Dear Editor:
We are writing to express our deep concerns regarding the accuracy and/or scientific integrity of a paper published in the August 2019 issue of The Journal of Clinical and Aesthetic Dermatology titled “Safe and Efficacious Use of Secretome From Fibroblasts and Adipose-derived (but not Bone Marrow–derived) Mesenchymal Stem Cells for Skin Therapeutics.” The title alone is alarming and contrary to the consensus view of the academic community devoted to stem cell research. After careful review of each of the paper’s 230 citations and the inferences the author has drawn therefrom, it is our conclusion that the subject paper misreports or misinterprets the actual findings of numerous of its referenced citations and shows systemic bias in the selection of references (Table 1).
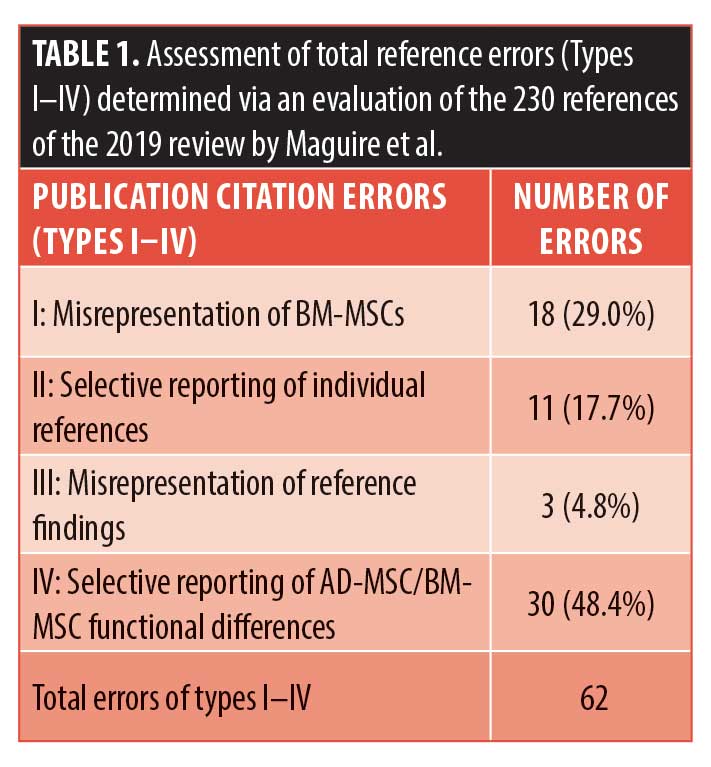 Five fundamental types of errors were identified, including 1) conflating studies of hematopoietic stem cells with those using bone marrow-derived stem cells (BM-MSCs), displaying a fundamental misunderstanding of stem cell niches;1–3 2) selective reporting of findings supporting the authors’ thesis while ignoring contrary evidence within the same reference; 3) invoking references that plainly don’t support statements made in text; and 4) biased reporting of functional characteristics/effects in favor of adipose-derived MSCs (AD-MSCs) (even if BM-MSCs have been shown to have the same function/attribute) or biased reporting of negative attributes of BM-MSCs when AD-MSCs have been shown to have the same functional characteristic.
Five fundamental types of errors were identified, including 1) conflating studies of hematopoietic stem cells with those using bone marrow-derived stem cells (BM-MSCs), displaying a fundamental misunderstanding of stem cell niches;1–3 2) selective reporting of findings supporting the authors’ thesis while ignoring contrary evidence within the same reference; 3) invoking references that plainly don’t support statements made in text; and 4) biased reporting of functional characteristics/effects in favor of adipose-derived MSCs (AD-MSCs) (even if BM-MSCs have been shown to have the same function/attribute) or biased reporting of negative attributes of BM-MSCs when AD-MSCs have been shown to have the same functional characteristic.
Selection bias, a fifth type of error, was determined by searching the National Library of Medicine for published works that would be expected to be included in a balanced review of the subject matter by an expert. As the headline conclusion of the subject paper unfavorably compares BM-MSCs and AD-MSCs regarding issues of oncogenesis, we then narrowed the search to papers invoking such concerns.
