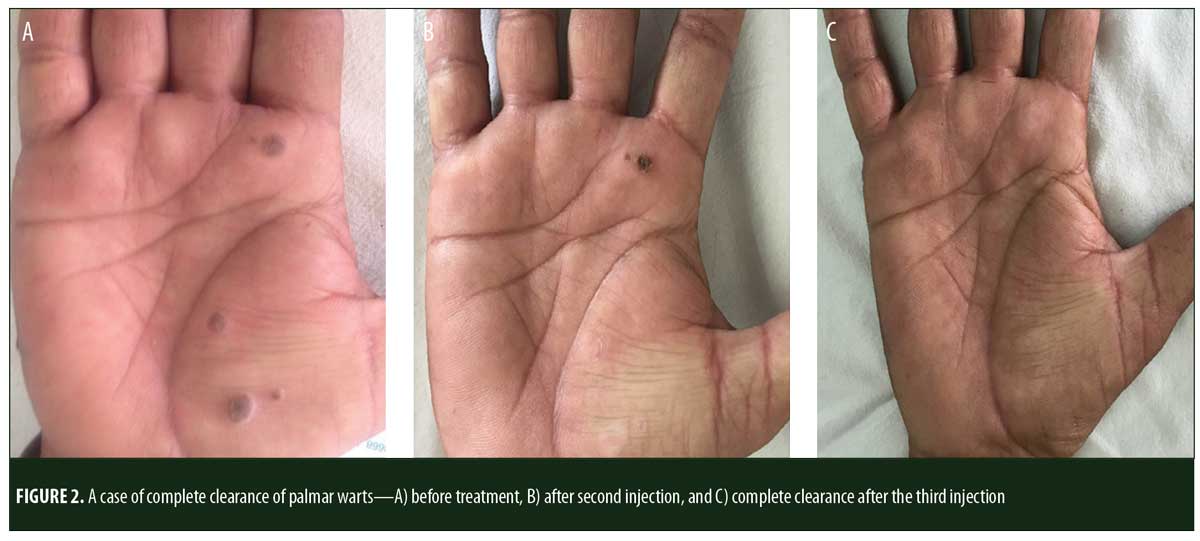
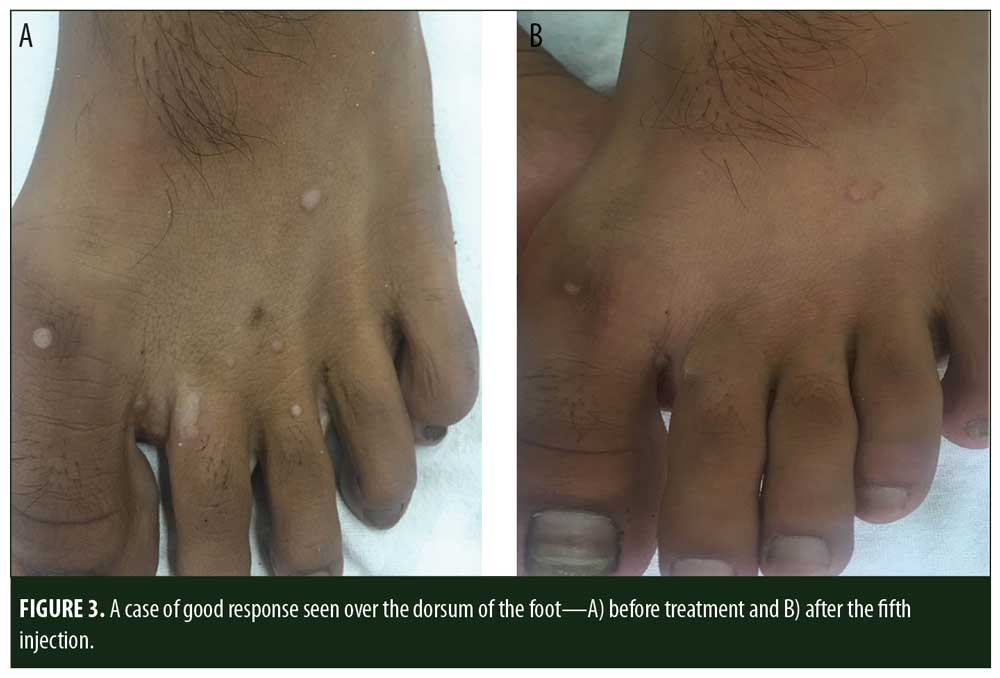
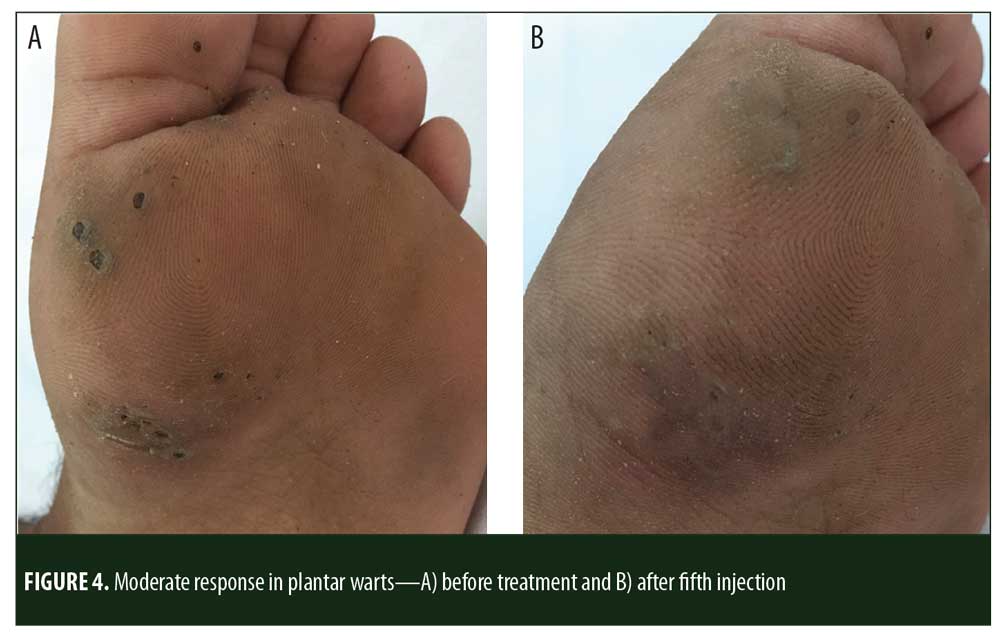
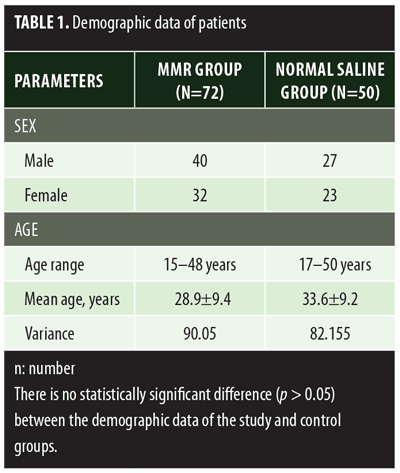
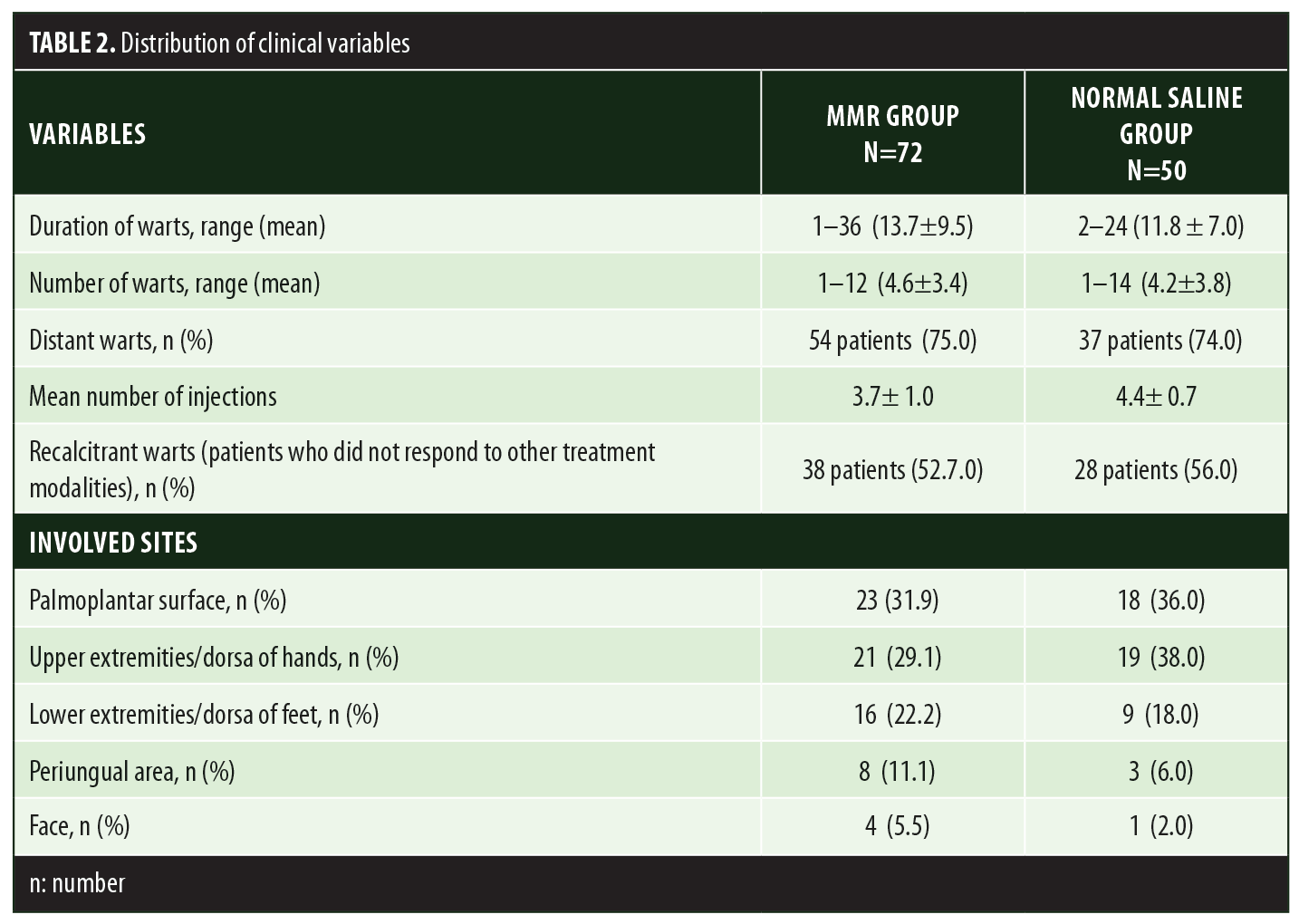
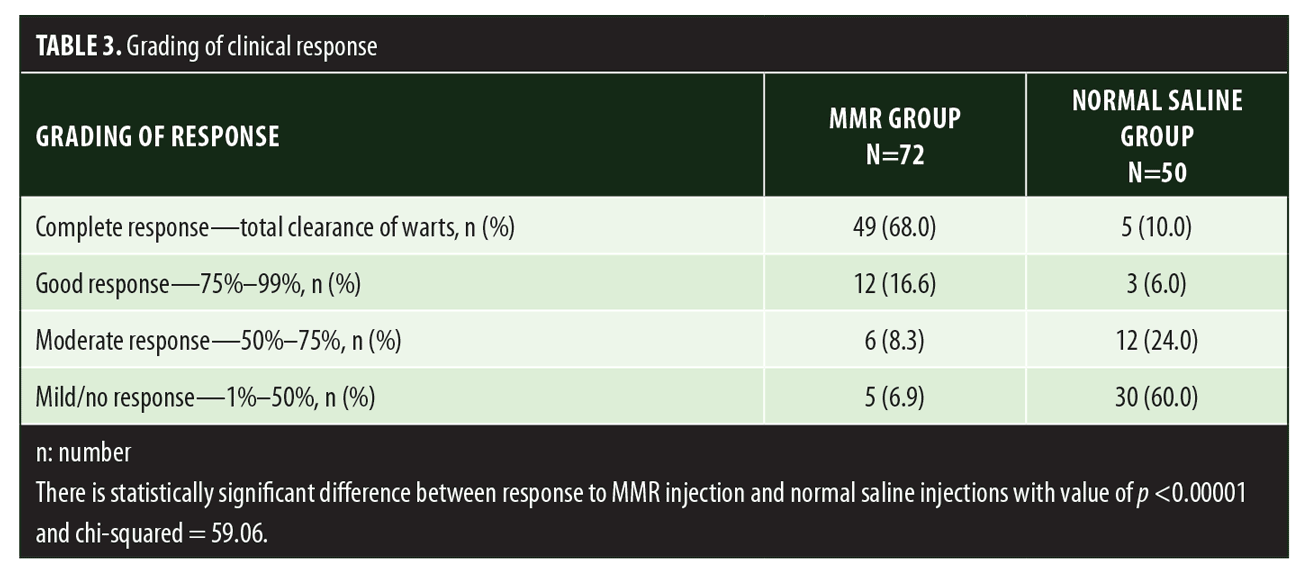
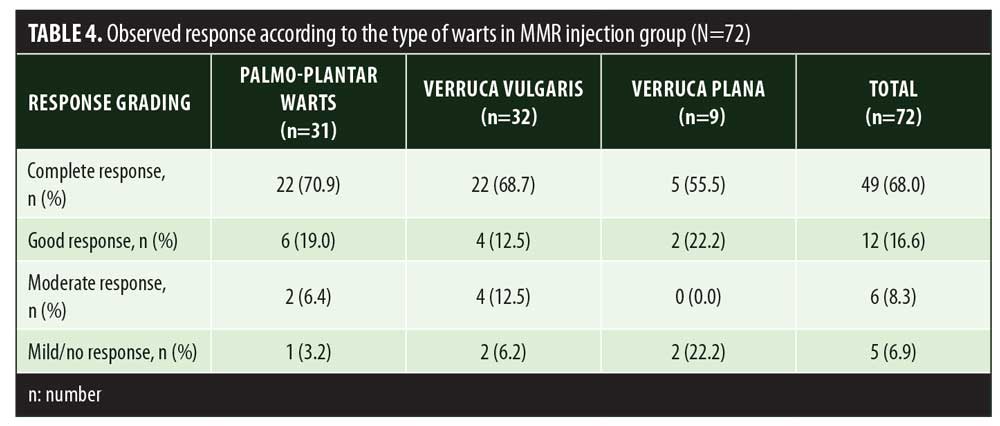
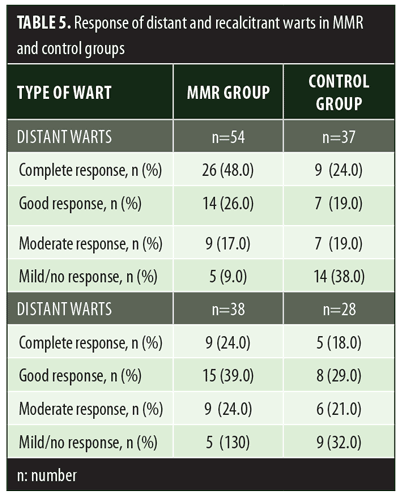
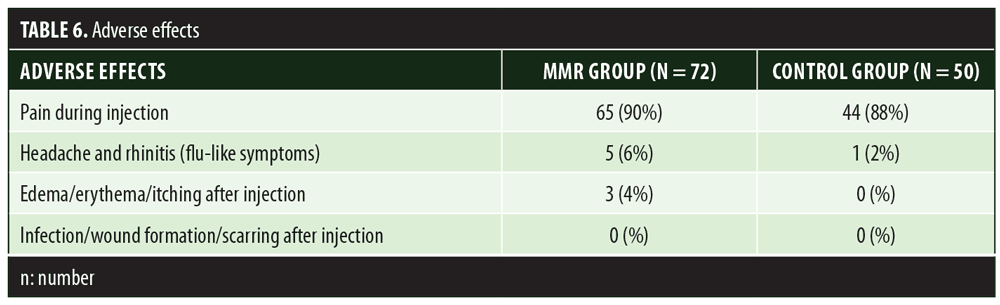
A total of 150 patients were included in this study (Figure 1). Of these, 28 patients did not complete the study or were lost to follow-up. Hence, the total number of patients in the sample size was 122, with 72 patients in the MMR study group and 50 patients in the control group. In the MMR group, there were 40 men and 32 women with a