
J Clin Aesthet Dermatol. 2023;16(2):11–13.
Atopic Dermatitis Therapeutic Algorithm of JAK-Inhibitors and IL-4/IL-13 Antagonists Utilizing Patient Profiles and Drug Efficacy
Dear Editor:
Atopic dermatitis (AD) is a chronic inflammatory disease that has increased in prevalence in the last 50 years and affects up to 20 percent of children and 3 percent of adults worldwide, and approximately 11 percent of children and 7 percent of adults in the United States.1
The pathophysiology of AD is likely multifactorial and includes a complex interaction between barrier dysfunction, genetics, allergens, microbiome, and immune dysregulation. Epidermal structural integrity may be compromised due to mutations in the filaggrin protein that allows for environmental antigen presentation and activation of the Th2-mediated immune response. The downstream effects of inflammatory cytokines and JAK/STAT molecular pathway activation have become more recently understood.
Previously, the mainstay of treatment for mild to moderate atopic dermatitis included emollients, topical corticosteroids, and topical calcineurin inhibitors. The treatment for moderate-to-severe atopic dermatitis included phototherapy, systemic steroids, systemic immunosuppressive medications, and methotrexate. Moderate-to-severe atopic dermatitis unresponsive to these first- and second-line treatments can be debilitating due to unresolved pruritis and skin lesions. Recent advances in the understanding of the underlying pathophysiology of atopic dermatitis has led to the development and approval of breakthrough medications including dupilumab, tralokinumab, abrocitinib, and upadicitinib. dupilumab and tralokinumab are injectable monoclonal antibodies which target the interleukin (IL)-13 and IL-4 pathways, while abrocitinib and upadacitinib are oral JAK inhibitors.2-5 As each of these medications have been approved for use in atopic dermatitis, the understanding of their comparative efficacy and best patient profiles for use are not well