There are several academic expert reviews that directly compare the universe of studies where BM-MSCs and AD-MSCs are directly compared in terms of promotion of tumor size or metastatic events. Lee et al4 recently published a review comparing stem cells of different niche sources in terms of antiproliferative (antitumor) effects on hematologic malignancies. The authors found only one study where AD-MSCs showed a positive effect. By contrast, there were eight studies showing antitumor the characteristics of BM-MSCs. An earlier review by Klopp et al5 also compares various niches in terms of tumor size and metastases, with a preponderance of studies favorable to the safety profile of BM-MSC in contrast with AD-MSC. These reviews and their source materials are conspicuously absent from the Maguire review. The selection bias here is glaringly evident and the conclusions to be drawn from a de novo review stand in stark contradistinction to the thesis, and title, of the subject paper.
The subject matter is one of particular sensitivity. The very title “The Safe and Efficacious Use of Secretome From Fibroblasts and Adipose-derived (but not Bone Marrow–derived) Mesenchymal Stem Cells for Skin Therapeutics” makes a direct implication that the secretome of MSCs from bone marrow are unsafe. In this era of easy internet access to scientific journals, members of the public could be influenced in their medical decision-making. Because MSCs of bone marrow origin are a therapy that is being employed in numerous clinical trials for a wide variety of conditions, the misinformation in this publication could have unforeseen untoward consequences. The ratio of clinical trials (ongoing and completed) where the treatment is derived from BM-MSCs is three times the number using AD-MSCs.6,7 One can contemplate the negative impact of this paper, and its title, on medical decision-making by nonexpert members of the public.
This paper presents as its sole disclosure: “Dr. Maguire is a part-owner of NeoGenesis, Inc.” Upon examination of that company’s website, it appears that Dr. Maguire’s company sells product(s) …derived from fibroblasts and adipose-derived (but not bone marrow–derived) mesenchymal stem cells. The very title of the paper thereby creates a marquee for the author’s product sales. He even references a competitor to make it clear that his intent is to gain advantage in the marketplace.
We assert with confidence that there is ample evidence that the aforementioned review, authored by Maguire, titled “Safe and Efficacious Use of Secretome From Fibroblasts and Adipose-derived (but not Bone Marrow–derived) Mesenchymal Stem Cells for Skin Therapeutics,” contains numerous examples of misrepresentation of scientific references in-text, selective reporting of scientific findings, and a profound degree of bias to create a polemic against BM-MSCs in favor of AD-MSCs.
Polemics have no place in peer-reviewed scientific journals. They are anathema to the ethics of scientific publishing. Scientific misinformation has real-world consequences. We all need to be cognizant that “science is a community built on trust; therefore, it is the responsibility of everyone to foster and promote a culture of scientific integrity.”8 In our opinion, this paper should be retracted.
With regard,
Jonathan R. T. Lakey, PhD, MSM; Samuel Rodrigues, BSc, and Michael Alexander, MSc
Affiliations. Dr. Lakey is with the Department of Surgery and the Department of Biomedical Engineering, University of California Irvine in Orange, California. Mr. Rodrigues and Mr. Alexander are with the Department of Biomedical Engineering, University of California Irvine in Orange, California.
Funding. No funding was provided for this study.
Disclosures. The authors have no conflicts of interest relevant to the content of this article.
Correspondence.
Jonathan R. T. Lakey, PhD, MSM; Email: jlakey@uci.edu
References
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74.
- Mogharbel BF, Abdelwahid E, Irioda AC, et al. Bone marrow–derived stem cell populations are differentially regulated by thyroid or/and ovarian hormone loss. Int J Mol Sci. 2017;18(10):2139.
- Gil-Sanchis C, Cervello I, Khurana S, et al. Contribution of different bone marrow-derived cell types in endometrial regeneration using an irradiated murine model. Fertil Steril. 2015;103(6):1596–1605. e1591.
- Lee MW, Ryu S, Kim DS, et al. Mesenchymal stem cells in suppression or progression of hematologic malignancy: current status and challenges. Leukemia. 2019;33(3):597–611.
- Klopp AH, Gupta A, Spaeth E, et al. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29(1):11–19.
