 by Jerry Bagel, MD, MS, and Elise Nelson, LPN, CCRC
by Jerry Bagel, MD, MS, and Elise Nelson, LPN, CCRC
Both authors are with the Psoriasis Treatment Center of Central New Jersey in East Windsor, New Jersey.
J Clin Aesthet Dermatol. 2018;11(5):27–29
Funding: This study was sponsored by Taro Pharmaceuticals U.S.A., Inc.
Disclosures: The authors have no conflicts of interest relevant to the content of this article.
Abstract: Objective. The goal of this study was to evaluate efficacy and safety of desoximetasone spray 0.25%, a topical corticosteroid, in the management of scalp and body psoriasis.
Design. This was an open-label, observational study.
Participants. Twenty adults aged 18 years or older with chronic scalp psoriasis present on at least 30 percent of the scalp surface area and an Investigator Global Assessment (IGA) scale score of scalp disease of at least 2 on a scale of 0 to 4 were included in the study.
Measurements. Study spray was applied twice daily for four weeks, followed by 12 weeks of twice-daily application for two consecutive days weekly.
Results. At Week 4, the mean Physician Global Assessment (PGA) scale score had decreased 54.8 percent, from moderate disease to almost clear. Body surface area (BSA) had decreased by 51.2 percent, BSA × PGA had decreased by 63 percent, and scalp IGA had decreased by 64.5 percent from moderate to almost clear. Additionally, mean Psoriasis Scalp Severity Index (PSSI) score was 27.3±10.0 at baseline and decreased 82.4 percent to 4.8±5.2 and scalp surface area (SSA) was reduced by 70.7 percent at Week 4. The initial Scalp Index score was a mean of 65.7±15.0 at baseline and was reduced by 44.3 percent and 40.8 percent at Weeks 4 and 16, respectively. The initial response was maintained after a change to twice-weekly, twice-daily dosing, with a 48.4-percent decrease in PGA, a 17.1-percent decrease in BSA, a 31.5-percent decrease in BSA × PGA, a 51.6-percent decrease in scalp IGA, a 63.4 percent decrease in PSSI, and a 42.3-percent decrease in SSA seen at Week 16. Minimal adverse events were experienced by seven subjects.
Conclusion. Desoximetasone spray 0.25% produced rapid improvements in PGA, BSA, BSA×PGA, scalp IGA, PSSI, SSA.
Keywords: Psoriasis, desoximetasone, topical corticosteroid, Scalpdex, body surface area
Introduction
Psoriasis is a chronic immunological disease characterized by infiltration of the skin with activated T-cells and by abnormal keratinocyte proliferation and differentiation, resulting in marked inflammation and thickening of the epidermis. Psoriasis affects 1 to 3 percent of the world’s population, making it one of the most prevalent inflammatory immunological diseases. It is a disabling disease, and the impact it can have on quality of life is often underestimated by healthcare providers.1 There are several clinical subtypes of psoriasis, with the most common being plaque psoriasis, affecting 75 to 80 percent of patients with psoriasis.2 Plaque psoriasis is characterized by epidermal hyperproliferation and inflammation of the epidermis and dermis. Clinically, it presents as raised silvery scales with underlying erythema, itching, and discomfort. Plaques might affect large areas of the skin and have a significant psychosocial impact on the lives of patients.
The scalp is one of the most common sites of psoriasis, often presenting at disease onset and continuing throughout the course of the disease.3 An estimated 50 to 80 percent of patients with psoriasis have scalp involvement.4 Psoriasis of the scalp is highly visible and has been found to have an outsized impact on the quality of life (QOL) of patients, even when only a small amount of body surface area (BSA) is affected. Visible plaques can affect self-esteem and negatively impact the ability of patients to socialize normally.3 The condition can appear as only localized scales or can manifest as widespread, hyperkeratotic plaques. Scales can extend visibly beyond the hair margins and involve the face, neck, and ears. Patients with scalp involvement might experience intense itching.5 Furthermore, topical therapies used to treat psoriasis can affect the cosmetic appearance of the hair. Application of topical therapy, along with the patient itch-and-scratch cycle, can produce a perpetual koebnerization that could perpetuate disease activity.3 Another issue for patients is being able to correctly apply topical therapies so as to bypass the hair. Taken together, these factors might affect adherence and efficacy of treatment.
