 J Clin Aesthet Dermatol. 2023;16(3 Suppl 1):S41–S46.
J Clin Aesthet Dermatol. 2023;16(3 Suppl 1):S41–S46.
by Nourhan Anis, MD; Nagwa Diab, MD; Magda Assaf, MD; Ahmed Soliman, MD; and Eman Salah, MD
Drs. Anis, Diabi, and Salah are with the Dermatology Department and Dr. Assaf is with the Pathology Department, Faculty of Medicine, at Zagazig University in Zagazig, Egypt. Dr. Soliman is with the Pathology Department, Medical Division, at the National Research Center in Cairo, Egypt.
FUNDING: No funding was received for this study or article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Basal cell carcinoma (BCC) is a locally aggressive skin tumor in which metastatic spread occurs infrequently, with a predominance of lymphatic involvement.
Objective. We sought to evaluate podoplanin-stained lymphatic vessels in different histological growth patterns of basal cell carcinoma, a well as the proliferative activity of tumor tissue, to determine if podoplanin provides any additional prognostic information.
Methods. The number of lymphatic vessels and total lymphatic vessel area was morphometrically analyzed in podoplanin-stained sections using anti-D2-40. The immunohistochemical study of epidermal Ki-67 in BCC and control skin specimens was also performed.
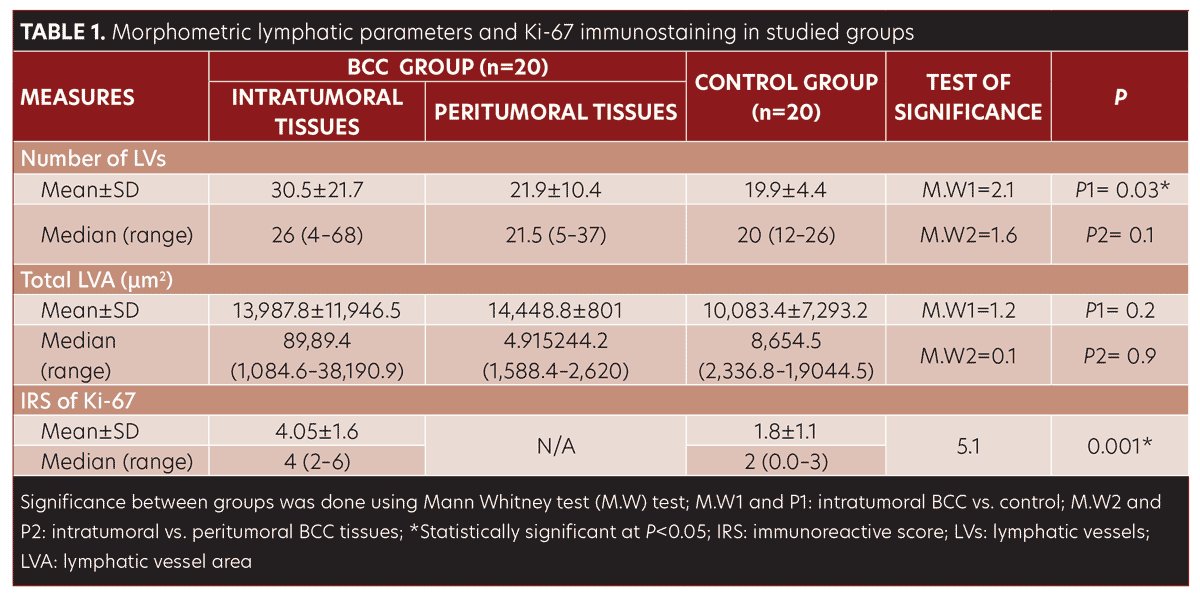
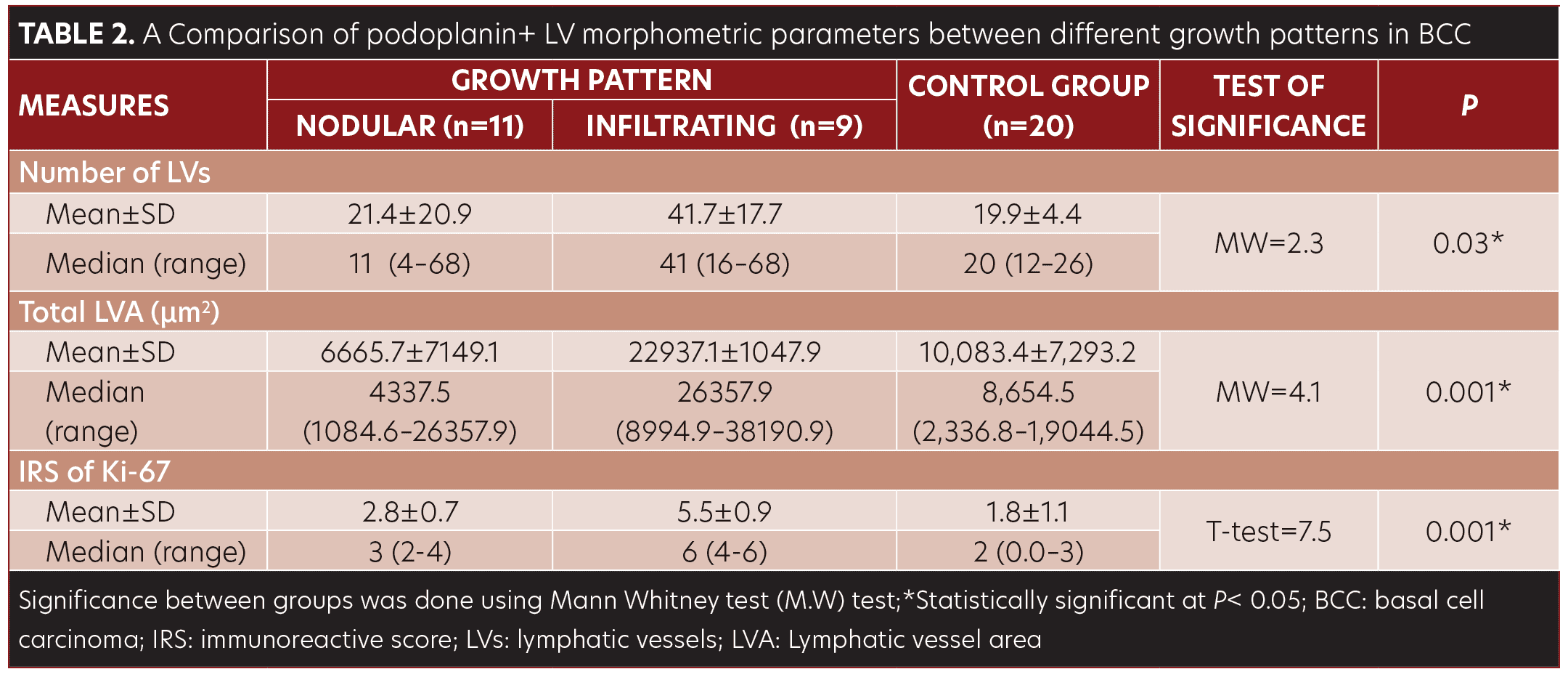
Results. The number of lymphatic vessels was higher in BCC cases compared to normal skin (P=0.03). In infiltrating growth pattern, the number of lymphatic vessels, total lymphatic vessel area, and Ki-67 immunoreactivity were higher than those in nodular lesions (P=0.03, P=0.001, P=0.001, respectively). Ki-67 immunoreactivity was correlated with the number of lymph vessels (r=0.6) (P=0.004) and total lymphatic vessel area (r= 0.6) (P=0.002).
Conclusion. Podoplanin expression in lymphatic vessels may be implicated in BCC carcinogenesis and is associated with cancer aggressiveness. Podoplanin expression can be further utilized as a marker reflecting tumoral local behavior.
