 J Clin Aesthet Dermatol. 2023;16(3 Suppl 1):S29–S33
J Clin Aesthet Dermatol. 2023;16(3 Suppl 1):S29–S33
by Rohan R. Shah, BA; Zaeem Nazar, MD; Alyssa Swearingen, BS; Nadia Waqas, MD; Shawana Sharif, MD; and Babar Rao, MD
Mr. Shah and Ms. Swearingen are with the Department of Dermatology at Rutgers New Jersey Medical School in Newark, New Jersey. Drs. Nazar, Waqas, and Sharif are with the Department of Dermatology at Benazir Bhutto Hospital in Rawalpindi, Pakistan. Dr. Rao is with the Department of Dermatology at Weil Cornell School of Medicine in New York, New York, and Rutgers Robert Wood Johnson Medical School of Medicine in Piscataway, New Jersey.
FUNDING: No funding was received for this study or article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Alopecia areata is an autoimmune-induced hair loss disorder affecting nearly two percent of the global population and is the third most common cause of dermatology visits among children. The prevalence and incidence of pediatric alopecia areata have increased significantly in certain regions of the world, including south Asia. Pediatric alopecia areata not only can affect patients psychosocially and emotionally, but also lacks proper therapeutic options as most treatments are beneficial only to adults. Topical anthralin is a commonly used ingredient in psoriasis treatment with potential application for pediatric alopecia areata.
Objective. We sought to assess the effectiveness of topical anthralin 1% for the treatment of alopecia areata in a pediatric population.
Methods. Our quasi-experimental study evaluated 190 patients ages 3 to14 years with alopecia areata. Each patient received daily application of topical anthralin 1% ointment to the area of scalp with hair loss and was observed at one, four, eight, and 12 weeks of treatment. Percentage of regrowth on the scalp was measured.
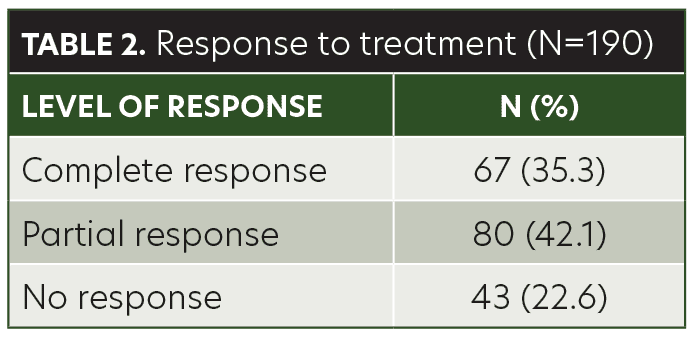
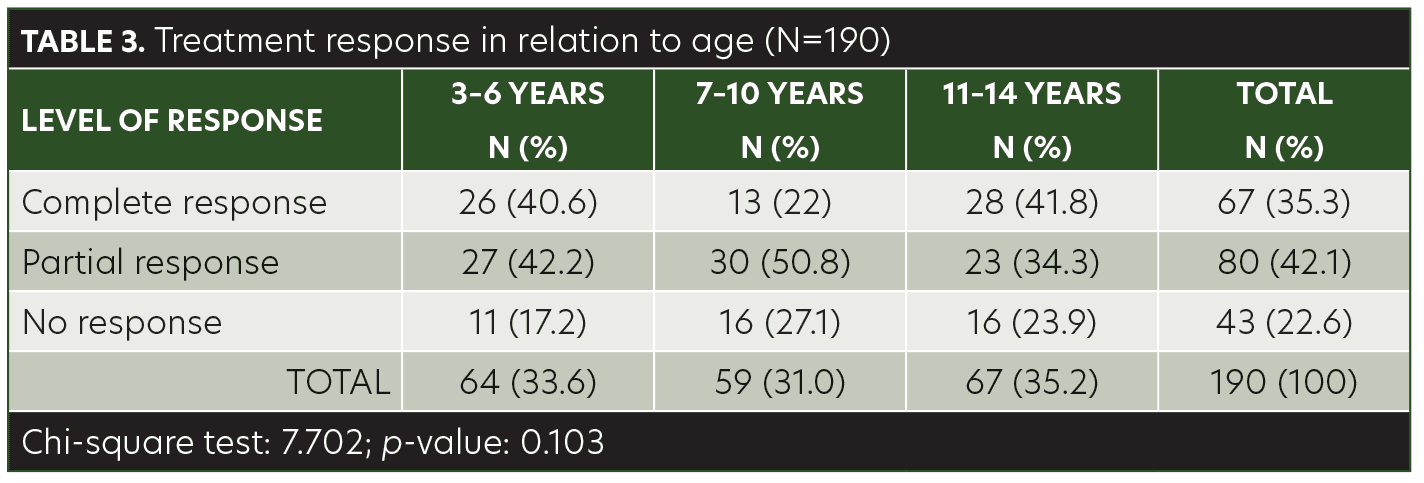
Results. Sixty-seven (35.3%) of the patients demonstrated complete response with anthralin therapy, 80 (42.1%) had partial response, and 43 (22.6%) had no response. There was no significant association between treatment response and age group, but treatment response was significantly associated with hair loss patch size.
