J Clin Aesthet Dermatol. 2025;18(8):41–43.
by Carlos Daniel Sánchez-Cárdenas, MD; Liliana Godinez Aldrete, MD; Zaira Ruelas Guzmán, MD; Diana Carolina Vega Sánchez, MD; Mayra Itzel Cano Viveros, MD; Nancy Pulido Díaz, MD; Roberto Arenas, MD; and Gabriela Moreno-Coutiño, MD
Drs. Sánchez-Cárdenas, Aldrete, Guzmán, Viveros, and Díaz are with the Dermatology Service at the La Raza National Medical Center in Mexico City, Mexico. Drs. Sanchez, Arenas, and Moreno-Coutiño are with the Mycology Service at the General Hospital Dr. Manuel Gea González in Mexico City, Mexico.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors declare no conflicts of interest relevant to the content of this article.
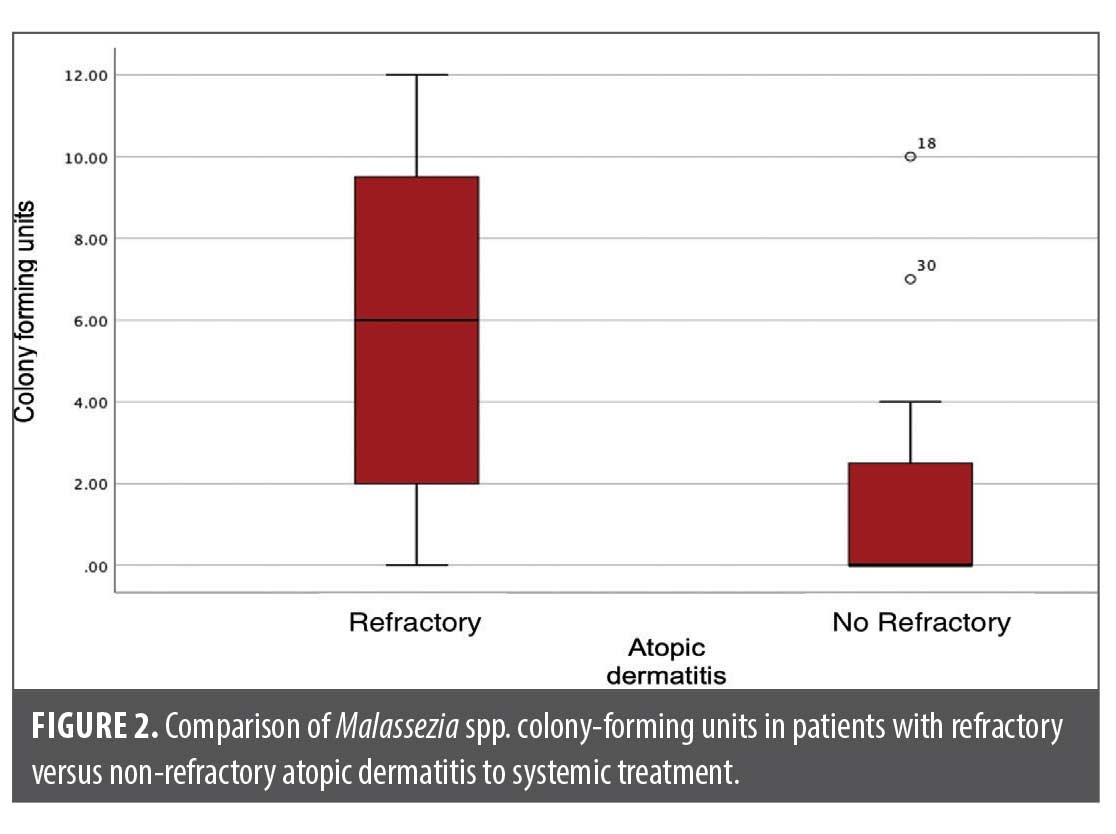
Abstract: It has been proposed that some cases of refractory atopic dermatitis (AD) are caused by an overgrowth of Malassezia spp. This study aimed to compare the colony-forming units of Malassezia spp. in patients with AD who were grouped based on their responsiveness to systemic treatment. Methods: This prospective, cross-sectional, and comparative study was conducted in patients with AD who were treated with prednisone, cyclosporine, and methotrexate, and separated in two groups, those with and those without response to their treatments, and who had not received treatment with antifungals (topical or systemic) within the prior six months. Scoring Atopic Dermatitis (SCORAD) was determined to evaluate disease severity and therapy response. A sample of epidermal cells from the glabellar region was obtained by scraping for direct examination with methylene blue and m-Dixon agar culture in search of colony-forming units. Fisher’s exact test and Mann-Whitney U test were performed to compare the groups. Results: A total of 15 patients were included in each group. The majority of the group who were refractory to treatment were women (60%), while the non-refractory group were predominantly men (66.7%). In addition, 73.3 percent of subjects with therapeutic resistance presented fungal growth in the cultures compared to 40 percent in the non-resistant group. A significant difference was found between direct examination (p=0.000) and colony-forming units of Malassezia spp. (p=0.016) in patients with AD resistant to systemic treatment. Conclusion: Patients with AD resistant to systemic treatment had a higher count of colony-forming units of Malassezia spp. Therefore, we propose intentionally seeking this fungus in patients with therapeutic failure to eliminate its overpopulation. Keywords: Atopic dermatitis, Malassezia spp., colony-forming unit, refractory systemic treatment