mean age of 28.9±9.4 years (Table 1). The duration of warts varied from 1 to 36 months in the MMR group and from 2 to 24 months in the control group. In the MMR study group, subjects had a range of 1 to 12 warts, and in the control group, subjects had a range of 1 to 14 warts. There was no statistically significant differences (p>0.05) between the two groups in terms of demographic data and clinical variables. The most common site of wart presentation in the MMR group was the palmoplantar surface in 23 patients, followed by the hands/upper extremities in 21 patients. In the control group, the most common sites were the upper extremities, including the hands, in 19 patients. The number of patients who presented with distant warts in the MMR group numbered 54 (75%) and, in control group, 37 (74%). The number of injections given in both groups ranged from 2 to 5 (Table 2). Response was observed on the basis of a decrease in size and number of lesions and according to the type of wart. A decrease in size of lesions by less than 50 percent was graded as no or mild response, a decrease of 50 to 75 percent was graded as a moderate response, a decrease of 75 to 99 percent was graded as a good response, and total clearance of all lesions was graded as a complete response (Table 3). A statistically significant difference with p<0.00001 was observed in terms of therapeutic response to MMR injection versus normal saline injection. A clinical response on the basis of type of warts was also observed in the MMR injection group: palmoplantar warts showed the best response, followed by common warts. Complete response in a patient with palmoplantar warts is shown in Figure 2. Figure 3 shows good response in a patient with Verruca plana. Complete clearance was seen in 70 percent of palmoplantar warts and 68 percent of common warts (Table 4). Good response was observed in 16.6 percent of total warts, with moderate response in 8.3 percent. Figure 4 shows moderate response in a case of plantar warts after five injections. Response in distant and recalcitrant warts was also observed (Table 5). Adverse effects were evaluated at every visit, then at six weeks and 16 weeks after the last injection. In the MMR group, 90 percent of the patients reported pain while receiving the injection, and in the control group, 88 percent patients reported pain during injection. Additionally, six percent patients in the MMR group reported rhinitis and headache (flu-like symptoms), which were relieved using paracetamol and antihistaminics. Erythema and edema after injection were observed in only four percent of the MMR group and zero in the control group (Table 6). Recurrence was observed in two (2.7%) patients who received MMR injections and three (6%) patients in the control group at 16 weeks after the last injection.