Conclusion. We suggest that anthralin may be beneficial for pediatric patients with alopecia areata, including younger patients in which steroids are not indicated or as a second-line option in patients with minimal response to glucocorticoids. We recommend the routine use of anthralin for pediatric alopecia areata management to improve patient satisfaction and disease outcome.
Key words: Alopecia areata, pediatric, anthralin, topical
Alopecia areata (AA) is an autoimmune type of nonscarring hair loss that affects up to two percent of the global population.1 It is characterized by relapsing and remitting patches of hair loss that may progress to more severe types, including alopecia universalis, alopecia ophiasis, or alopecia totalis.1 While AA is commonly considered an adult condition, approximately 40 percent of patients with AA have their first episode of hair loss by 20 years of age and one in five cases of AA occur during infancy.2 As such, AA is the third most common cause of dermatology visits among children.2 The annual prevalence of pediatric AA in the United States has increased steadily from 2009 to 2019 (0.057% to 0.11%), nearly doubling in that timespan.3 Additionally, US rates during this timespan were highest among Hispanic and Asian children, followed by Black and White children.3 Similarly, pediatric AA has increased significantly in prevalence and incidence in South Asia—an epidemiological study from Eastern India and Kuwait recorded a prevalence rate of up to 20.4 percent in certain populations of pediatric patients.4 This was higher when compared to previous studies from China, Singapore, and Saudi Arabia.5–7
Although AA is a physioloigically harmless condition without any systemic findings, it can affect the patient emotionally and psychosocially.2 Furthermore, AA occurring before puberty has been associated with a poorer prognosis than AA in adults.2 Other negative prognostic factors include a positive family history, seen in 20 percent of pediatric AA cases, atopy, and nail involvement.1 Because the pediatric population is more susceptible to the psychosocial consequences of AA, adequate treatment is critical to prevent further morbidity associated with this condition.1
While various therapies, including topical and systemic modalities, are available for AA treatment, they are focused on the adult population. Thus, therapeutic options for children and adolescents are limited. One reason for this is the lack of studies on pediatric AA. The second reason stems from incongruent guidelines among physicians regarding the definition and management of pediatric AA. For instance, according to the British Association of Dermatologists, pediatric patients with AA should be treated with a similar regimen as adults.8 However, the Italian Association of Dermatologists suggest children below the age of 10 years receive a different therapeutic regimen from their adult counterparts.9 Finally, many currently available treatments have potential adverse side effects, which are less manageable and less desirable in children.10
Anthralin, also known as dithranol, is a commonly used active ingredient in psoriasis treatments. The free radicals generated by anthralin induce an anti-inflammatory response localized to the site of application in contrast to systemically active glucocorticoids10 This lack of systemic toxicity makes anthralin a promising candidate for pediatric AA.10 In this single-institution study, we assessed the effectiveness of topical anthralin 1% therapy on the scalp area in pediatric patients with AA.
Methods
Our quasi-experimental study was performed within the inpatient dermatology department of a Pakistani hospital. A total of 190 male and female pediatric patients were included in the study, based on the World Health Organization (WHO) sample size calculator11 using a consecutive sampling method. Patients 3 to 14 years of age with a diagnosis of AA of the scalp, regardless of previous treatment, were included in the study. Patients with a prior history of skin disease that can cause hair loss, other chronic medical conditions (e.g., diabetes mellitus, nephritic syndrome, connective tissue disease), history of skin cancer, or known allergy to anthralin compounds were excluded from the study.