Topical therapy of scalp psoriasis has been shown to improve QOL outcomes.6 It has been suggested that newer vehicles, including sprays and foams, are the most appropriate methods for scalp medication application because they are less messy and are better able to penetrate to the scalp through the hair.5 It has recently been shown that patients with plaque psoriasis prefer sprays more than 2 to 1 over creams, ointments, lotions, gels, and foams.7
In this report, we present the findings of an open-label, observational study evaluating the efficacy, tolerability, and patient satisfaction of desoximetasone 0.25% spray (Topicort® Spray 0.25%; Taro Pharmaceuticals, Inc., Hawthorne, New York) in the treatment of psoriasis of the scalp. Desoximetasone spray 0.25% is a Class I, super-potent corticosteroid that has been shown to provide rapid and effective relief of scaling in plaque psoriasis.7 It is the first super-potent formulation of desoximetasone.8 The use of a spray might improve patient adherence. The Psoriasis Process of Care Consensus Panel noted that spray is the preferred vehicle for the scalp, male chest, and other hair-bearing locations, as well as when there is extensive skin involvement.5
Pilot Study Methods
Study design. Twenty patients with plaque psoriasis of the scalp were enrolled in this 16-week study. They used desoximetasone spray 0.25% twice daily for four weeks, followed by twice-daily application on two consecutive days per week for an additional 12 weeks. Subjects also applied the study material to other areas affected by plaque psoriasis, excluding the face, groin, and axillae.
Inclusion/exclusion criteria. The main inclusion criteria were men and women aged 18 years or older with a diagnosis of chronic plaque psoriasis of the scalp, with a scalp surface involvement of at least 30 percent and an Investigator Global Assessment (IGA) scale score of at least 2 on a 0- to 4-point scale. Main exclusion criteria were subjects with non-plaque psoriasis (e.g., guttate or pustular); those with less than 30-percent scalp involvement; and those using study-prohibited medications and therapies within 2 to 4 weeks (depending on medication/therapy being used) of baseline. Subjects were permitted to use non-steroidal topical medications on the face, groin, and axillae during the study period.
Study endpoints. Efficacy endpoints included IGA scale score of scalp involvement, using a 5-point (0–4) scale that recorded overall disease severity based on average scores for erythema, induration, and scaling of affected areas made at each visit. The IGA scale used was 0=clear, 1=almost clear, 2=mild, 3=moderate, and 4=severe.
Psoriasis Scalp Severity Index (PSSI) and scalp surface area (SSA) were also calculated. The average degree of scalp erythema, induration, and desquamation severity were assessed and assigned a score of 0 to 4 (0=mild, 4=severe). The SSA was estimated as a percentage. The PSSI score is the sum of the erythema, induration, and desquamation scores multiplied by the SSA.
Another efficacy endpoint was Physician Global Assessment (PGA) Scale score, a 5-point scale that records the overall disease severity at each clinical evaluation based on the average degree of erythema, induration, and scaling of all areas affected by psoriasis. The PGA scale used was 0=clear, 1=almost clear, 2=mild, 3=moderate, and 4=severe.
BSA was determined for all subjects throughout the study. BSA was calculated by using the palm sizes of subjects to represent one percent of BSA.5
Patient-reported outcomes included subject self-assessments of pain, itching, and scaling of the scalp in the prior 24 hours on a Likert scale of 1 to 10 (1=minimal, 10=severe) at Weeks 0, 4, and 16. In addition, Scalpdex, a self-administered QOL instrument developed for scalp dermatitis, was used.9 This 23-item survey evaluates patients with cutaneous scalp involvement across the domains of pain, function, and emotion over the preceding three weeks. It was also administered at Weeks 0, 4, and 16.