Keywords: Basal cell carcinoma, growth pattern, lymphangiogenesis, morphometry, podoplanin
Lymphatic vessels (LVs) have been largely investigated in health and disease. LVs transport interstitial fluid from tissue into the blood circulatory system.1 They also serve as channels for trafficking immune cells, cytokines, and foreign antigens from the periphery to the regional lymph nodes.2
Lymphangiogenesis is a term to describe the formation of new LVs from an existing lymphatic network in response to regional stimuli, a process that primarily occurs during the developmental phase of human growth.3,4 LV remodeling in which existing LVs become enlarged can also occur.4 Lymphangiognesis typically does not occur in healthy adults, an exception being during the female reproductive cycle in the breasts and ovaries.4 However, englargement of existing LVs and creation of new LVs in adults can be triggered by acute and chronic inflammatory conditions and diseases, such as the wound healing process and cancers.4
Lymphangiogenesis in primary tumors facilitates tumor dissemination to regional lymph nodes. It is well-known that enlarged lymphatic vessel density (LVD) correlates with a higher incidence of metastasis and poorer survival in multiple cancers. In tumors, lymphangiogenesis may occur both within the primary tumor mass (intratumoral LVs) and/or in the tumor periphery (peritumoral LVs).4
Basal cell carcinoma (BCC), the most prevalent skin cancer, is associated with the induction of the proliferative activity of basal cells.5 While BCC can be aggressive locally, metastatic spread rarely occurs, with a predominance of lymphatic involvement.6,7 Metastatic BCC occurs more frequently in male patients, and characterizations include largely neglected lesions, predominance in the head and neck regions, resistance to multiple local treatments, and being of basosquamous or morphoeic subtype.7 The study of lymphangiogensesis and tumor proliferation is considered an essential tool in predicting lesional growth.8
Podoplanin is a LV-specific marker. It is a 38-kDa mucin-type transmembrane protein that is expressed exclusively on lymphatic endothelial cells (LECs), but not in blood endothelial blood cells.9
We assessed podoplanin-stained LVs to determine the extent of lymphangiogenesis in different histological growth patterns of BCC. We sought to correlate the extent of lymphangiogenesis with the proliferative activity of tumor tissue to determine whether podoplanin levels have prognostic value in BCC management.
Methods
Patients. This study was approved by the ethics committe of the authors’ institution, and all participants gave informed consent before enrollment.
Patients were recruited from the outpatient clinic of the dermatology department of an Egyptian hospital. Adult male and female patients under the age of 60 years (to avoid the age-dependent variability of skin microcirculation10) with a clinically and pathologically confirmed diagnosis of BCC were included in the study. Patients under 18 years of age or over 59 years of age, who were pregnant or lactating, who had previously received treatment for BCC, and/or who had other skin or systemic disorders were excluded.
Histopathological evaluation. A skin biopsy using a 5mm disposable punch was taken from the edge of the lesions. Biopsy specimens from both BCC and control groups were exposed to fixation using a 10% buffered formalin solution. The specimens were then stored in paraffin-embedded blocks. Serial sections of 4μm thickness were taken from each block and stained with routine hematoxylin and eosin (H&E) to confirm the diagnosis. BCC samples were histologically classified according to growth pattern—a nodular growth pattern (i.e., well-circumscribed, round, dermal islands) was categorized as low risk and an infiltrating pattern (i.e., thin infiltrative strands in the dermis, particularly at the deeper advancing edge of the tumor) was categorized as high risk.11
Immunohistochemical staining. Cutaneous podoplanin detection was achieved via a primary antipodoplanin antibody (monoclonal mouse anti-human D2-40 IgG1, clone D2-40, code: IR072, ready-to-use; Dako Denmark, Glostrup, Denmark). A primary antibody against Ki-67 (monoclonal mouse anti-human Ki-67 IgG1, clone MIB-1, code: IR626, ready-to-use; Dako Denmark) was used to evaluate epidermal hyperproliferation. The processing was handled according to manufacturer instructions using a labeled streptavidin-biotin visualization system (BenchMark Ultra Automated IHC/ISH staining instrument; Roche Tissue Diagnostics, Oro Valley, Arizona, USA).
Immunostaining interpretation. A slide was considered positive if it showed a brown cytoplasmic and/or membranous precipitate for D2-40. All D2-40-stained sections were morphometrically analyzed using Leica Qwin plus Image Analyzer Computer System (Leica Microsystems Inc. Deerfield Park, Illinois, USA). All solitary-stained endothelial cells and endothelial cell groups separate from another microvasculature were considered a vessel.12
Three lymphangiogenesis hotspots were identified at x100 magnification. Three intratumoral and three peritumoral hotspots were identified for BCC cases. As proposed in the literature,13 intratumoral LVs were defined as stained vessels located within the tumor mass. Peritumoral LVs were defined as stained vessels located in the surrounding stroma at a maximum distance of 2mm from the tumor periphery. The LVs in the control samples were defined as those located in the stromal area, within 2mm of the underlying basement membranes. Under x200 magnification and using the automatic image analyzer system, the following parameters were assessed: 1) the count of D2-40-stained lymphatic vessels, 2) the surface area of the lumen of each vessel (in μm2). We then automatically collected the total lymphatic vessel area (LVA) in the three hotspot areas for each case. Digital pictures of microscopic views were taken with a Leica DFC290 HD colored video camera connected to the Leica DM3000 LED Microscope (Leica Microsystems Inc.).
