J Clin Aesthet Dermatol. 2024;17(7):16–18.
J Clin Aesthet Dermatol. 2024;17(7):16–18.
Mohs Micrographic Surgery Tourism: Can We Accurately Verify Credentials of International Surgeons?
Dear Editor:
For centuries, patients seeking medical evaluation and treatment have traveled to other countries for care. However, verifying credentials of providers and health centers outside the United States and Canada on the internet may not be possible.
An online internet search conducted in October 2022 for the terms “Mohs surgery tourism”, “Mohs surgery abroad”, and “Micrographic surgery tourism”, on the search engines Google and Bing revealed web pages advertising Mohs surgical treatment in health centers located in the Americas, Europe, Asia, and Africa. The official web pages for these centers were reviewed to identify Mohs surgeons and dermatologists associated with them. Once these physicians were identified (N=191), their names were searched on the database of the American College of Mohs Surgery (ACMS), the American Board of Dermatology (ABD), the European Society for Micrographic Surgery (ESMS), the Australasian College of Dermatologists (ACD), and the American Society for Mohs Surgery (ASMS).1–5
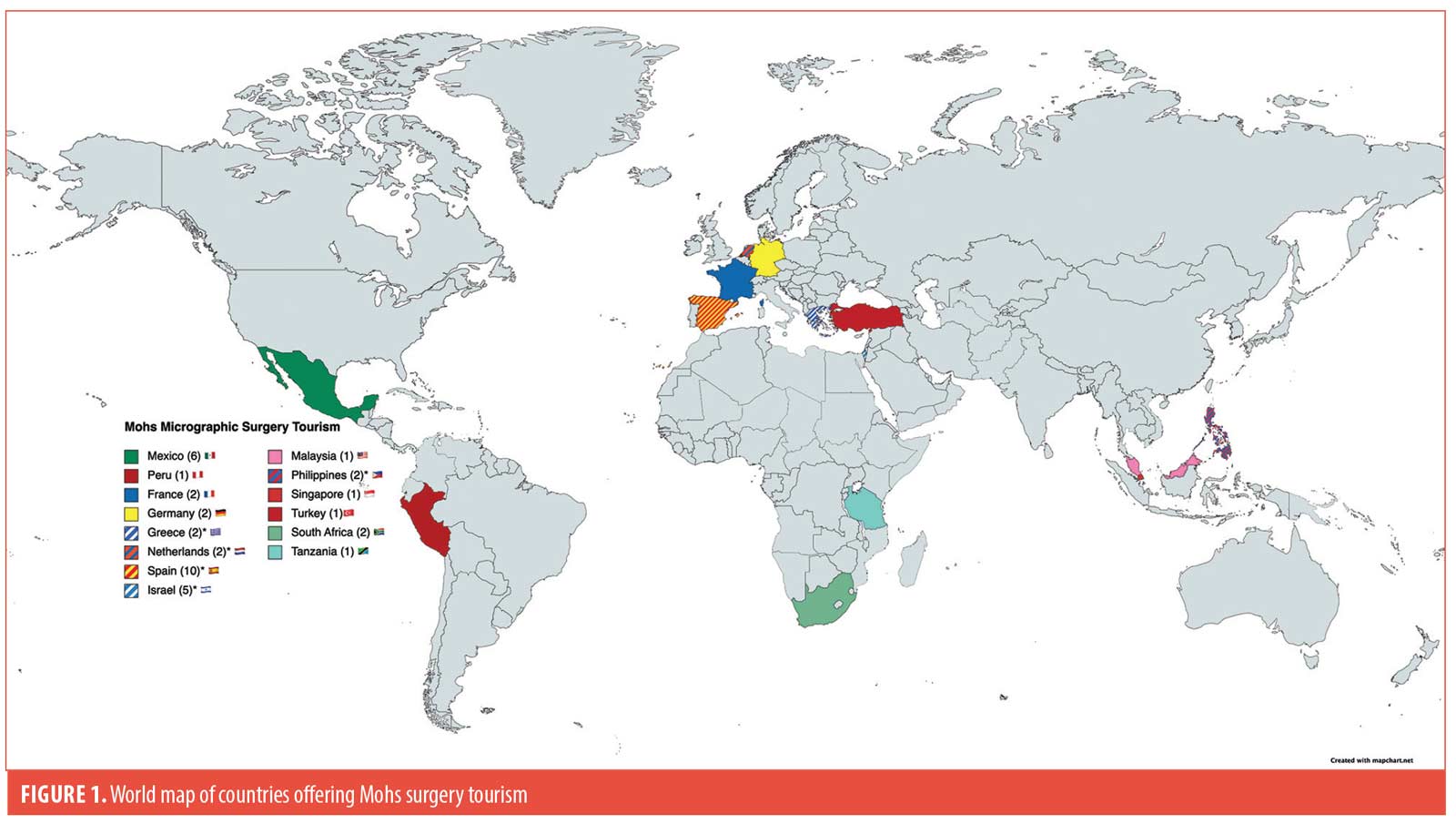
A total of 14 countries (Figure 1) outside of the United States and Canada with 42 centers offering Mohs surgery for international patients were identified. Countries with the greatest number of centers advertising Mohs surgery were Spain (n=10) and Mexico (n=6). Spain, Netherlands, Greece, Israel, and Philippines have Mohs surgeons recognized by ACMS (n=5) or ESMS (n=23) offering treatment to international patients.