The study was approved by the hospital’s ethics committee, and the guardians of each patient provided informed consent. Patients and their parents were asked about any underlying medical conditions or drug allergies. All patients were briefed on the study’s purpose and examined for AA. Photographs were taken of affected areas and were measured in millimeters by a dermatologist (study author Z.N.). All patients and their guardians were instructed on how to apply the 1% anthralin ointment daily at the affected scalp areas. Follow-up assessments occurred at Weeks 1, 4, 8, and 12 of treatment. At each visit, the same dermatologist examined the affected areas and measured their size. The scalp area where regrowth took place was measured in terms of percentage regrowth. An area where complete hair regrowth was evident was documented as 100-percent scalp regrowth. The length of time to achieve a minimum of 50-percent scalp regrowth and 100-percent scalp regrowth were measured in weeks. All results were documented in a specifically designed report and included the patient’s sex, age, and number of AA patches involved. All data were analyzed using SPSS version 23. Age, sex, and patch size were all stratified, followed by a chi-square test with a p value less than 0.05.
Results
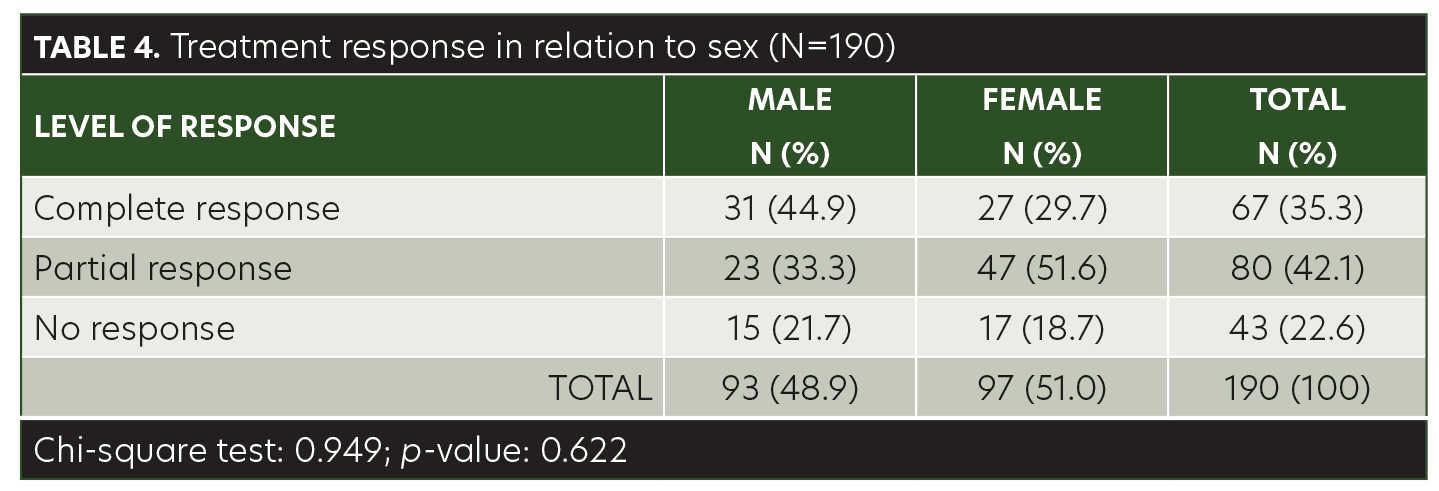
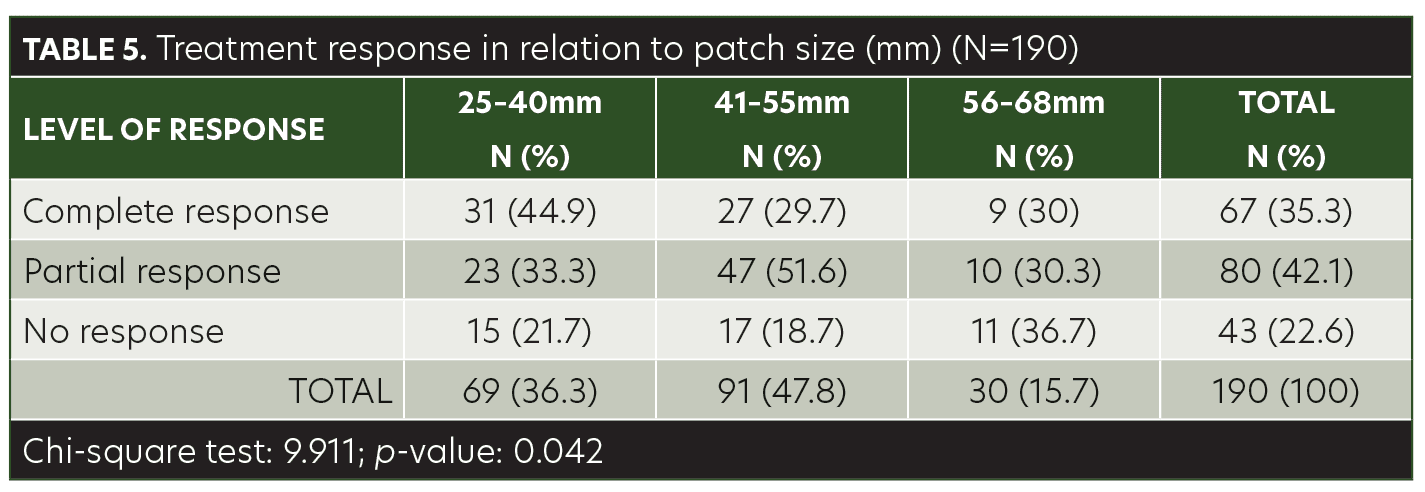
The mean age of the children in this study was 8.40±3.48 years (range, 3–14 years). Among the patients, 48.9 percent were male and 51.1 percent were female. AA was present on the anterior scalp of 76 (40%) patients, on the posterior scalp of 99 (52.1%) patients, and on the eyebrows of 15 (7.9%) patients (Table 1). The mean size of a single patch was 44.42±10.97mm (range, 25–68mm). Sixty-seven (35.3%) of the patients achieved complete response with anthralin therapy, 80 (42.1%) patients achieved partial response, and 43 (22.6%) had no response (Table 2). While a higher frequency of complete response was observed in patients 3 to 6 years of age (40.6%) and 11 to 14 years of age (41.8%), there was no significant association between