A slide showing a brown nuclear precipitate was considered positive for Ki-67. The immunoreactive score (IRS), as described by Remmele and Stegner,14 was used to measure the percentage of Ki-67-positive cells and the intensity of the staining reaction.
• The percentage of positive cells was scored from 0 to 4 points as follows: 0=no positive cells, 1≤10 percent, 2=11 to 50 percent, 3=51 to 80 percent, and 4≥80 percent.
• The staining intensity was scored from 0 to 3 points as follows: 0=no detectable stain, 1=weak stain, 2=moderate stain, 3=strong stain.
• Total IRS=percentage of Ki+ cells (0–4) × intensity of stain (0–3), ranging from 0 to 12 points: 0=no immunoreactivity, 1 to 2=weak immunoreactivity, 3 to 4= moderate immunoreactivity, and 6 to 12=strong immunoreactivity. Three fields (×100) with the highest density of stained cells were selected for each sample. The mean score was measured (×400) in relation to the total tumor cell population for BCC cases and in the lower third of the normal epidermis.
Statistical analysis. The statistical package SPSS (SPSS Inc., Chicago, Illinois, USA) Version 23 was used for data analysis using Mann-Whitney test (M.W), T-test, and Pearson correlation coefficient. The significance threshold was fixed at five percent P-value for all tests. P-values less than 0.05 were considered statistically significant.
Results
This study included 40 subjects—20 treatment-naive patients with a pathologically confirmed diagnosis of primary BCC (13 male and 7 female; age range: 42–59 years; mean age: 54.3±3.5 years) and 20 standard control subjects (10 female and 10 male; age range: 23–55 years; mean age: 40.2±10.6 years). Ten percent of the patients were Fitzpatrick Skin Type (FST) II, 45 percent were FST III, 40 percent were FST IV, and five percent were FST V. The most frequently involved site among the BCC group was the cheek (45.0%), followed by the scalp (20.0%), and the nose (15.0%). BCC lesions ranged in size from 0.6cm to 0.7cm, with a mean of 2.25±1.48cm.