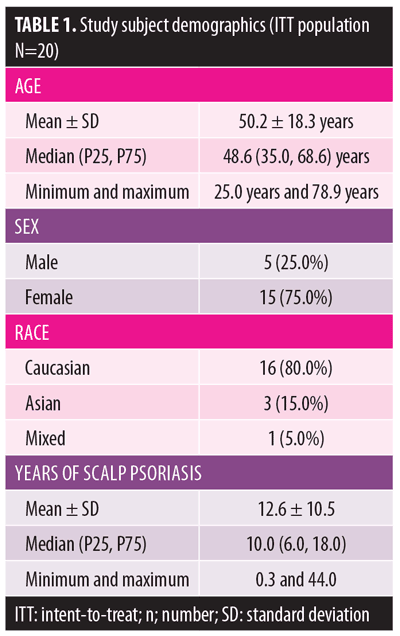
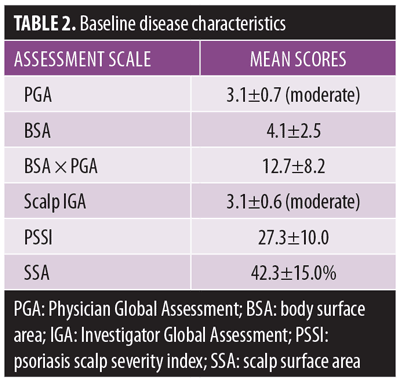
Demographics and baseline characteristics. The intent-to-treat (ITT) population (n=20) was used for analysis. The mean age of the subjects was approximately 50 years. The majority of subjects (75%) were female and Caucasian (80%). Subjects had had scalp psoriasis for a mean of 12.6 years (Table 1). The mean PGA at baseline was 3.1±0.7, the mean BSA was 4.1±2.5, the mean BSA×PGA was 12.7±8.2, the mean scalp IGA was 3.1±0.6, the mean PSSI was 27.3±10.0, and the mean SSA at baseline was 42.3±15.0 (Table 2). Baseline subject reports included a mean baseline pain score of 4.0±2.5, itch score of 7.1±2.7, and a scaling score of 7.0±2.4. The mean baseline Scalpdex score was 65.7±15.0.
Results
Efficacy results. The response to treatment was rapid across all efficacy parameters. By Week 4, mean PGA had decreased from 3.1±0.7 (moderate disease) to 1.4±1.0 (almost clear). This represents a decrease from a baseline value of 54.8 percent. At Week 16, mean PGA was 1.6±1.3, a decrease of 48.4 percent from baseline. BSA decreased from 4.1±2.5 at baseline to 2.0±2.9 at Week 4 for a decrease of 2.1±1.7 or 51.2 percent. At Week 16, the mean BSA was 3.4±3.7, representing a change from baseline of 0.4±2.9 or 17 percent. BSA×PGA was reduced from 12.7±8.2 to 5.0±11.4, a decrease of 7.4±6.8 (63%) from baseline. By Week 16, the BSA×PGA change from baseline was 3.0±8.9 or 31.5 percent. At Week 4, scalp IGA was reduced by 2.0±0.9 to 1.1±0.7 (i.e., from moderate disease to almost clear). By Week 16, mean scalp IGA was 1.5±1.2, demonstrating a change of 1.6±1.2 from baseline and representing a decrease of 64.5 percent.This improvement was maintained with a change from baseline of 51.6 percent. PSSI was reduced by 82.4 percent at Week 4, with a decrease of 23.6±7.1 from baseline to 4.8±5.2. At Week 16, the change from baseline was 17.0±13.8 (63.4%) (Figure 1). SSA was reduced to 12.4±16.0 percent at Week 4 from 42.3±15.0 percent at baseline, representing a change of 31.0±16.8 percent or 70.7 percent. At Week 16, mean SSA was 24.4±24.4 percent, representing a change of 17.9±28.1 percent or 42.3 percent from baseline.