treatment response and age group (p= 0.103) (Table 3). Similarly, no significant difference was observed between male and female subjects regarding treatment response (p=0.622) (Table 4). However, treatment response was significantly associated with patch size, with patch sizes in the 25- to 40mm range demonstrating the highest rate of complete response (44.9%), followed by 41- to 55mm (29.7%) patch sizes and 56- to 68mm (30%) patch sizes (p=0.042) (Table 5).
Adverse events. Besides minor skin irritation at the applied site, there were no adverse effects reported in our study. Adverse events reported elsewhere include skin irritation, soreness at application site, and minor swelling.10

Discussion
Treatment options for AA in children are limited compared to that of adults. While anthralin is utilized in pediatric AA, there remains a lack of controlled trials measuring its efficacy in hair regrowth in this patient population. According to current therapeutic evidence for pediatric AA, topical anthralin has demonstrated effectiveness in patients who did not respond to first-line treatments with topical or intralesional corticosteroids.12 In our study, 35.3 percent of the patients achieved complete response and 42.1 percent achieved partial response using topical anthralin 1% (Figures 1A and 1B). Although age and sex did not have a significant association with treatment response, patch size did: Hair loss patches ranging from 25- to 40mm in size had the greatest response; thus, anthralin may be best suited for small- to average-sized lesions.
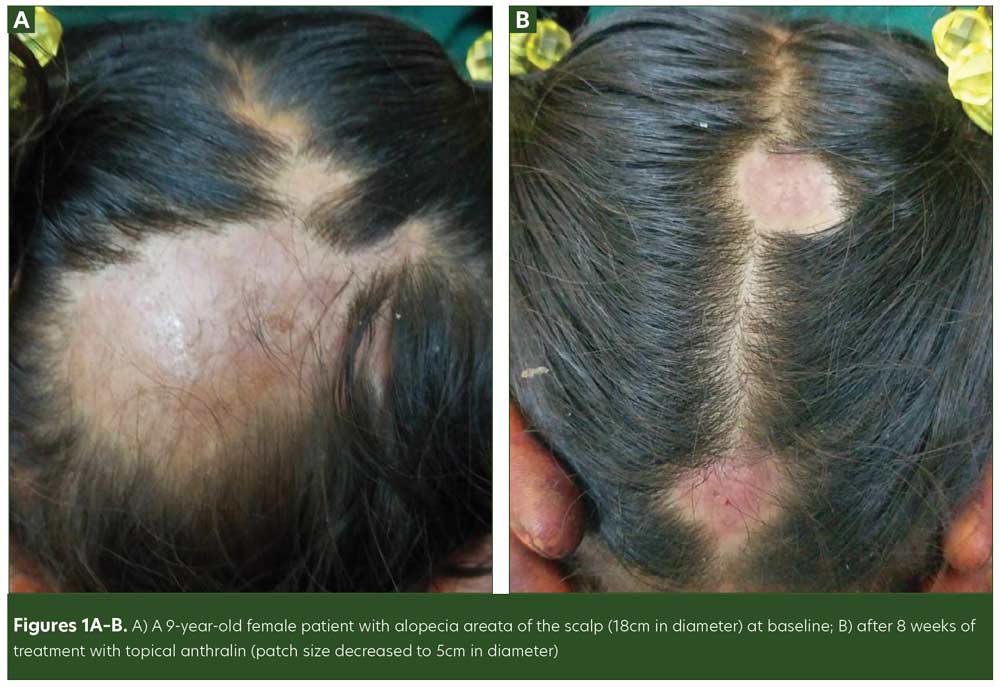
In a previous randomized, controlled trial, 70 percent of pediatric patients with intense refractory AA exhibited either partial or complete response with 1% anthralin after 12 months.12 In a separate study of pediatric AA, only 26 percent of the patients experienced 50-percent or greater regrowth using 0.5% or 1.0% anthralin at the six-month follow-up visit.10 Our study exhibited similar results to these two studies, though our study had a different follow-up duration and interval schedule.