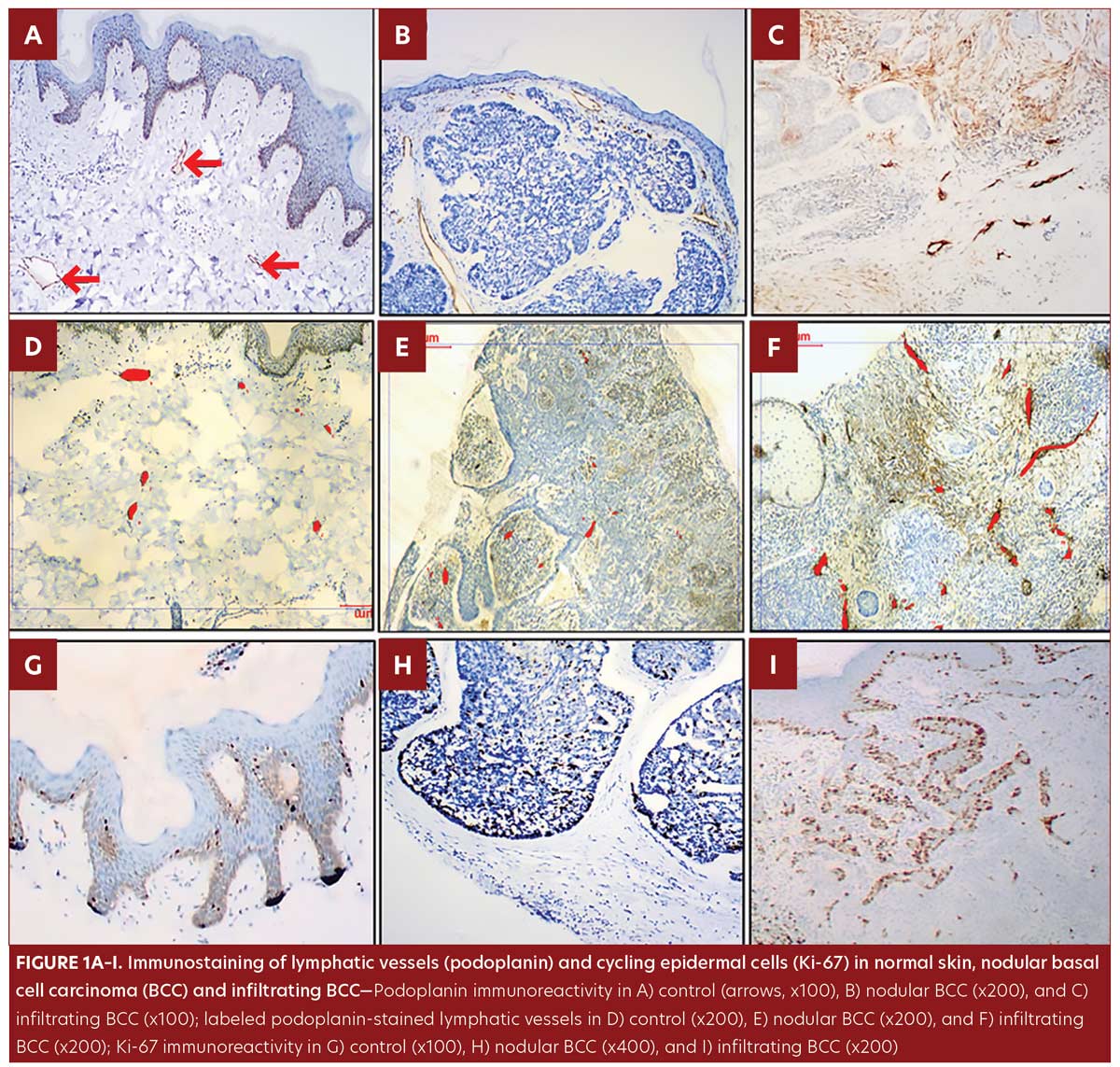
In the control group, podoplanin immunoreactivity was detected in all skin specimens as a weak positive membrano-cytoplasmic immunostaining of the LV endothelial cells and some basal keratinocytes. However, all dermal venules and capillaries were negative for podoplanin immunostaining (Figure1A).
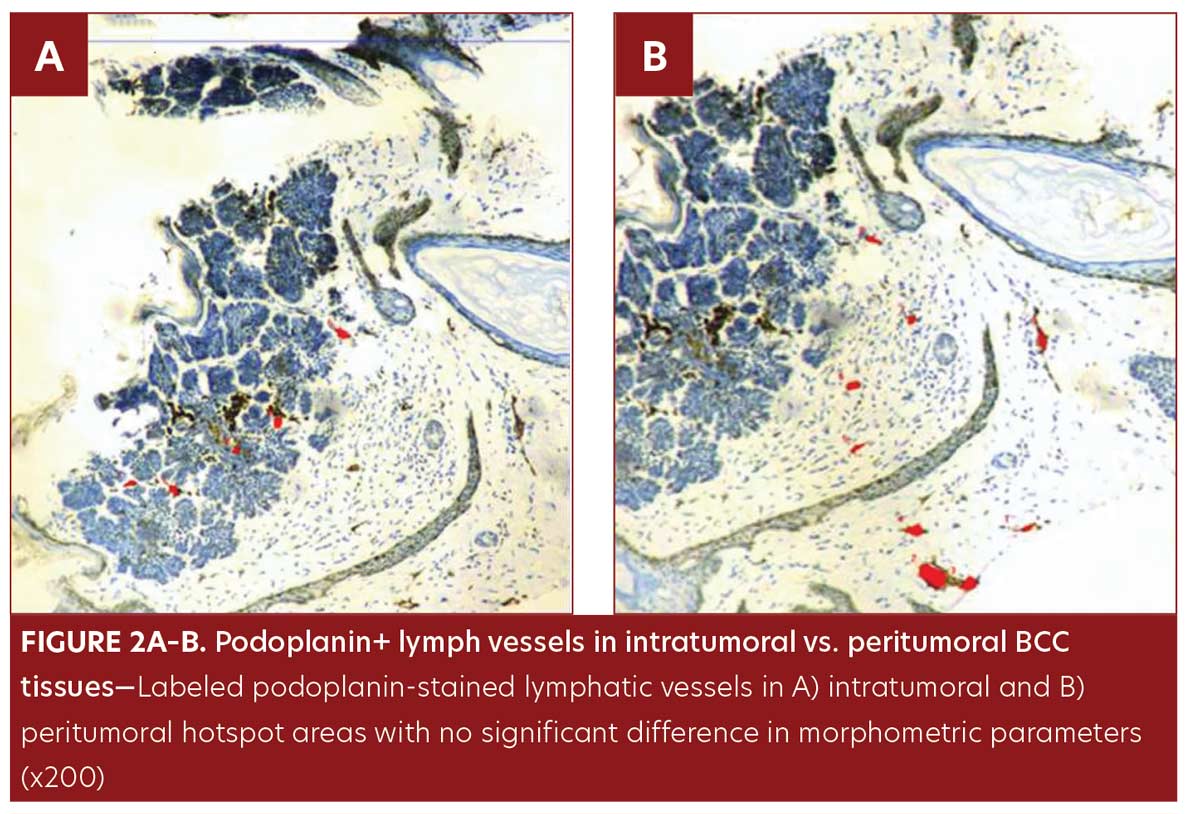
All samples from the BCC group were of the undifferentiated, solid type; nodular pattern was identified in 11 patients (Figure 1B) and infiltrating pattern was identified in nine patients (Figure 1C). Podoplanin+LVs were observed in BCC patients, both intratumoral and peritumoral.
The LV count was higher in the BCC group compared to control group (P=0.03) (Table 1, Figure 1D–F).
No significant difference was observed in morphometric parameters between intratumoral and peritumoral tissues in the BCC group samples (Table 1, Figure 2). The number of LVs and total LVA were greater in infiltrative BCC, compared to nodular BCC (p=0.03 and p=0.001, respectively) (Table 2, Figure 1E–1F).
Ki-67 was detected in control samples as sparse positive nuclear immunostaining restricted to the basal keratinocytes and in a few suprabasal cells with a lacking dermal staining reaction (Figure1G). In the BCC group, Ki-67 immunostaining presented as a diffuse pattern showing positive nuclear staining throughout the tumor islands in 100 percent of the samples. IRS of Ki-67 was significantly higher in BCC samples compared to control samples (p=0.002) (Table 1, Figure 1G–1I). IRS of Ki-67 was higher in the infiltrating pattern samples compared to the nodular pattern samples (p=0.001) (Table 2, Figure 1H–1I.
Ki-67 immunoreactivity was correlated with the number of LVs and total LVA (r=0.6, p=0.004; r= 0.6, p=0.002, respectively).