Introduction
Atopic dermatitis (AD) is an inflammatory dermatosis characterized by impaired skin barrier function and subsequent skin microbiota alteration. It is frequently seen in infants and children (20–30%), while in adults, the prevalence is only between 7 to 10 percent worldwide.1 Therapy choices depend on the extent of the dermatosis, using topical therapy for mild-to-moderate cases and systemic therapy for severe cases.
Most cases of AD respond to conventional therapies, including topical steroids and calcineurin inhibitors. For systemic treatment, prednisone, cyclosporine, methotrexate and azathioprine are commonly used.2 However, some cases can be resistant to these therapies, and some authors have proposed it could be caused by Malassezia overpopulation.1,3 The present study aimed to compare Malassezia colony-forming units in AD patients with and without response to systemic therapy.
Methods
We designed a descriptive, cross-sectional, prospective, and comparative study in patients with AD seen at the Dermatology Service of the La Raza National Medical Center in Mexico City, from January to June 2022. The study was approved by the ethics and research committee of La Raza National Medical Center, and participants signed an informed consent. Patients aged at least 18 years with a minimum two-year history of AD with at least six months on systemic treatment with cyclosporine, prednisone, or methotrexate were included and divided into two groups, according to their response to therapy for AD: (1) a refractory group in which patients did not respond to topical treatment and more than one systemic therapy, and (2) a non-refractory group in which patients showed a clinical response after receiving a systemic therapy at four weeks. The subjects did not receive topical or systemic antifungal therapy in the six months prior and had no history of seborrheic dermatitis or added infections.
During the consultation at the outpatient clinic, patients with AD were invited to participate, and those that accepted signed an informed consent. Scoring Atopic Dermatitis (SCORAD) was determined in all participants. We considered a score of 25 or more as moderate-severe disease and less than 25 as mild disease.
The patients were requested to attend a subsequent consultation without applying a topical treatment, makeup, or creams in the facial region and without performing body hygiene 12 hours prior to the study. Samples were taken for smears and culture.
