J Clin Aesthet Dermatol. 2022;15(3):12–14.
Surgical Pearl: Noninterrupted Secure Knot Technique for Running Sutures
Dear Editor:
Using a running suture, as opposed to simple interrupted sutures, for wound closure has its advantages and disadvantages. The advantages include: 1) faster suturing times to complete wound closure; 2) the ease of continuous repair motion without requiring an assistant to repeatedly cut the suture, and; 3) saving suture materials. However, it conventionally requires placement of a few simple interrupted sutures after the entire wound is closed to reduce the higher risk of wound dehiscence (Figure 1).1 This step limits the efficiency of the process. We solve this problem by introducing the noninterrupted secure knots (NISK) technique for running sutures.
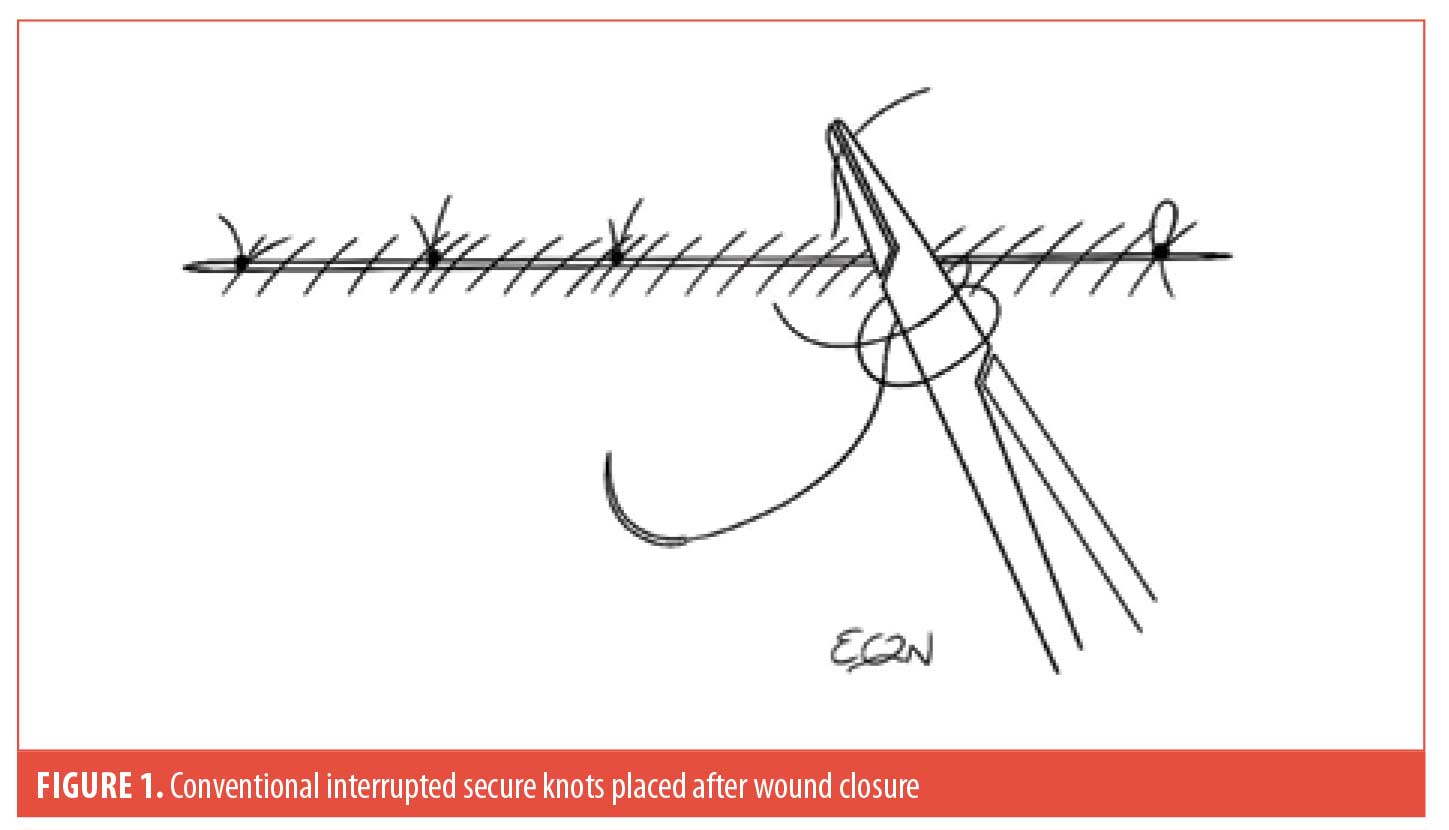
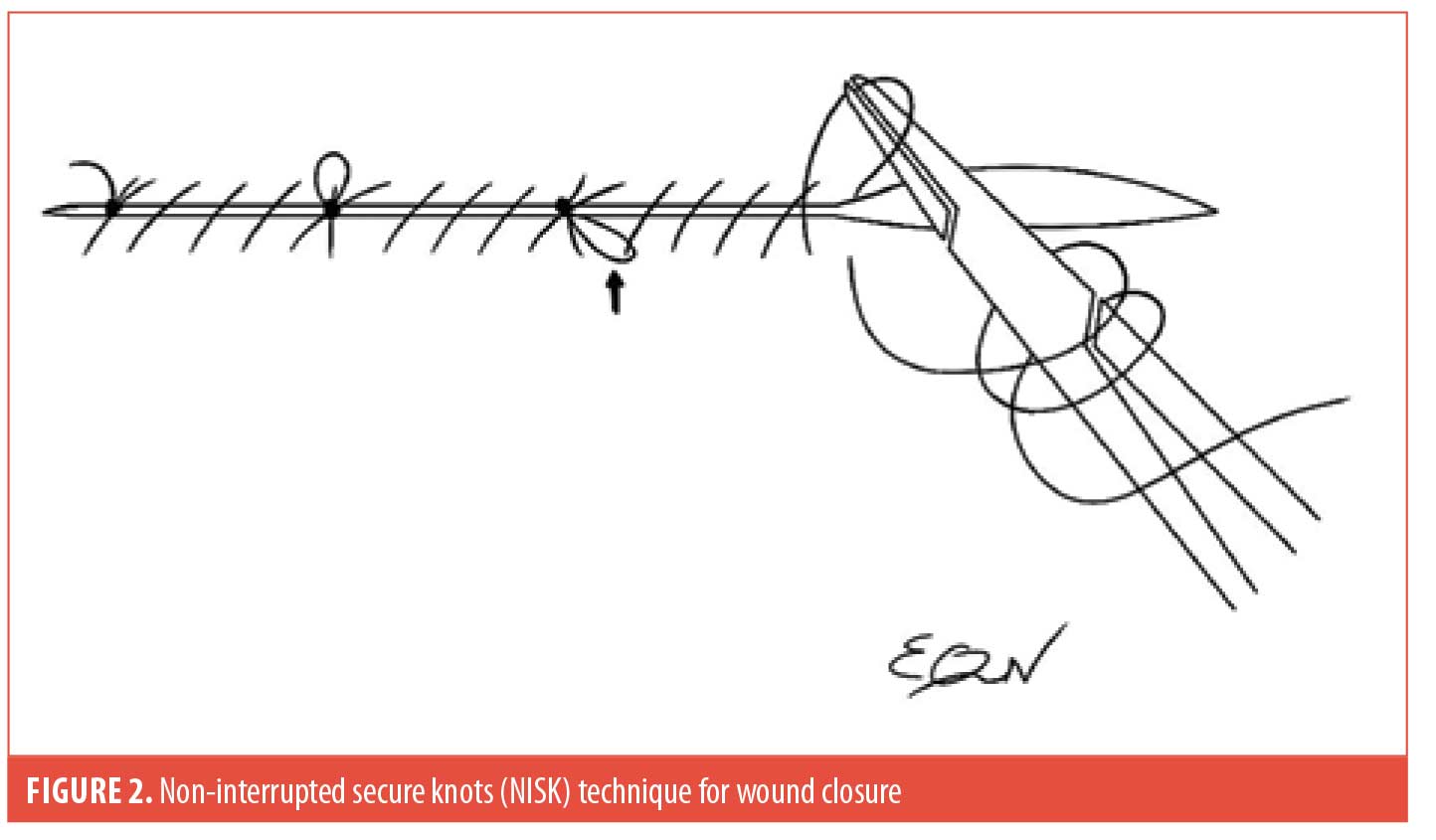
The NISK technique allows dermatologists to optimize their time, perform procedures without the need for staff assistance (i.e., cutting sutures), and potentially reduce office costs by saving suture material. This method consists of making a loop every 4 to 6 sutures across the length of the surgical defect in order to tie a square knot before the needle is reintroduced to continue the running suture. As an option, the subsequent suture after the knot should pass through the previous loop to trap it down (Figure 2, see arrow), further enhancing security by preventing slip knots due to tissue swelling. Thus, this modified technique eliminates the need to put in simple interrupted sutures at the end of wound closure.
With regard,
Wendy Li, MD, and Ethan Nguyen, MD
Affiliations. Drs. Li and Nguyen are with the Riverside Community Hospital & University of California Riverside in Riverside, California. Dr. Nguyen is also with UC Riverside School of Medicine in Riverside, California
Funding. No funding was provided for this article.