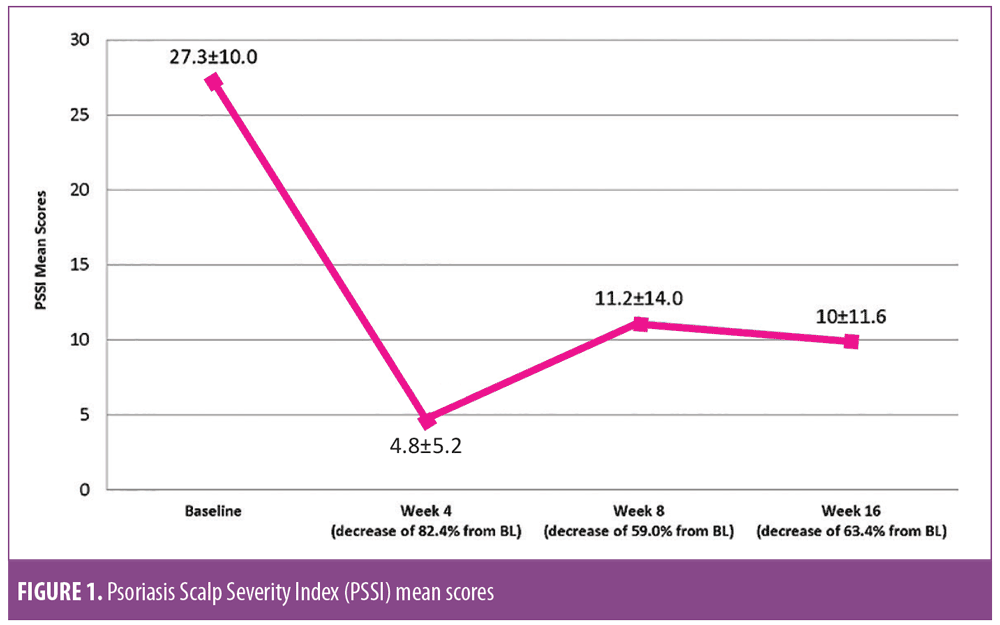
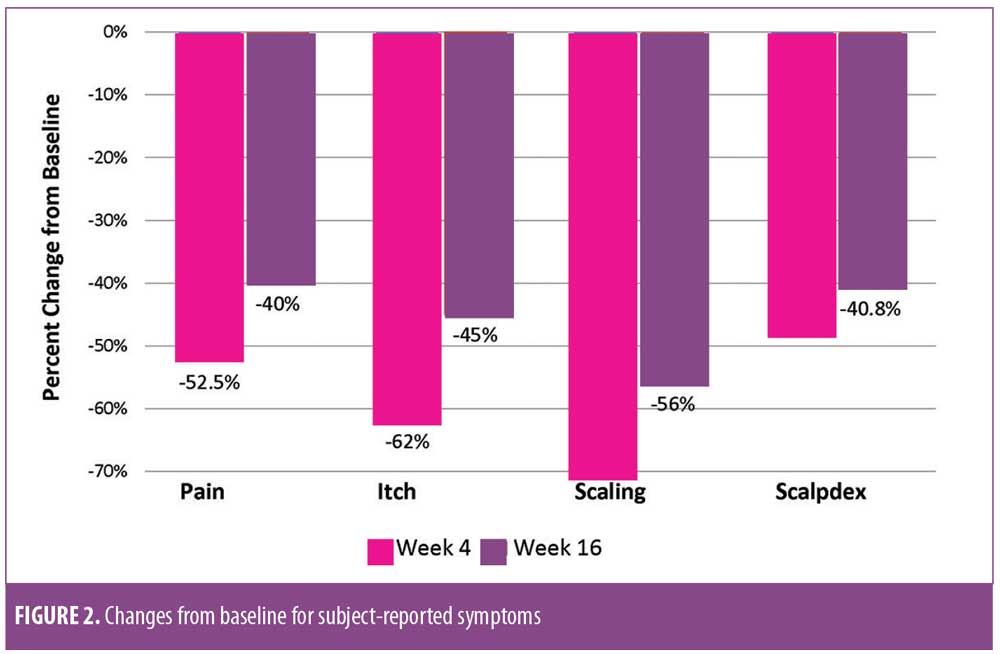
Figure 2 shows the changes in mean subject-reported scores from baseline to the study’s end. Patient-reported pain was 1.9±1.8 at Week 4, representing a decrease of 2.1±2.6 (52.5%) from baseline. At Week 16, the mean pain score was 2.4±2.0—a decrease of 40 percent (1.5±2.6) from baseline. Itch was reduced by 62 percent at Week 4 to 2.7±2.0 for a decrease of 4.1±2.5 from baseline. At Week 16, the mean itch score was 3.9±2.8, demonstrating a change of 45.1 percent from baseline. Scaling decreased 4.4±2.8 from baseline to Week 4 and, at Week 16, the mean scaling score was 3.1±2.3 for a change from baseline of 37.5 percent. The mean Scalpdex score was reduced 28.5±22.5 points from baseline to a mean score of 36.6±18.9 points at Week 4 and 38.9±20.0 points at Week 16, representing a decrease of 25.9±18.5 points (40.8%) from baseline.