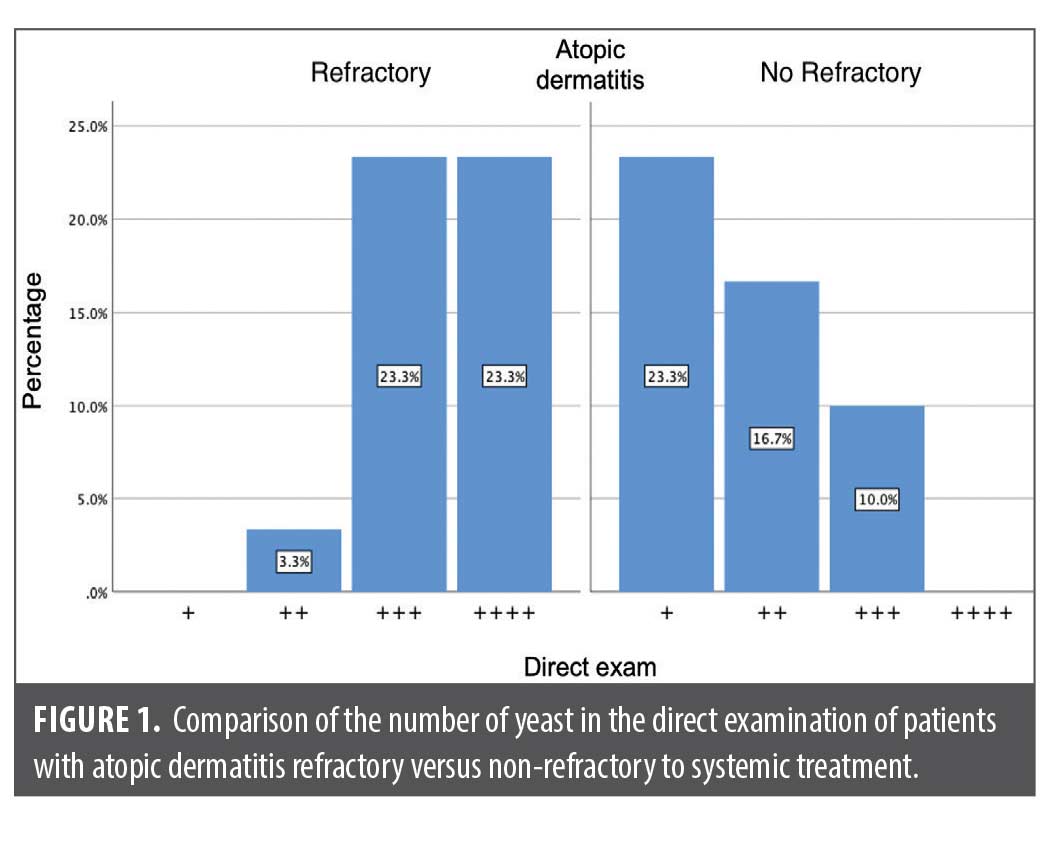
To take the samples, a drop of injectable water was applied to a Scalpel Blade No. 20. An atraumatic scraping of the glabellar area was performed to obtain a sample of epidermal cells from the stratum corneum. The sample was divided into two; one half was stained with methylene blue to quantify yeasts, dried at room temperature, and fixed with 96% ethyl alcohol and direct heat. Then, a drop of methylene blue was applied for one minute, washed with water, and the sample was left to dry. The samples were evaluated under a 100x light microscope with immersion oil. The results were interpreted as follows: +: 1 to 5 yeasts; ++: 6 to 10 yeasts; +++: 11 to 15 yeasts; and ++++: more than 20 yeasts. The other half of the sample was cultured on m-Dixon agar (Condalab, Spain) at 37ºC. The cultures were checked every 48 hours for two weeks to evaluate colony growth. Finally, the colony-forming units were counted.
Data were analyzed with the statistical software SPSS v25. The normality testing for variables was determined with the Shapiro-Wilks test. Chi-square and Mann-Whitney U tests were performed for group comparison. A p-value less than 0.05 was considered significant.
Results
A total of 30 patients were included, 15 in each group, resistant and non-resistant to systemic therapy.