Mohs surgery is practiced worldwide with multiple centers around the world offering the procedure to international patients. Verifying the credentials of physicians offering Mohs surgery abroad from internet searches may not be possible.
With regard,
by Juan Pinto-Cuberos, MD; Frank Winsett, MD; Andrew Armenta, MD; and Richard F. Wagner Jr, MD
Affiliations. All authors are with the Department of Dermatology at the University of Texas Medical Branch in Galveston, Texas.
Funding. No funding was provided for this article.
Disclosures. The authors report no conflicts of interest relevant to the content of this article.
References:
- American College of Mohs Surgeons 2022, American College of Mohs Surgeons website. Accessed October 8,2022. https://mohscollege.org.
- American Board of Dermatology 2022, American Board of Dermatology website. Accessed October 8, 2022. https://abderm.org.
- European Society for Micrographic Surgery, European Society for Micrographic surgery website. Accessed October8 2022. https://esms-mohs.eu.
- The Australasian College of Dermatologist 2022, The Australasian College of Dermatologist website. Accessed October 8, 2022. https://dermcoll.edu.au.
- American Society for Mohs Surgery 2022, American Society for Mohs Surgery website. Accessed October 8, 2022. https://mohssurgery.org.
Growth in the Cost of Biologics in Medicare Beneficiaries, 2017 to 2019