documented in the literature. Therefore, providers may not be adequately treating patients with moderate-to-severe atopic dermatitis. The aim of developing this treatment algorithm was to review standardized efficacy data and offer practice guidelines which can be utilized to select patients who would be the ideal candidates for specific therapies.
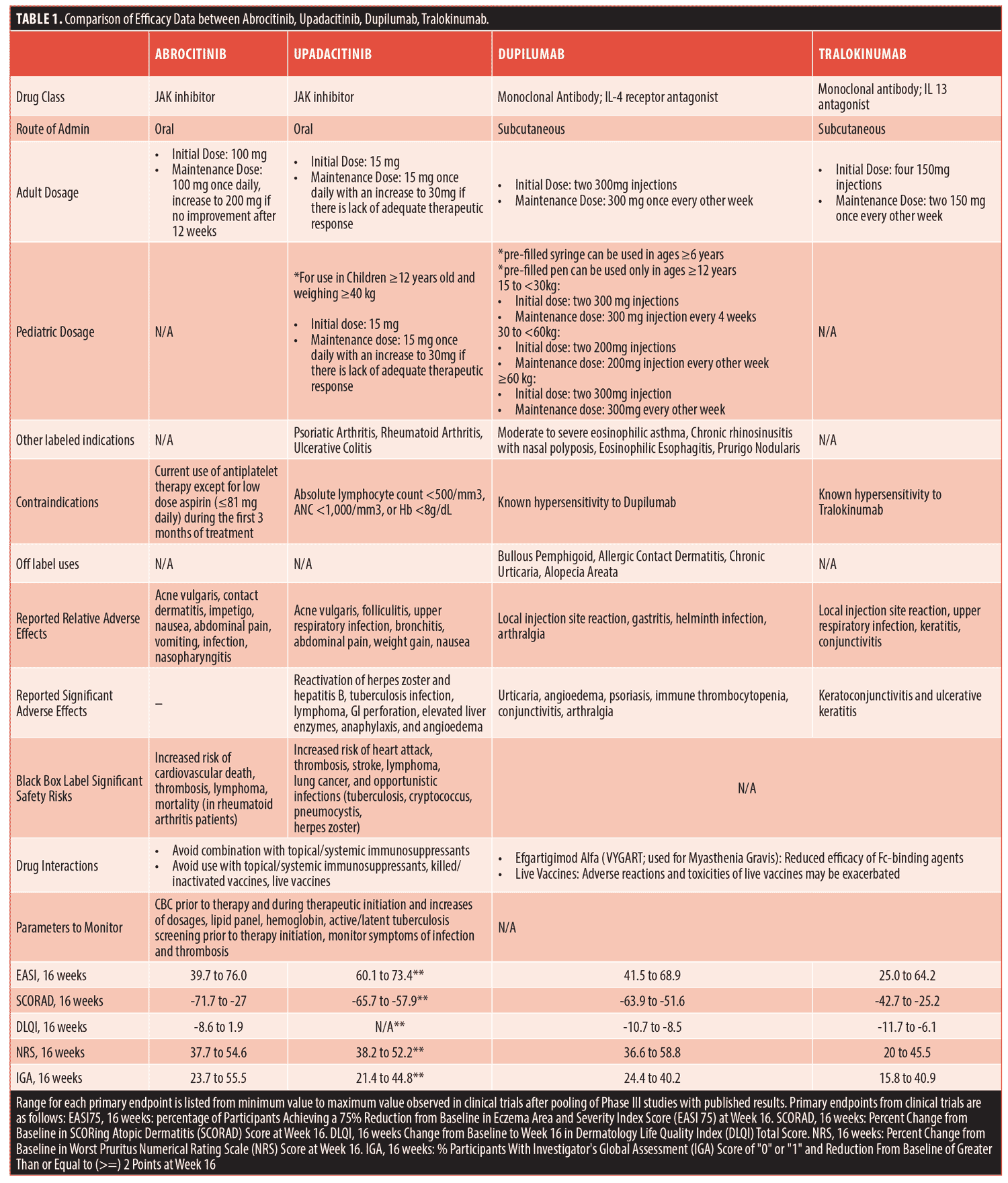
Published Phase III clinical trial data for each therapy was reviewed for pertinent information. The endpoints compare clinical trial treatment efficacies percentage of participants achieving a 75-percent reduction from baseline in Eczema Area and Severity Index score (EASI-75) at Week 16, percent change from baseline in SCORing atopic dermatitis (SCORAD) score at Week 16, change from baseline to 16 weeks total score of Dermatology Life Quality Index (DLQI), percent change from baseline in worst Pruritus Numerical Rating scale (NRS) score at Week 16, and percentage of participants with Investigator’s Global Assessment (IGA) score of “0” or “1”, and reduction from baseline of greater than or equal to 2 Points at Week 16.
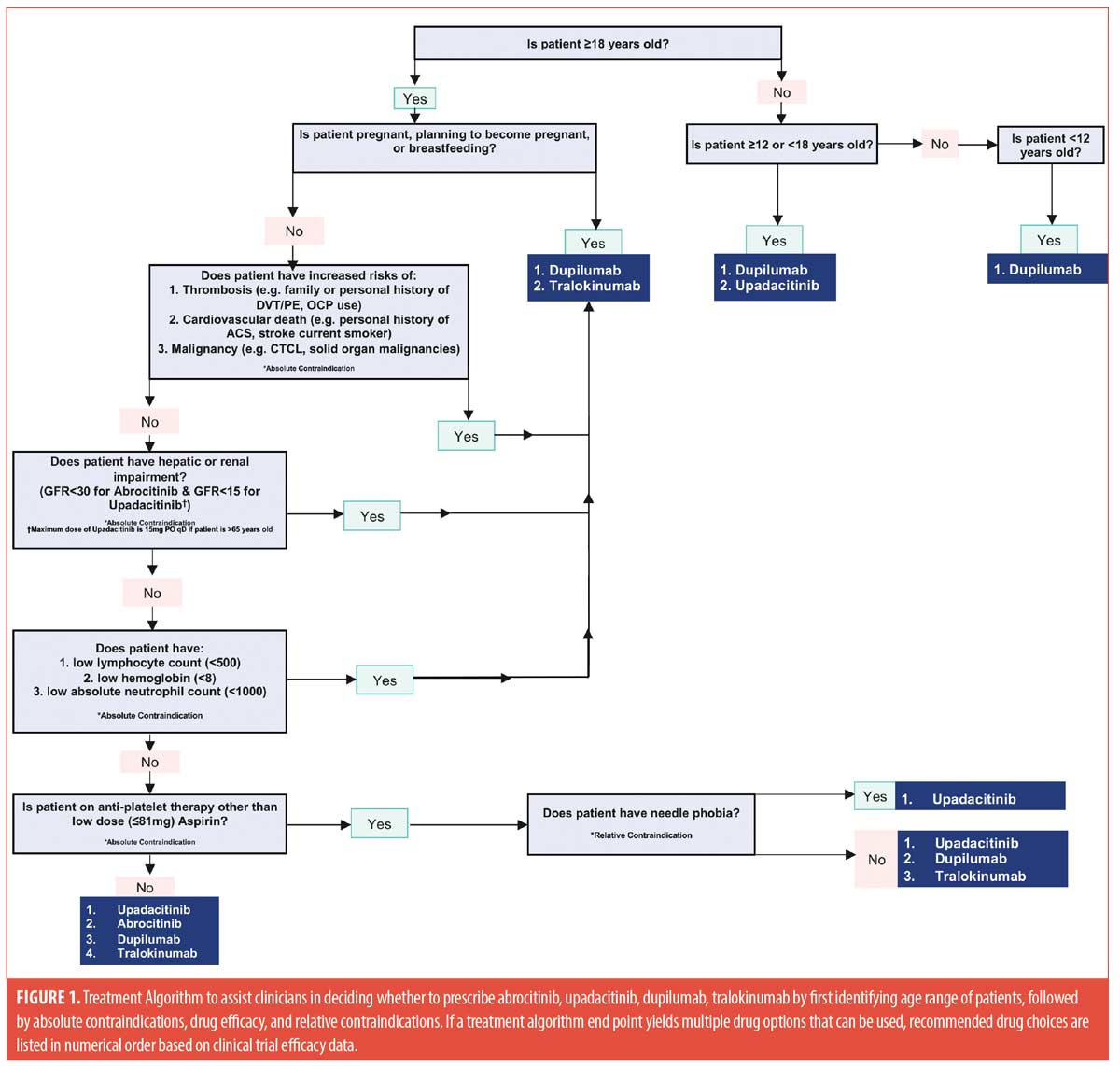
We acknowledge the utility of oral JAK inhibitors for AD treatment in lieu of monoclonal antibodies, but want to highlight the importance of adequately screening patients to tailor treatment regimens for optimal outcomes. We recommend using screening questions that identify patients at increased risks of adverse side effects to determine which therapy to use. Prior screening questions should assess patients’ age, smoking status, history of deep vein thrombosis or pulmonary embolism, contraceptive use/pregnancy plans, TNF-alpha-inhibitor use, personal/family history of cancer, history of prior opportunistic infections, as well as presence of comorbidities, including: ulcerative colitis, psoriatic arthritis, rheumatoid arthritis, eosinophilic asthma, and chronic rhinosinusitis with nasal polyps.