Discussion
BCC is associated with the induction of the proliferative activity of basal cells with the capacity of local invasion and tissue destruction. Although BCC is locally aggressive, metastatic spread rarely occurs.6,7 Podoplanin is a specific marker to delineate the LECs. The role of podoplanin during lymphangiogenesis was previously explored in different disorders.9,15
The significantly higher number of podoplanin-stained LVs in the BCC group, compared to control group, along with nonsignificant increase in LVA, indicate that the formation of neolymphatic network and lymphangiogenesis in BCC carcinogenesis is more important than LV remodeling. Unlike our results, Neinaa et al16 did not find a statistically significant increase in LV density in BCC samples compared to those of normal skin.
A higher count of LVs and lower total LVA in intratumoral tissue, compared to peritumoral stroma, have been noted in previous literature, which described small and collapsed intratumoral LVs.17 This finding has been explained by the high interstitial pressure within the tumor lesion and/or loss of typical tissue architecture.17 In contrast, peritumoral LVs have been described as dilated, twisted vessels that are filled with cells, leading to the idea that these vessels provide the primary tumor with a drainage route for fluid and cells.4 In our study, though we did observe differences between intratumoral tissues and peritumoral stroma, they did not reach statistical significance. This could be attributed to the rare metastatic potential of BCC, which was observed by Wysong et al.6 Wojciechowska-Zdrojowy et al18 observed higher LV density in stromal cells than in the BCC lesion mass. These contradictory results could be attributed to tumor heterogeneity and the possible multifactorial pathogenesis of BCC.