In a study by Wu et al,10 32 percent of their pediatric study subjects achieved full scalp regrowth and 68 percent achieved at least 50-percent scalp regrowth. The average time to achieve 50-percent scalp regrowth was 3.4 months, but 64 percent of these patients were affected by relapses.10 A Turkish study assessing 1% anthralin in pediatric patients with AA reported that of the 33.4 percent who achieved complete response, 20 percent achieved complete response by six months, 50 percent by nine months, and 30 percent by 12 months.13 These variable results regarding anthralin’s effectiveness likely stem from a lack of practice guidelines, differences in follow-up time, and cultural dissimilarities.
Contextualizing anthralin as a therapeutic option for pediatric AA is important. Intralesional glucocorticoids are effective in adults with AA but are less commonly used in children due to potential fear of injections and pain.2 According to the Australian Expert Consensus, intralesional glucocorticoid injections are not recommended in pediatric cases of AA due to required use of sedation prior to injection.14 Oral glucocorticoids tend to result in the most rapid response of all systemic options for AA and are recommended for use in chronic cases of AA among adolescents between 13 and 18 years of age.15 In children under seven years of age, systemic glucocorticoids are not approved, primarily due to side effects, such as weight gain and abdominal pain.16 Based on these guidelines and our observations, a well as those reported in previous studies, topical anthralin is perhaps best suited for children with AA who are too young to be treated with systemic steroids and for children over 10 years of age with chronic, patchy hair loss. Additionally, anthralin can be used as a second-line treatment for all pediatric patients with AA if response to systemic or intralesional steroids is limited. Further studies are needed to determine whether anthralin is a sufficient option to pair with either topical, systemic, or intralesional steroids.
To our knowledge, this study is the first to exclusively sample a completely South Asian population for evaluating anthralin’s effectiveness in pediatric AA. All of our patients had Fitzpatrick Skin Types IV, V, or VI and most patients had black or dark brown hair. A study by Yousaf et al indicated that AA is modulated by natural hair color, preferentially affecting darker hair.17 These results support the model that immunity in AA is directed against melanogenesis-associated proteins in the anagen hair follicle.17 Importantly, a Saudi Arabian study found that a greater proportion of children were affected by AA in certain South Asian countries compared to Western nations. Additionally, when pediatric patients were affected by AA, the level of severity was greater compared to their adult counterparts.13
On reviewing AA data from Pakistan, we found only one hospital-based study delineating the clinical features of children with AA.18 A complex condition such as AA, which is multifactorial in nature, requires a better understanding of the environmental and genetic factors that contribute to pathogenesis and severity so that treatment modalities can be better allocated to certain populations depending on their individual risk factors.
AA often results in significant psychological detriment, and the pediatric population is particularly susceptible to these consequences of AA.19 Thus, adequate treatment is critical to prevent further morbidity associated with this disease. Because therapeutic options for children with AA are limited, anthralin provides an opportunity for clinicians to treat AA in this vulnerable population when first-line options approved for adults are either too dangerous or not clinically indicated for the AA pediatric population.