Dear Editor:
A study by Singh et al1 in 2019 found significant price increases for Medicare beneficiaries from 2013 to 2016 for the biologics adalimumab, etanercept, infliximab, ustekinumab, and abatacept; however, data on interleukin (IL)-12/23, IL-17, and IL-23 inhibitors were not evaluated.1 Therefore, we explored trends in the Medicare Part D spending and prescription patterns from 2017 to 2019 for 11 biologics that are approved by the United States (US) Food and Drug Administration for psoriasis: etanercept, infliximab, adalimumab, certolizumab pegol, ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab, tildrakizumab, and risankizumab.
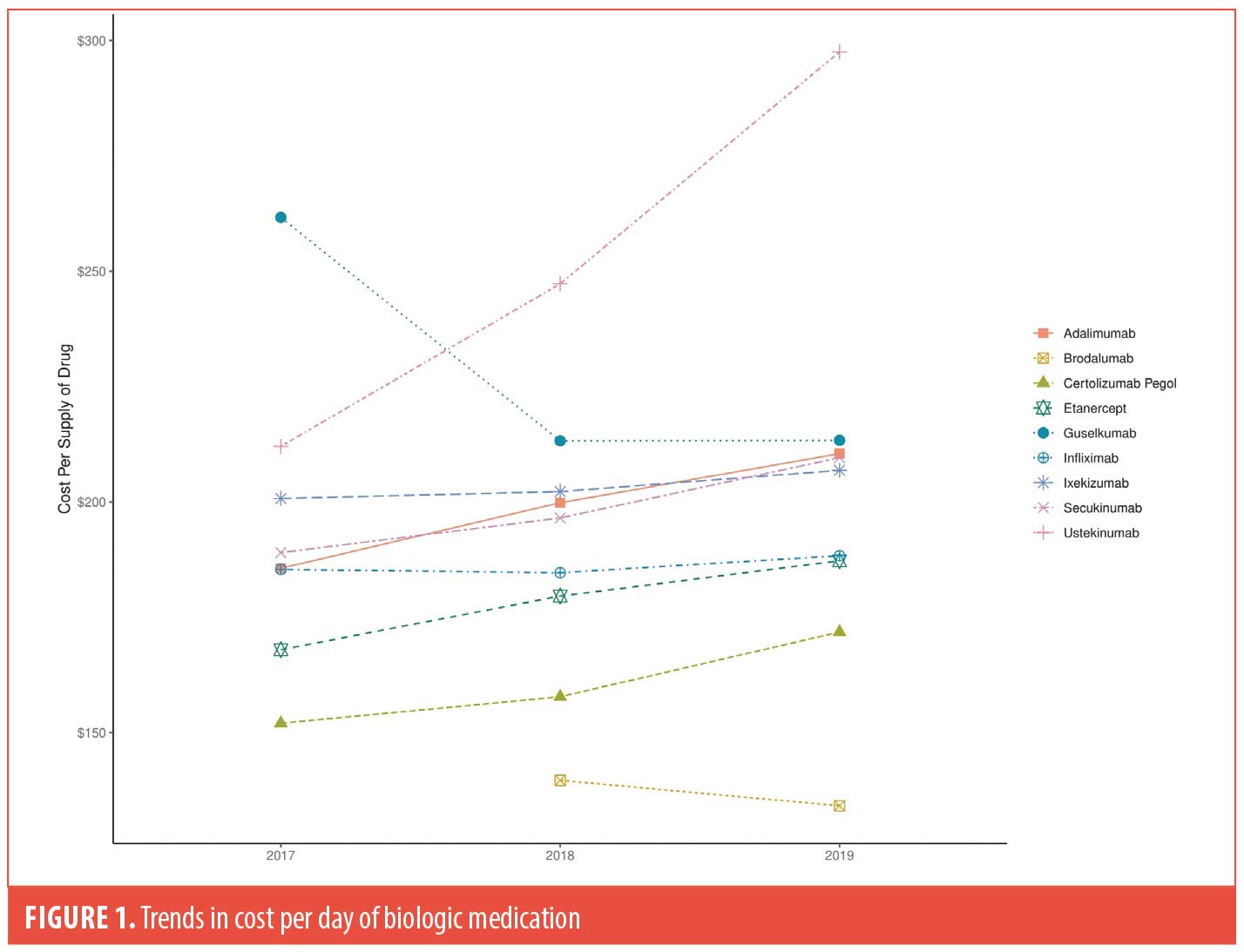
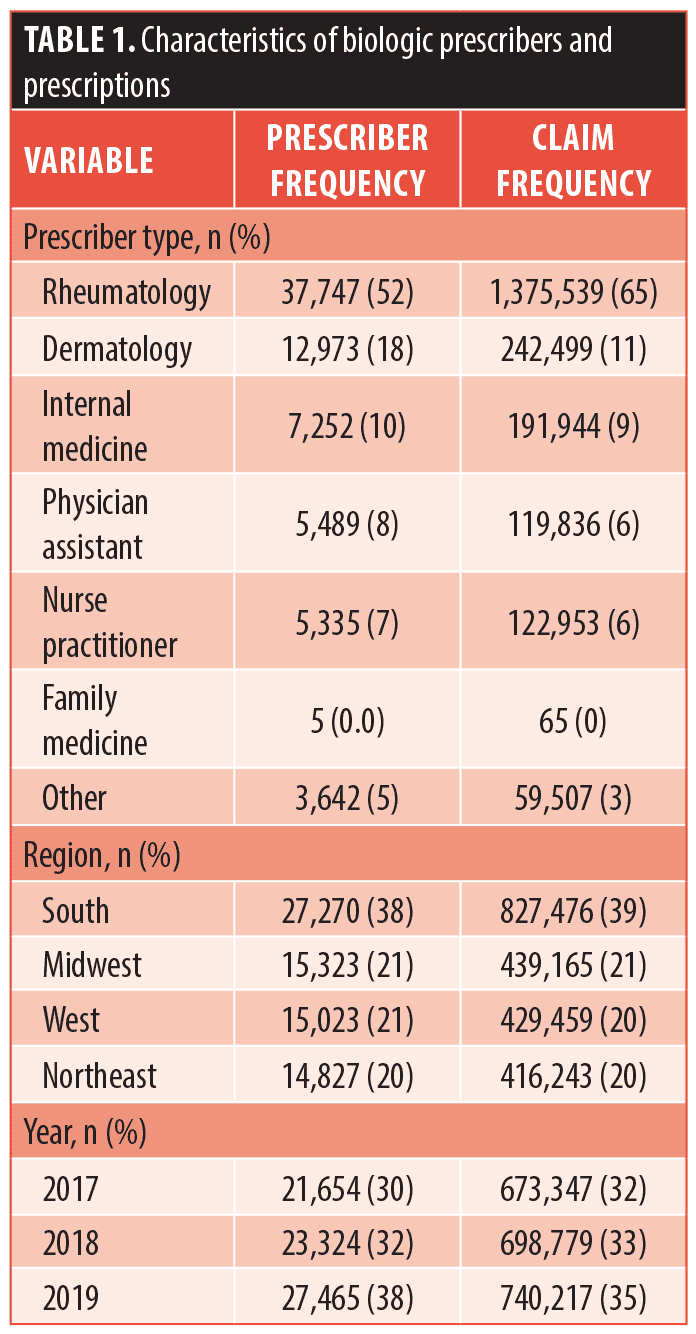
Methods. The Centers for Medicare and Medicaid Services (CMS) Part D Public Use Files from 2017 to 2019 were utilized to examine trends in cost per biologic, including biosimilars for infliximab (infliximab-dyyb and infliximab-abda).2 Prescriber type and geographic region were also analyzed (Table 1). The mean drug cost per day of medication was calculated as the cost of dispensed drug divided by the aggregate number of day’s supply dispensed. Prices were adjusted for inflation to 2019 US dollars using the Bureau of Labor Statistics Consumer Price Index.3 Trends in costs for each biologic were compared across the calendar years (Figure 1).