The mean age was 35.2±9.6 years (Table 1). All treatment-resistant patients presented with predominantly head and neck lesions with dissemination to the upper part of the trunk and flexor areas of the upper extremities, as well as on hands. All patients in the treatment-respondent group presented lesions in the flexor areas of upper and lower extremities, as well as in the hands.
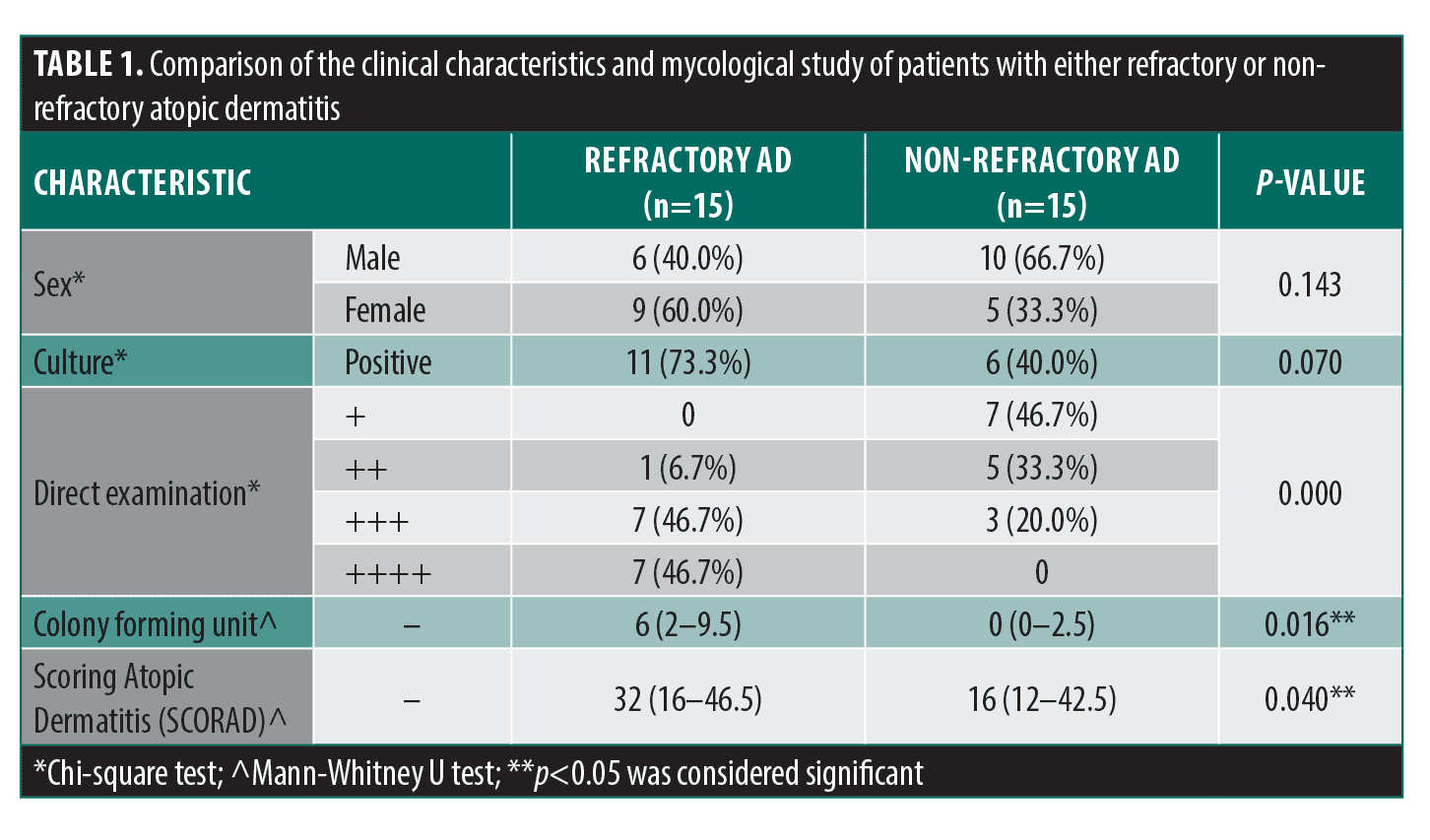
A significant difference was found between the direct examination (p=0.000) and the Malassezia colony-forming units (p=0.016) between both groups (Table 1, Figures 1 and 2).