- United States Food and Drug Administration. Clinical Trials.gov search. Available at: https://clinicaltrials.gov/ct2/results?term=bone+marrow+ mesenchymal+stem+cells&Search=Apply
&recrs=a&recrs=f&recrs=d&recrs=e&age_v=&gndr=&type=&rslt=. Accessed November 5, 2019. - United States Food and Drug Administration. ClinicalTrials.gov search. Available at: https://clinicaltrials.gov/ct2/ results?term= adiposederived+mesenchymal+stem+cells &Search= Apply&recrs=a&recrs= f&recrs=d&recrs=e&age v=&gndr= &type=&rslt=. Accessed November 5, 2019.
- Kretser A, Murphy D, Bertuzzi S, et al. Scientific integrity principles and best practices: recommendations from a scientific integrity consortium. Sci Eng Ethics. 2019;25(2): 327–355.
Author Response
The consensus in 2020, according to physician-scientists trained in oncology and dermatology, is that “adipose mesenchymal stem cells (ADSCs) have generated great interest and are perceived as the most preferred cell type for tissue engineering and regenerative medicine.”1 My recent review in another medical journal highlighted the dangers of bone marrow (BM) stem cell transplants that contain BM-derived MSCs (BMSCs).2 The safety of fat transplants that contain adipose mesenchymal stem cells (ADSCs), fibroblasts (FBs), and adipocytes is evidenced by many formalized clinical studies.3 In my original article, I used great care to search, review, and interpret the available data fairly and, like Conese et al,1 engaging in polemics was not the intended purpose when I described the superiority of ADSCs and FBs over BMSCs for use in therapeutic development in dermatology. Critically, when comparing experimental data of BMSCs to ADSCs from the same human donor, “ADSCs have a ‘younger’ phenotype,” according to stem cell scientists.4 Indeed, Burrow et al4 found that BMSCs have, among other negative attributes relative to ADSCs, an increased level of senescence in comparison with matched ADSCs. Senescent cells develop the senescence-associated secretory phenotype (SASP), a proinflammatory set of molecules wherein the local tissue effects of a SASP or specific SASP components have been found to be involved in a wide variety of age-related pathologies in vivo, such as hyperplastic diseases, including cancer. One of the many reasons why ADSCs are preferred compared to BMSCs is that ADSCs express a low level of major histocompatibility complex (MHC) Class I molecules and do not express MHC Class II or costimulatory molecules. Even the exosomes of BMSCs express MHC Class II proteins. These problems in BMSCs are amplified when using donor, allogeneic BMSCs that have been replicated many times, essentially aging the cells, during expansion to develop the therapeutic.
Lakey et al state that I conflate BMSCs with hematopoietic stem cells (HSCs). Perhaps the confusion arises because of the complexity of the BM niche. To be clear, the properties I discuss here are those of BMSCs, not HSCs. Because of the complexity, many BMSC phenotypes exist, including disease-causing phenotypes that are varied and hard to distinguish—a part of the problem inherent in using BMSC for therapeutic development. This complication, unlike that for ADSCs, includes recirculated cells, particularly recirculated cancer cells.6 The BM is a site of BMSCs that may differentiate into HSCs and recirculating blood cells that may differentiate into BMSCs.7,8,9 BMSCs are also found outside of the niche in peripheral blood and home into sites of injury and cancer tissue, where they are educated into becoming a pro-cancerous phenotype.10,11,12 Recirculated melanoma and myelogenous leukemia cells in BM interact with BMSCs to change the phenotype of the BMSC to one that is cancer-promoting by enhancing their proliferation, migration, and invasion and altering the production of proteins involved in the regulation of the cell cycle.13,14 Indeed, melanoma tumor cells start to disseminate to BM during the initial steps of tumor development.15 In patients with breast cancer, the detection of recirculated cancer cells that disseminated in BM predicts the recurrence of the cancer.16 Cancer cells can fuse with BMSCs and change their phenotype or release exosomes to change the phenotype of BMSCs to a cancer-promoting one.17,18 Indeed, breast tumor cells fuse spontaneously with BMSCs.19 This fusion might facilitate the exchange of cellular material from the cancer cell to the BMSC, rendering the fused cell more oncogenic.20 Further, others have observed the same result of this fusion and exchange of cellular material, which has been found to increase metastasis. For example, Li et al21 found that human hepatocellular carcinoma cells with a low metastatic potential exhibit a significantly increased metastatic potential following fusion with BMSCs in vitro and in xenograft studies. In the end, the BMSCs and their molecules/exosomes, having been conditioned by tumor cells, were found to increase the probability of cancer in human patients.22
Unlike in Lakey et al’s letter, in my original article, I fully disclosed my affiliation with NeoGenesis; however, Lakey’s own direct involvement with the company AnteAge, a manufacturer of skin care products that uses “stem and growth factor cytokines derived from human bone marrow,” should be clearly disclosed to the readers to frame the comments made by Lakey et al fairly and avoid any occlusion regarding Lakey’s own commercial interests. In 2019, I offered as a stem cell scientist and, in 2020, Conese et al as physician-scientists offered the readers data and interpretations so that they may form their own conclusions as to the superiority of ADSCs and FBs when compared with BMSCs in dermatological products and procedures.