Discussion
In our practice, the prevalence of cutaneous warts has been increasing. Frequent relapses and resistance to treatment have become challenging problems for dermatologists.Despite the availability of multiple therapeutic options for the treatment of warts, recurrence and resistance to treatment are still commonly seen.8 Lately, the role of the immune system has been widely studied in patients with cutaneous warts. Spontaneous resolution of warts has led to exploration of the role of the immune system theory.9,10 As per the literature, there is increase in Th1 cytokines along with the infiltration of T cells (e.g., CD4+,CD8+) around the wart, which is responsible for its spontaneous regression.11 Keeping in mind this cell-mediated mechanism, various immunotherapeutic options have been used, including oral zinc sulphate, levamisole, cimetidine, and topical contact sensitizers (e.g., dinitrochlorobenzene, diphencyprone, squaric acid dibutyl ester, and imiquimod).12 Despite the immunomodulatory role of these agents, however, the results obtained generally are not significantly satisfactory. Recently, the use of injectable immunotherapy using antigens such as MMR has gained popularity. Better results with reduced adverse effects and lower recurrence rates have been reported with this therapy.13
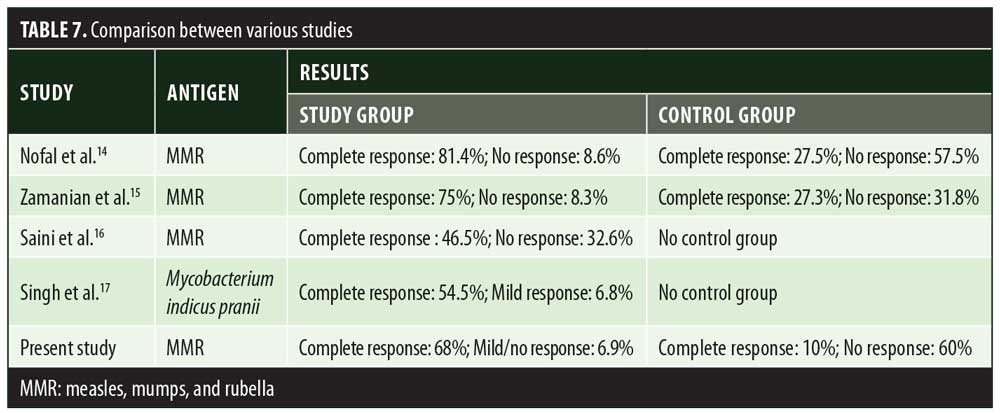
In the present study, significant difference in therapeutic response was observed between the MMR and control groups. A study done by Nofal et al14 also reported a significant difference in results between patients receiving MMR injection and those receiving placebo. In Nofal’s study, 81.4 percent patients showed complete clearance of warts with minimal side effects. These findings were slightly better than the results of our study, where complete clearance was observed in 68 percent of the patients who received MMR injection. Similar to Nofal et al’s14 observation, Zamanian et al15 also observed a higher response (75%) to MMR injection compared to our study. Saini et al16 observed a lower rate of complete clearance (46.5%) with MMR injection (Table 7). Demographic distribution and other clinical variables such as the number and site of warts did not alter the therapeutic response to MMR injection. In a study done by Horn et al,15 immunotherapy with single antigens (mumps) yielded a 54-percent response in patients. Use of the three antigens—measles, mumps, and rubella— together help in eliciting a stronger immune response against HPV through the production of various cytokines like interleukin (IL)-2, IL-4, IL-5, and tumor necrosis factor-?. In the present study, 68-percent complete clearance was seen with a mean number of 3.7±1.0 injections. Comparable results were seen in the study by Singh et al17 where a 54.5-percent complete response rate was achieved with a mean number of 3.4±1.1 injections. Singh et al17 also emphasized the activation of Th1 response with immunotherapy, which was effective for treating not only injected warts but also distant warts. In a study by Dhakar et al,18 significant clearance was seen in distant warts with the use of Mycobacterium vaccine immunotherapy when compared to cryotherapy. In another clinical trial,19 78 percent of patients showed clearance of anatomically distant warts with mumps and Candida immunotherapy. Maximum complete clearance was seen in palmoplantar warts (70.9%). Palmoplantar warts are comparatively resistant to other treatment modalities. In our study, patients with palmoplantar warts were more in number, owing to treatment resistance compared to other types of warts; hence, better response was seen with this particular type. This was in contrast to complete remission seen in Verruca vulgaris (66.7%) with Mycobacterium indicus pranii vaccine immunotherapy.20
Immunotherapy with MMR antigen has been shown to be a safer modality than other options, without precipitating any serious adverse effects.
Pain during injection and flu-like symptoms were common side effects reported by the patients included in our study. These findings were comparable with various other studies.15,19,20 A 2.7-percent recurrence rate in the MMR group and a six-percent rate in the control group were seen in our study. This was in contrast to the studies done by Nofal et al14 and Zamanian et al15 where no relapse was seen. In a study by Johnson et al,19 relapse occurred in two percent of patients who received mumps antiserum.