Some studies have shown that the infiltrating BCC growth pattern is more frequently associated with deeper invasion and high recurrence rates compared to other subtypes.19 Higher LV counts and greater total LVA were observed in BCC legions with infiltrating growth patterns compared to those with nodular growth patterns.19 These results suggest the role of podoplanin in skin carcinogenesis and its association with cancer aggressiveness. Podoplanin may be further utilized as a marker reflecting tumoral local behavior. As far as we know, the correlation between podoplanin and the proliferative marker Ki-67 for BCC has not been described in the literature. Regarding Ki-67 immunoreactivity, most previous studies evaluated only the density of Ki-67+ cells. However, Esmaeili et al20 examined the density and staining intensity of Ki-67+ cells separately. Using the IRS is more accurate due to its consideration of both the density and the intensity of the staining reaction. Numerous studies have investigated Ki-67 expression levels in BCC.20,21 The values were variable, as in our work, but most, if not all, of BCC lesions were positive for this marker.22 This variability in Ki-67 expression may be attributed to the working protocol, different Ki-67 clones, and some tumor heterogeneity.
Higher Ki-67 immunoreactivity was observed in BCC lesions with infiltrating growth patterns, compared to those with nodular growth patterns. Investigators have also reported the highest values of Ki-67 expression in the more aggressive histopathological cases of BCC.11,21 However, other researchers found no significant correlation between Ki-67 expression and histopathological types of BCC.20,22
In the presented study, our findings demonstrated a positive correlation between Ki-67 staining, LV count, and total LVA. We also detected more podoplanin+ LVs in hyperproliferative and infiltrative BCC growth patterns, confirming the value of neolymphatic network formation in tumor growth and progression.
The correlation between epidermal proliferation and lymphangiogenesis in BCC may be attributed to the Hedgehog signaling pathway; this signaling pathway is the predominant player in the tumorigenesis of BCC through activation of the Gli family of transcription factors with subsequent proliferation and differentiation of adult stem cells.23 The hedgehog pathway has also been shown to be a key mediator of lymphangiogenesis and likely tumor survival.24,25
Our findings support the significant role of podoplanin in tumor lymphangiogenesis and progression. Podoplanin stimulates the formation and release of exosomes and microvesicles into the tumor microenvironment. Podoplanin-containing exosomes induce lymphangiogenesis by transporting genetic information and proteins from tumors to surrounding cells.26 Podoplanin+ cancer-associated fibroblasts stimulate lymphangiogenesis by the secretion of lymphangiogenic growth factors, facilitating cancer entry into lymphatic vessels.27
LVs are possible therapeutic targets that can be exploited to reach symptomatic control and an improved prognosis in diseases such as BCC.28 Much progress has been made concerning inhibiting lymphangiogenesis and metastasis by targeting the VEGF-C/VEGF-D/VEGFR-3 axis.29 Additionally, antipodoplanin antibodies were developed as a trial to inhibit podoplanin-induced cancer metastasis.9 Antipodoplanin antibodies can disrupt podoplanin binding to C-type lectin-like receptor- 2 (CLEC2) presenting on the platelet surface, inhibiting platelet aggregation and hematogenous metastasis.30 Thus, podoplanin may be considered an innovative therapeutic target for lymphangiogenesis-mediated disorders.31
Limitations. Our study has limitations. This study only indicates the lymphangiogenesis fraction and not the rates at which this process occurs. In addition, other histological variants of BCC were not included in the study, and studies with larger sample sizes are needed to support our findings.
Conclusion
The process of lymphangiogenesis can be more deeply and adequately explored using the quantitative morphometric analysis. The expression of podoplanin in LVs could be involved in the pathogenesis and progression of BCC. Therefore, the possibility of podoplanin as a biomarker for BCC warrants further studies. Podoplanin blockers show potential as a new therapeutic option for patients with BCC.
References
- Moore Jr JE, Bertram CD. Lymphatic system flows. Annu Rev Fluid Mech. 2018;50:
459–482. - Hansen KC, D’Alessandro A, Clement CC, Santambrogio L. Lymph formation, composition and circulation: a proteomics perspective. Int Immunol. 2015;27(5):
219–227. - Hantusch B. Morphological and functional characteristics of blood and lymphatic vessels. In: Geiger M (ed). Fundamentals of Vascular Biology. Switzerland: Springer Nature;2019: 1–43.
- Dieterich LC, Detmar M. Tumor lymphangiogenesis and new drug development. Adv. Drug Deliv Rev. 2016; 99: 148–160.