With regard,
Greg Maguire, PhD
References
- Conese M, Annacontini L, Carbone A, et al. The role of adipose-derived stem cells, dermal regenerative templates, and platelet-rich plasma in tissue engineering–based treatments of chronic skin eounds. Stem Cells Int. 2020;2020:7056261.
- Maguire G. Transplanted stem cells survive a long time: do they make you sick? J Roy Soc Medicine. 2019;112(10):412–414.
- Raj S, Abu-Ghname A, Davis MJ, et al. Safety and regulation of fat grafting. Semin Plast Surg. 2020;34(1):59–64.
- Burrow KL, Hoyland JA, Richardson SM. Human adipose-derived stem cells exhibit enhanced proliferative capacity and retain multipotency longer than donor-matched bone marrow mesenchymal stem cells during expansion in vitro. Stem Cells Int. 2017;2017:2541275.
- Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: a link between cancer and age-related degenerative disease?. Semin Cancer Biol. 2011;21(6):354–359.
- Wolock SL, Krishnan I, Tenen DE, et al. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep. 2019;28(2):302–311.e5.
- Freisinger E, Cramer C, Xia X, et al. Characterization of hematopoietic potential of mesenchymal stem cells. J Cell Physiol. 2010;225(3):888–897.
- Tondreau T, Meuleman N, Delforge A, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23(8):1105–1112.
- Cardenas C, Kwon JY, Maeng YS. Human cord blood–derived CD133+/C-Kit+/Lin- cells have bipotential ability to differentiate into mesenchymal stem cells and outgrowth endothelial cells. Stem Cells Int. 2016;2016:7162160.
- Chong P, Selvaratnam L, Abbas AA, et al. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2011;30: 634–642.
- Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745.
- Sai B, Dai Y, Fan S, et al. Cancer-educated mesenchymal stem cells promote the survival of cancer cells at primary and distant metastatic sites via the expansion of bone marrow-derived-PMN-MDSCs. Cell Death Dis. 2019;10(12):941.
- Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25(11):1315–1321.
- Ma Z, Cui X, Lu L, et al. Exosomes from glioma cells induce a tumor-like phenotype in mesenchymal stem cells by activating glycolysis. Stem Cell Res Ther. 2019;10(1):60.
- Rocken M. Early tumor dissemination, but late metastasis: insights into tumor dormancy. J Clin Invest. 2010;120(6):1800–1803.
- Tjensvoll K, Nordgård O, Skjæveland M, et al. Detection of disseminated tumor cells in bone marrow predict late recurrences in operable breast cancer patients. BMC Cancer. 2019;19(1):1131.
- Terada N, Hamazaki T, Oka M. et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416(6880):542–545.
- Nakata R, Shimada H, Fernandez GE, et al. Contribution of neuroblastoma-derived exosomes to the production of pro-tumorigenic signals by bone marrow mesenchymal stromal cells. J Extracell Vesicles. 2017;6(1):1332941.
- Noubissi FK, Harkness T, Alexander CM, Ogle BM. Apoptosis-induced cancer cell fusion: a mechanism of breast cancer metastasis. FASEB J. 2015;29(9):4036–4045.
- Chitwood CA, Dietzsch C, Jacobs G, et al. Breast tumor cell hybrids form spontaneously in vivo and contribute to breast tumor metastases. APL Bioeng. 2018;2(3):031907.
- Li H, Feng Z, Tsang TC, et al. Fusion of HepG2 cells with mesenchymal stem cells increases cancer associated and malignant properties: an in vivo metastasis model. Oncol Rep. 2014;32(2):539–547.
- Medyouf H, Mossner M, Jann JC, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14(6):824–837.