Discussion
AD is characterized by a defective epidermal barrier function due to mutations in the filaggrin gene, increased trans-epidermal water loss and surface pH, with decreased hydration of the stratum corneum, and downregulated expression of tight junction components.1,4 The latter enables the proliferation of the skin microbiota, which generates considerable skin inflammation and further exacerbates the disease.2
Some studies have found colonization of Malassezia species in AD patients when compared to healthy subjects.5 However, in other studies, no significant differences were found between the two populations; thus, their role in the pathogenesis of this disease has yet to be clarified.2
Despite the above, in certain types of AD, positive responses have been observed using antifungals (mainly azoles), such as the head and neck variant, with high concentrations of IgE specific to Malassezia detected.1,3
Head and neck AD occurs in adolescents and adults. It affects the head, face (primarily eyelids and lips), neck, and upper trunk. As for the clinic characteristics, it presents with eczematous plaques, lichenification, erosions, erythema, and scales.6 Usually, this AD variant is recalcitrant to conventional treatments for AD but has a good response to treatment with itraconazole, showing decreased Malassezia colonization in the seborrheic areas.2
Malassezia is a lipid-dependent commensal fungus, which is part of the normal skin flora.6,7 However, the pathogenic role of Malassezia in seborrheic areas still requires confirmation since it is difficult to distinguish its transition from commensal to pathogenic. This fungus becomes pathogenic due to the activation of several lipase enzymes. In addition, it interacts with various components of the skin’s immune system, such as IgG, and IgM, especially in individuals sensitized by allergens produced by the yeast.2,8,9
The mechanisms by which Malassezia is thought to participate in the pathogenesis of AD include genetic, environmental, and epidermal barrier defects. The fungus’ phospholipase production decreases the lipid production of the epidermis, which is already involved in AD. The increased skin pH in patients with AD causes the production of Malassezia allergens, which penetrate the skin through the already damaged epidermal barrier. These allergens are recognized by Toll-like receptors expressed on keratinocytes and dendritic cells, which release pro-inflammatory cytokines, such as IL-4, 1, 5, 6, 13, CXCL-8, and IL-12p40. The production of B lymphocytes is also observed, prompting IgE specific for Malassezia activated by dendritic cells and T cells. This IgE produces mast cells, increasing inflammation in AD patients. In addition, autoreactive T cells may cross-react with the fungus and manganese-dependent human superoxide dismutase, perpetuating the inflammation.1,10 Inflammasomes play an important role, as they are part of the pattern recognition receptors (PRRs) of the keratinocytes surface, which use the NOD-like receptor (NLR) as a sensor of the fungus proteins, the apoptosis-associated speck-like protein (CARD), and the protein protease caspase-1, which are part of the response mechanisms of the innate immune system of different pathogens.9,11,12
Guglielmo et al5 conducted a retrospective study to determine the role of Malassezia spp in the pathogenesis of head and neck AD. Thirty-one patients were identified with AD and fungal proliferation, including 17 adolescents and 14 adults. A positive correlation was observed between the history of AD and the exclusive presentation of head and neck dermatitis in adolescents (p<0.05). In adults, dermatitis predominated in the same location but with lesions spreading to the flexural areas of the upper and lower limbs, trunk, hands, and nipples.6 In our study, all patients were adults presenting with lesions in the same topography as previously described. In addition, further proliferation of the fungus was found in patients resistant to systemic treatment (p<0.05), with a tendency to have greater disease severity.