- Wei R, Lv M, Li F, et al. Human CAFs promote lymphangiogenesis in ovarian cancer via the Hh-VEGF-C signaling axis. Oncotarget. 2017;8(40):67315.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149(5):615–616.
- Tang S, Thompson S, Smee R. Metastatic basal cell carcinoma: case series and review of the literature. Aust J Dermatol. 2017;58(2): 40–43.
- Bgatova NP, Lomakin AI, Fursov SA, et al. Expression of molecular markers of angiogenesis, lymphangiogenesis, and proliferation depending on the stage of skin. B Exp Biol Med. 2016;161;(4):542–546.
- Deepa AG, Angelin D, Joseph TI, Das CN. Podoplanin: an insight into its role in tumor invasion and metastasis. Oral Maxillofac Pathol J. 2017;8(2):118–125.
- Výbohová D, Mellová Y, Adamicová K, et al. Quantitative changes of the capillary bed in aging human skin. Histol Histopathol. 2012;27:961–967.
- Koseoglu RD, Sezer E, Eyibilen A, et al. Expressions of p53, cyclinD1 and histopathological features in basal cell carcinomas. J Cutan Pathol. 2009;36(9):
958–965. - Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36(2):169–180.
- Zhang SQ, Yu H, Zhang LL. Clinical implications of increased lymph vessel density in the lymphatic metastasis of early-stage invasive cervical carcinoma: a clinical immunohistochemical method study. BMC Cancer. 2009; 9(1);64.
- Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140.
- Henno A, Blacher S, Lambert C, et al. Altered expression of angiogenesis and lymphangiogenesis markers in the uninvolved skin of plaque type psoriasis. Br J Dermatol. 2009;160:581–590.
- Neinaa YMEH, El-Ashmawy AA, Alshenawy HAS, Dorgham WL. The prognostic value of podoplanin expression in nonmelanoma skin cancers: correlation with lymphatic vessel density. Am J Dermatopathol. 2020;42(6):432–438.
- Stacker SA, Williams SP, Karnezis T, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–172.
- Wojciechowska-Zdrojowy M, Szepietowski JC, Matusiak L, et al. Expression of podoplanin in nonmelanoma skin cancers and actinic keratosis. Anticancer Res. 2016;36:1591–1598.
- Rippey JJ, Rippey E. Characteristics of incompletely excised basal cell carcinomas of the skin. Med J Austral. 1997;166(11):
581–583. - Esmaeili R, Seyedin Khorasani M, Monsef A. Immunohistochemical expression of P53 and Ki-67 in different histopathological variants of basal cell carcinoma. Middle East J Cancer. 2015;6(1):29–34.
- Florescu D, Stepan AE, Mărgăritescu C, et al. Proliferative activity in basal cell carcinomas. Curr Health Sci J. 2018;44(1):55.
- Son KD, Kim TJ, Lee YS, et al. Comparative analysis of immunohistochemical markers with invasiveness and histologic differentiation in squamous cell carcinoma and basal cell carcinoma of the skin. J Surg Oncol. 2008;97(7):615–620.
- Gupta AK, Daigle D, Martin G. Basal cell carcinoma. Skin Med. 2014;12(1):33–38.
- Shen B, Xia HX, Liu TW, et al. Abnormal hedgehog signaling activation promotes lymphangiogenesis and lymph node metastasis in nasopharyngeal carcinoma. Int J Clin Exp Med. 2016; 9(11):22835–22840.
- Wei R, Lv M, Li F, et al. Human CAFs promote lymphangiogenesis in ovarian cancer via the Hh-VEGF-C signaling axis. Oncotarget. 2017;8(40): 67315.
- Quintanilla M, Carrasco-Ramírez P, Montero-Montero L, et al. Podoplanin promotes malignancy through a diversity of strategies. Vascular. 2016;3:1–7.
- Stacker SA, Williams SP, Karnezis T, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–172.
- Mumprecht V, Roudnicky F, Detmar M. Inflammation-induced lymph node lymphangiogenesis is reversible. Am J Pathol. 2012;180(3):874–879.
- Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31(42):4499–4508.
- Krishnan H, Rayes J, Miyashita T, et al. Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Sci. 2018;109:1292–1299.
- Maruyama Y, Maruyama K, Kato Y, et al. The effect of podoplanin inhibition on lymphangiogenesis under pathological conditions. Investig. Ophthalmol Vis Sci. 2014;55:4813–4822.