Disclosures. The authors declare no conflicts of interest related to the content of this article.
References
- Wong NL. Review of Continuous Sutures in Dermatologic Surgery. J Dermatol Surg Oncol. 1993;19(10):923–931
The Area Capable of Being Covered by the Application of 250mg of Tirbanibulin Ointment
Dear Editor:
The United States (US) Food and Drug Administration (FDA) recently approved the field treatment for actinic keratoses (AK) on the face and scalp with topical tirbanibulin ointment 1%. Tirbanibulin inhibits tubulin polymerization and alters Src kinase signaling, resulting in antiproliferative and pro-apoptotic effects. FDA approval was based on the application of the contents of one packet of 2.5mg tirbanibulin in 250 mg ointment to a 25cm2 contiguous area of skin on the face and scalp, once daily for five consecutive days. However, clinically relevant field treatment of actinic keratoses most often requires application to an area greater than 25 cm2. Although the area able to be covered by 250mg of imiquimod 5% cream has been studied1, to our knowledge, there are no published data regarding the surface area of skin able to be covered by a single packet of 2.5mg tirbanibulin in 250mg ointment (TRB).
Case report. The contents of one TRB was evenly applied to the balding scalp and forehead to the hairline of a 72-year-old male patient with multiple actinic keratoses. In addition, two targeted facial AKs were treated with a thin layer of the remaining residual ointment from the sachet. This was repeated daily for five consecutive days. A 3D image of the subject’s scalp and forehead was created using a 3D high-resolution imaging technique (Cherry Imaging, Ltd, Yokneam, Israel). Using the Trace™ software, three separate measurements of area of application were calculated to be 317.82 cm2 (Standard Deviation=2.06).
The area of application of the contents of one 250-mg packet of TRB applied to the hair-bearing area of the midback was visualized by dusting the midback with a green, fluorescent powder (Art ‘N Glow, Plano, Texas) which selectively adhered to the applied ointment (Figure 1). Using the 3D high resolution imaging technique, the area of the fluorescent powder adhering to the evenly applied ointment was calculated to be 210.27cm2 (SD=2.10).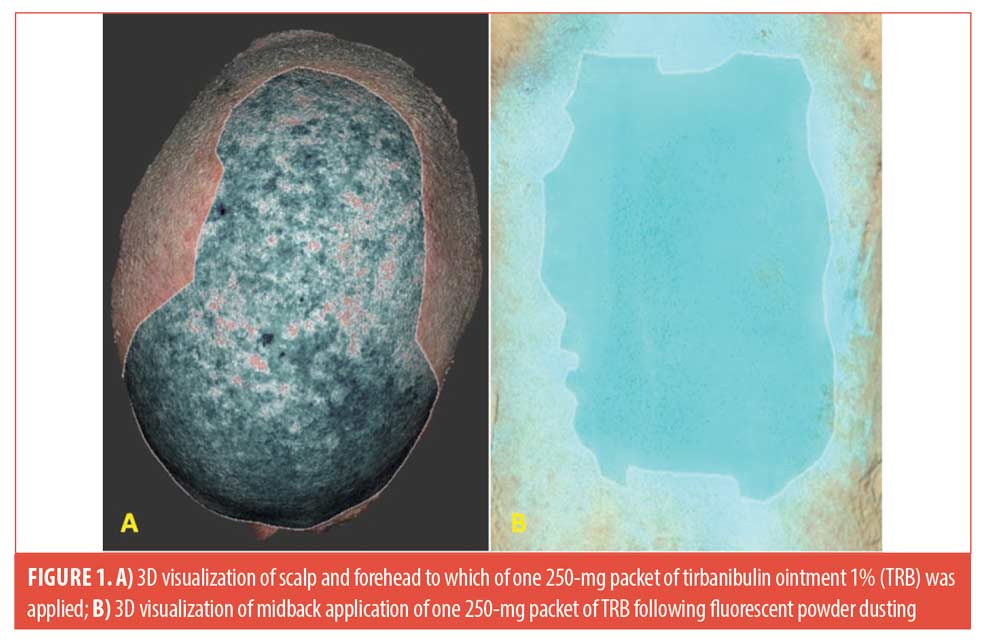 Conclusion