Bjerre et al12 conducted a study to determine the microbiome characteristics of different affected areas between patients with AD and healthy subjects, including bacteria, fungi, and viruses at various body sites and lesion stages. Definite differences were found between the microbiome, mainly in the flexural and neck regions. The absence of Malassezia spp. was noted in patients with AD. Jain et al13 conducted a study attempting to isolate Malassezia from the affected skin areas of AD patients in 38 patients with AD and 38 healthy subjects. They found a high rate of Malassezia isolates in patients with AD compared to the control group. In our study, the examined area was exclusively the glabellar region of the head, as it was the site where the disease was predominantly observed in all patients resistant to systemic treatment. In addition, in concordance with Bjerre and Jain, our study showed that the sites presenting greater disease severity also showed more significant colonization of Malassezia.
Conclusion
Patients with AD refractory to systemic treatment presented a higher number of colony-forming units of Malassezia spp. and greater severity of the disease. Therefore, we propose intentionally seeking this fungus in these patients to eliminate its overpopulation.
References
- Nath S, Kumari N, Bandyopadhyay D, et al. Dysbiotic Lesional Microbiome With Filaggrin Missense Variants Associate With Atopic Dermatitis in India. Front Cell Infects Microbiol. 2020;10:570423.
- Kurniadi I, Wijaya WH, Timotius KH. Malassezia virulence factors and their role in dermatological disorders. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31(2):65–70.
- Abdillah A, Ranque S. Chronic Diseases Associated with Malassezia Yeast. J Fungi (Basel). 2021;7(10):855.
- Navarrese-Triviño FJ, Ayén-Rodríguez Á. Study of hypersensitivity to Malassezia furfur in patients with atopic dermatitis with head and neck pattern: Is it useful as a biomarker and therapeutic indicator in these patients? Life. 2022;12(2).
- Guglielmo A, Sechi A, Patrizi A, et al. Head and neck dermatitis, a subtype of atopic dermatitis induced by Malassezia spp: Clinical aspects and treatment outcomes in adolescent and adult patients. Pediatr Dermatol. 2021;38(1):109–114.
- Sparber F, de Gregorio C, Steckholzer S, et al. The Skin Commensal Yeast Malassezia Triggers a Type 17 Response that Coordinates Anti-fungal Immunity and Exacerbates Skin Inflammation. Cell Host Microbe. 2019;25(3):389–403.
- Glatz M, Bosshard P, Schmid-Grendelmeier P. The Role of Fungi in Atopic Dermatitis. Immunol Allergy Clin North Am. 2017;37(1):63–74.
- Koh LF, NGO RY, Common JE. Skin microbiome of atopic dermatitis. Allergol Int. 2022;71(1):31–39
- Nowicka D, Nawrot U. Contribution of Malassezia spp. to the development of atopic dermatitis. Mycoses. 2019;62(7):588–596.
- Park HR, Oh JH, Lee YJ, Park SH, Lee YW, Lee S, et al. Inflammasome-mediated Inflammation by Malassezia in human keratinocytes: A comparative analysis with different strains. Mycoses. 2021;64(3):292–9.
- Woo YR, Cho M, Han Y, Lee SH, Cho SH, Lee JD, Kim HS. Characterization of Distinct Microbiota Associated with Scalp Dermatitis in Patients with Atopic Dermatitis. J Clin Med. 2022;11(6):1735.
- Bjerre RD, Holm JB, Palleja A, Sølberg J, Skov L, Johansen JD. Skin dysbiosis in the microbiome in atopic dermatitis is site-specific and involves bacteria, fungus and virus. BMC Microbiol. 2021;21(1):256.
- Jain C, Das S, Ramachandran VG, et al. Malassezia yeast and cytokine gene polymorphism in atopic dermatitis. J Clin Diag Res. 2017;11(3):D C01–5.