Results. We found rheumatologists (52.1%) to be the most frequent prescribers of biologics, followed by dermatologists (17.9%). The number of prescribers continued to increase each year between 2017 and 2019, with most prescribers located in the southern states. Annualized growth of expenditure for the biologics were as follows: brodalumab (-4.0%), guselkumab (-9.7%), infliximab (0.4%), ixekizumab (1.5%), secukinumab (5.3%), etanercept (5.6%), certolizumab pegol (6.3%), adalimumab (6.5%), and ustekinumab (18%). Guselkumab was initially the most expensive biologic drug for psoriasis in 2017 but was overtaken by ustekinumab in 2018, whereas brodalumab and certolizumab pegol were the least expensive. Furthermore, cost analysis per prescriber type revealed that family medicine physicians, nurse practitioners, and physician assistants were most impacted by the rising costs (Table 1).
Discussion. In this study, we observed price increases for infliximab, ixekizumab, secukinumab, etanercept, certolizumab pegol, adalimumab, and ustekinumab in increasing order as well as price decreases for guselkumab and brodalumab in decreasing order. The rapid price increase of ustekinumab may be due to the drug being double the price for 90mg compared to 45mg and shortening of the usual interval to eight weeks for many patients who have recurrence of psoriasis in the last weeks before their next planned injection.4 However, we predict a decrease in its price in the coming years, given the US patent for ustekinumab expired in 2023, and biosimilars will be available soon.5 In contrast, the cost of guselkumab (approved in July 2017) rapidly declined from 2017 to 2019, likely driven down by the approval of two other IL-23 inhibitors. Compared to CMS files from 2013 to 2016, annualized growth rates from 2017 to 2019 have declined for adalimumab, etanercept, and infliximab. This may in part be due to biosimilars being approved for the aforementioned drugs during those years.
Limitations. Regarding study limitations, our data was restricted to Medicare Part D enrollees only, and our analysis focused on the list prices of drugs without accounting for manufacturer rebates and other discounts. In addition, it is not possible to determine the indication for each prescription given biologics can have multiple indications. Future studies in a larger diverse population including non-Part D Medicare enrollees are warranted to better understand these trends and navigate such cost barriers.
With regard,
by Jennifer Laborada, MD; Leah Shin, MD; Claudia Lee, MD; Shahin Shahsavari, BS; Alexander Egeberg, MD, PhD, DMSc; and Jashin J Wu, MD
Affiliations. Drs. Laborada and Lee are with the University of California Riverside School of Medicine in Riverside, California. Dr. Shin is with Loma Linda University School of Medicine in Loma Linda, California. Mr. Shahsavari is with the Geisel School of Medicine at Dartmouth College in Hanover, New Hampshire. Dr. Egeberg is with the Department of Dermatology at Bispebjerg Hospital in Copenhagen, Denmark. Dr. Wu is with the Department of Dermatology at University of Miami Miller School of Medicine in Miami, Florida.
Keywords. Biologics, cost, healthcare spending, immunomodulators, medicare, psoriasis
Funding. No funding was provided for this article.
Disclosures. Dr. Laborada, Dr. Shin, Dr. Lee, Mr. Shahsavari, and Dr. Egeberg report no conflicts of interest. Dr. Wu is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health (Ortho Dermatologics), Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, and Zerigo Health.
References:
- Singh P, Silverberg JI. Growth in the cost of biologics in Medicare beneficiaries, 2013 to 2016. J Am Acad Dermatol. 2019;80(1):
281–282. - Centers for Medicare and Medicaid Services website. Medicare provider utilization and payment data: part D prescriber. https://www.cms.gov/Research-Statistics-Data- and-Systems/Statistics-Trends-and-Reports/Medicare-Provider- Charge-Data/Part-D-Prescriber.html. Accessed November 1, 2023.
- CPI Inflation Calculator. U.S. Bureau of Labor Statistics. https://www.bls.gov/data/inflation_calculator.htm. Accessed November 1, 2023.
- Savage LJ, Wittmann M, McGonagle D, et al. Ustekinumab in the Treatment of Psoriasis and Psoriatic Arthritis. Rheumatol Ther. 2015;2(1):11–16.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in pediatric patients (≥ 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183(4):664-672.