Safety results. There were a total of 19 adverse events (AEs) that occurred in nine subjects. The most common AEs overall were burning sensation in five subjects and itching in three subjects. Nine of the 19 AEs were considered treatment-related and occurred in seven subjects, or 35 percent of the study population. Additional AEs that were considered treatment-related were folliculitis in one subject and acne in two subjects.
Discussion
This open-label, observational study of desoximetasone 0.25% topical spray to treat scalp psoriasis indicates that this treatment is safe and effective. Onset of action was rapid across all efficacy parameters, with significant improvements seen as early as Week 4 after initiation of treatment, thus optimizing initial control. PGA, BSA, BSA×PGA, scalp IGA, and PSSI were reduced from baseline by 55, 51, 63, 65, and 82.4 percent, respectively. After four weeks, desoximetasone 0.25% spray was decreased from twice-daily application to twice-daily application on two consecutive days per week from Week 4 through Week 16. Improvements from baseline were maintained at Week 16. At Week 16, PGA and scalp IGA were reduced from baseline by approximately 50 percent. Additionally, PSSI was reduced by 63.4 percent at Week 16.
Conclusion
Taken together, our findings demonstrate that desoximetasone 0.25% topical spray prompts rapid and significant improvement in scalp psoriasis. This level of efficacy combined with the convenience and the use of a patient-preferred spray vehicle suggest that this is an effective therapy for scalp psoriasis. A larger, randomized, controlled trial should be initiated in order to corroborate and extend these results.
References
- Greaves MW, Weinstein GD. Treatment of psoriasis. New Engl J Med. 1995;332(9):581–588.
- Camp RDR. Psoriasis. In: Champion RH, Burton JL, Burns DA, Breathnach SM, eds. Textbook of Dermatology. Vol. 2. 6th ed. Hoboken, NJ: Wiley-Blackwell; 1999: 1589–1649.
- Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26(5):448–459.
- Kragballe K. Management of difficult to treat locations of psoriasis. Scalp, face, flexures, palm/soles and nails. Curr Probl Dermatol. 2009;38:160–171.
- Zeichner JA, Lebwohl MG, Menter, A et al. Optimizing topical therapies for treating psoriasis: a consensus conference. Cutis. 2010; 86(3 Suppl): 5–31.
- Ortonne JP, Ganslandt C, Tan J, et al. Quality of life in patients with scalp psoriasis treated with calcipotriol/betamethasone dipropionate scalp formulation: a randomized controlled trial. J Eur Acad Dermatol Venereol. 2009;23(8):919–926.
- Keegan BR. Desoximetasone 0.25% spray for the relief of scaling in adults with plaque psoriasis. J Drugs Dermatol. 2015; 14(8):835–840.
- Kircik L, Lebwohl MG, Del Rosso JQ, et al. Clinical study results of desoximetasone spray 0.25% in moderate to severe plaque psoriasis. J Drugs Dermatol. 2013;12(12):1404–1410.
- Chen SC, Yeung J, Chren M. Scalpdex: a quality of life instrument for scalp dermatitis. Arch Dermatol. 2002;138(6):803–807.