Conclusion
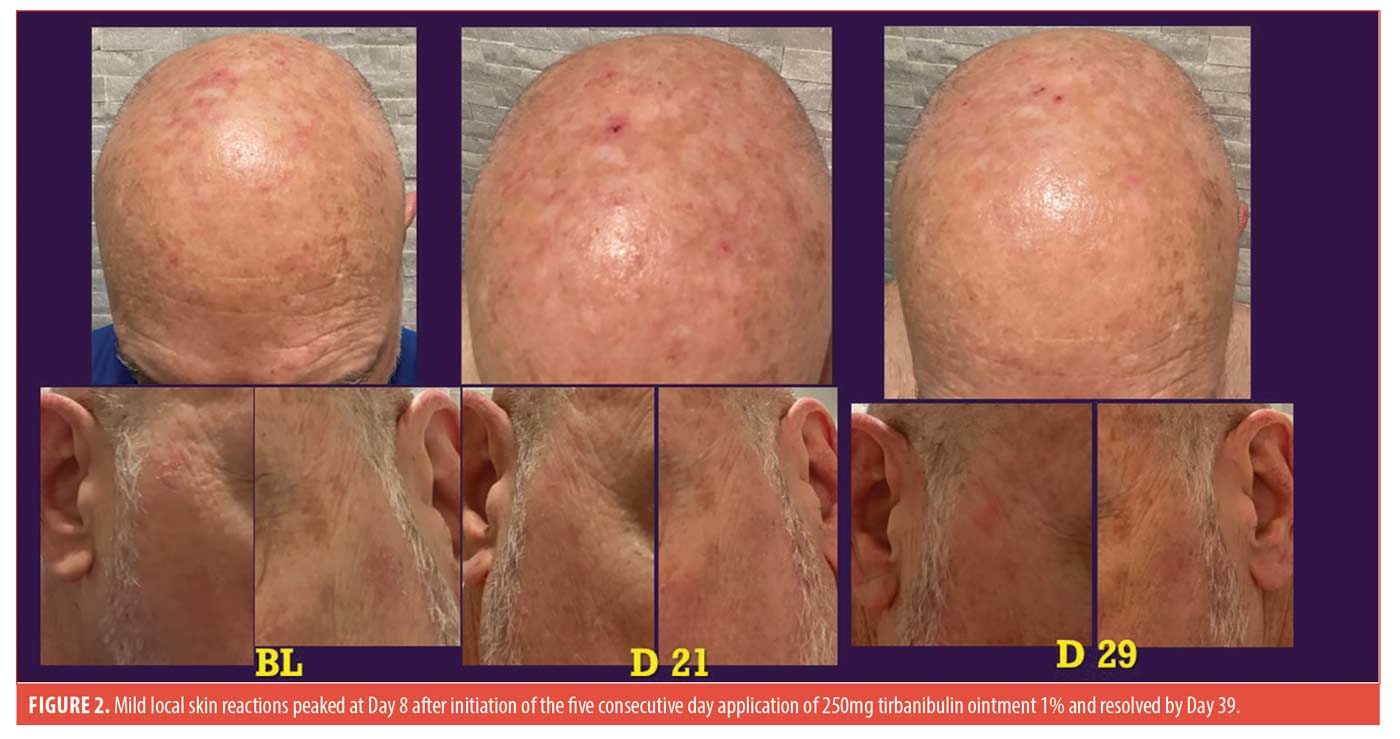
A single TRB was sufficient to be applied to the patient’s balding scalp and forehead up to the hairline and down to the top of the eyebrows. In addition, two targeted AKs on the left and right lateral canthal areas were treated with a thin layer of the final remaining ointment from the packet. As expected, local skin reactions (LSRs) peaked at Day 8 in the treatment areas and resolved by Day 39 (Figure 2), similar to the findings in the Phase III trials.2 Presence of LSRs, and clearance of the two target AK lesions last to be treated at each of the five applications suggest that the layer of ointment applied was sufficient to be effective. The calculated 317.82cm2 area of application in the patient was over 12 times the 25-cm2 area treated in the Phase III trials. These results suggest that the contents of a single packet of 250mg tirbanibulin 1% ointment can be applied therapeutically to areas larger than 25cm2, allowing for successful treatment of whole cosmetic units within pharmacy dispensing limitation to one packet/day for the five-day treatment.
With regard,
Austin Dunn, Do; Haowei Han, DO; Anita Gade, DO; and Brian Berman, MD, PhD
Affiliations. All authors are with the Center for Clinical and Cosmetic Research in Aventura, Florida.
Funding. No funding was provided for this article.
Disclosures. Dr. Brian Berman is a consultant, a speaker, and has served on the advisory board for Almirall. Dr. Berman has received compensation from Almirall in the form of honoraria. Drs. Dunn, Gade, and Han have no conflicts of interest to disclose.
Correspondence. Austin Dunn, DO; Email: a.dunn@admcorp.com
References
- Berman B, Ricotti CA Jr, Cazzaniga A, Davis SC. Determination of the area of skin capable of being covered by the application of 250 mg of 5% imiquimod cream. Dermatol Surg. 2004;30(5):784-786.
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis. N Engl J Med. 2021;384(6):512-520.