Figure 1 displays a decision tree that combines clinically useful outcome data, along with the above mentioned screening questions, to help support clinicians in deciding which therapy to prescribe. Table 1 highlights and compares key clinical prescribing information and Phase III clinical trial efficacy information regarding the four approved medications for atopic dermatitis.
The last several years have rapidly changed the way atopic dermatitis can be treated and managed. Therefore, it is critical for clinicians to be acutely aware of both the advantages and disadvantages of these novel atopic dermatitis therapeutics to practice optimal patient-centered, personalized treatment plans.
With regard,
Adel Haque, MD; Fahad Ahmed, BA; and Amir Amanullah, BS
Affiliations. Dr. Haque is with the Department of Medicine at Jefferson Health Northeast in Philadelphia, Pennsylvania. Mr. Ahmed is with the Department of Dermatology at Perelman School of Medicine at the University of Pennsylvania in Philadelphia, Pennsylvania. Mr. Amanullah is with the Department of Dermatology at Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania.
Keywords. Atopic dermatitis, JAK inhibitor, monoclonal antibody, practice guidelines.
Funding. No funding was provided for this article.
Disclosures. The authors report no conflicts of interest relevant to the content of this article.
References
- Avena-Woods, C. Overview of Atopic Dermatitis. American Journal of Managed Care. 2017.23(8): p. S115–S123.
- Munera-Campos, M. Carrascosa JM. Innovation in Atopic Dermatitis: From Pathogenesis to Treatment. Actas Dermo-Sifiliograficas. 2020. 111(3): p. 205–221.
- Chan, S.S. Biologics in atopic dermatitis. Current Opinion in Allergy and Clinical Immunology. 2021.21(3): p. 297–302.
- Deeks, ED and Duggan S. Abrocitinib: First Approval. Drugs. 2021. 81(18): p. 2149–2157.
- Guttman-Yassky E, Teixeira HD, Simpson EL et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double- blind, randomised controlled phase 3 trials. Lancet. 2021. 397(10290): p. 2151–2168.