Limitations. Although the MMR vaccine appears to be a promising immunotherapy agent, certain limitations exist. Due to lack of resources, our investigators were not blinded, as there would have been difficulties in the assessment of efficacy and safety of the therapeutic agent used. The role of the MMR vaccine in the treatment of anogenital warts, warts in children, and warts in pregnant women was not evaluated in this study. Lastly, the follow-up period of our study was only 16 weeks from the last injection. Longer follow-up periods are necessary in order for more meaningful conclusions to be drawn.
Conclusion
Immunotherapy with MMR intralesional injection has therapeutic potential as a safe and effective treatment modality for cutaneous warts. It is a simple, cost-effective, and non-destructive method that has shown efficacy and good tolerability. With less side effects and a lower relapse rate compared to other treatment modalities, MMR immunotherapy can potentially be used as a first-line treatment for warts.
References
- Leto MGP, Santos Jr GF, Porro AM, Tomimori J. Human papillomavirus infection: etiopathogenesis, molecular biology and clinical manifestations. An Bras Dermatol. 2011;86(2): 306–317.
- Antonsson A, Forslund O, Ekberg H, et al. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of theses viruses. J Virol. 2000;74(24):11636–11641.
- de Villiers EM, Fauquet C, Broker TR, et al. Classification of papillomaviruses. Virology. 2004;324(1):17–27.
- Bruggink SC, de Koning MN, Gusseklooa J, et al. Cutaneous wart-associated HPV types: prevalence and relation with patient characteristics. J Clin Virol. 2012;55(3):
250–255. - Berman A, Winkelmann RK. Involuting common warts. J Am Acad Dermatol. 1980;3(4):356–362.
- Bacelieri R, Johnson SM. Cutaneous warts: an evidence-based approach to therapy. Am Fam Physician. 2005; 72(4):647–652.
- Lipke MM. An armamentarium of wart treatments. Clin Med Res. 2006;4(4): 273–293.
- Leung L. Recalcitrant nongenital warts. Aust Fam Physician. 2011 Jan-Feb; 40(1-2):40-2.
- Goncalves MA, Donadi EA. Immune cellular response to HPV: current concepts. Braz J Infect Dis. 2004;8(1):1–9
- Shepherd PS, Hebert A. Tcell responses to HPV in cervical dysplasia. Papillomavir Rep. 1999;10:53–78.
- Grassegger A, Rollinger-Holzinger I, Zelger BW, et al. Spontaneous or interferon-? induced Tcell infiltration, HLA-DR and ICAM1 expression in genitoanal warts are associated with TH1 or mixed TH1/TH2 cytokine mRNA expression profiles. Arch Dermatol Res. 1997;289(5):243–250.
- Dall’oglio F, D’Amico V, Nasca MR, Micali G. Treatment of cutaneous warts: an evidence-based review. Am J Clin Dermatol. 2012;13(2):73–96.
- Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida and trichophyton skin test antigens: a single-blinded, randomized and controlled trial. Arch Dermatol. 2005;141(5):589–594.
- Nofal A, Nofal E. Intralesional immunotherapy of common warts: successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol. Venereol 2010;24(10):
1166–1170. - Zamanian A, Mobasher P, Jazi GA. Efficacy of intralesional injection of mumps-measles-rubella vaccine in patients with wart. Adv Biomedic Res. 2014;3:107.
- Saini P, Mittal A, Gupta LK, Khare AK, Mehta S. Intralesional mumps, measles and rubella vaccine in the treatment of cutaneous warts. Indian J Dermatol. Venereol Leprol. 2016;82(3):343–345.
- Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts . Indian J Dermatol Venereol Leprol. 2014;80(6):509–514.
- Dhakar AK, Dogra S, Vinay K, et al. Intralesional Mycobacterium w vaccine versus cryotherapy in treatment of refractory extragenital warts: a randomized, open label, comparative study. J Cutan Med Surg 2016;20(2):123–129.
- Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or candida skin test antigens: a novel immunotherapy for warts. Arch Dermatol. 2001;137(4):
451–455. - Johnson SM, Horn TD. Intralesional immunotherapy for warts using a combination of skin test antigens: a safe and effective therapy. J Drugs Dermatol. 2004;3(3):263–265.