Limitations. There are limitations to our study. First, this was a quasi-experimental study rather than randomized, control study. Patients were not randomly assigned to treatment or control groups, which limits the generalizability of the study’s findings. Further investigation with an appropriate control and treatment group, along with appropriate randomization is warranted. One additional limitation is the frequency of follow-up. Even though thorough follow-up was conducted at Weeks 1, 4, 8, and 12 of treatment, future studies should emphasize weekly follow-up.
Conclusion
The results of this study demonstrate that topical anthralin 1% may be an effective treatment option for pediatric AA. Our study indicates that sex and age groups within the pediatric population did not appear to affect treatment response to anthralin, but patch size did. In comparing anthralin with current treatment options for adults and certain children, we suggest that anthralin has many applications for the pediatric patient with AA, including its use in patients for whom steroids are not indicated or as a second-line option in patients with minimal response to glucocorticoids. We recommend the routine use of topical anthralin 1% for pediatric AA management to improve patient satisfaction and disease outcome. Furthermore, our study is unique in that almost all patients had black or dark brown hair and all patients had Fitzpatrick Skin Types IV to VI. The relevance of this to AA is still not understood and requires further investigation.
References
- Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:675–682.
- Waśkiel-Burnat A, Kołodziejak M, Sikora M, et al. Therapeutic management in paediatric alopecia areata: a systematic review. J Eur Acad Dermatol Venereol. 2021;35:1299–1308.
- McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol. 2022;158:547–551.
- Bhardwaj P, Basu D, Podder I , Gharami RC. Clinico-epidemiological profile of childhood alopecia areata along with dermoscopic correlation: a cross-section, observational study. Ind Dermatol Online J. 2021;12:
250–257. - Xiao FL, Yang S, Liu JB, et al. The epidemiology of childhood alopecia areata in China: a study of 226 patients. Pediatr Dermatol. 2006;23:13–8
- Tan E, Tay YK, Giam YC. A clinical study of childhood alopecia areata in Singapore. Pediatr Dermatol. 2002;19:298–301.
- Alshahrani AA, Al-Tuwaijri R, Abuoliat ZA, Alyabsi M. Prevalence and clinical characteristics of alopecia areata at a tertiary care center in Saudi Arabia. Dermatol Res Pract. 2020;2020:7194270.
- Messenger AG, McKillop J, Farrant P, et al. British Association of Dermatologists’ guidelines for the management of alopecia areata. Br J Dermatol. 2012;166(5):916–926.
- Messenger AG, McKillop J, Farrant P, McDonagh AJ. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166:916–926.
- Wu SZ, Wang S, Ratnaparkhi R , Bergfeld WF. Treatment of pediatric alopecia areata with anthralin: a retrospective study of 37 patients. Pediatr Dermatol. 2018;35:817–820.
- World Health Organization site. Noncommunicable disease surveillance, monitoring and reporting. Planning and sampling tools. Sample size calculator. https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps/planning-sampling. Accessed 17 Jan 2023.
- Özdemir M, Balevi A. Bilateral half-head comparison of 1% anthralin ointment in children with alopecia areata. Pediatr Dermatol. 2017;34:128–132.
- Durdu M, Özcan D, Baba M, Seçkin D. Efficacy and safety of diphenylcyclopropenone alone or in combination with anthralin in the treatment of chronic extensive alopecia areata: a retrospective case series. J Am Acad Dermatol. 2015;72:640–650.
- Cranwell WC, Lai VW, Photiou L, et al. Treatment of alopecia areata: an Australian expert consensus statement. Australas J Dermatol. 2019;60:163–170.
- Meah N, Wall D, York K, et al. The Alopecia Areata Consensus of Experts (ACE) study: results of an international expert opinion on treatments for alopecia areata. J Am Acad Dermatol. 2020;83:123–130.
- Rossi A, Muscianese M, Piraccini BM, et al. Italian guidelines in diagnosis and treatment of alopecia areata. G Ital Dermatol Venereol. 2019;154:609–623.
- Yousaf A, Lee J, Fang W, Kolodney MS. Association between alopecia areata and natural hair color among White individuals. JAMA Dermatol. 2021 Mar 10;e210144. Online ahead of print.
- Ahmed I, Nasreen S , Bhatti R. Alopecia areata in children. J Coll Physicians Surg Pak. 2007;17:587–590.
- Barton VR, Toussi A, Awasthi S , Kiuru M. Treatment of pediatric alopecia areata: a systematic review. J Am Acad Dermatol. 2022;86:1318–1334.

