 J Clin Aesthet Dermatol. 2020;13(7):18–23
J Clin Aesthet Dermatol. 2020;13(7):18–23
by Sanjeewani Fonseka, MD, PhD; Chandrika N. Wijeyaratne, DM, MD; Indika B. Gawarammana, MD, PhD; Nishan S. Kalupahana, PhD; Shanthini Rosairo, MD; Neelakanthi Ratnatunga, MD, PhD; and Ranjith Kumarasiri, MD
Dr. Fonseka is with the Department of Pharmacology, Faculty of Medicine at the University of Peradeniya in Sri Lanka. Dr. Wijeyaratne is with the Department of Obstetrics and Gynocology, Faculty of Medicine at the University of Colombo in Sri Lanka. Dr. Gawarammana is with the Department of Medicine, Faculty of Medicine at the University of Peradeniya in Sri Lanka. Dr. Kalupahana is with the Department of Physiology, Faculty of Medicine at the University of Peradeniya in Sri Lanka. Dr. Rosairo is with the Department of Radiology, Faculty of Medicine at the University of Peradeniya in Sri Lanka. Dr. Ratnatunga is with the Faculty of Medicine at the University of Peradeniya in Sri Lanka. Dr. Kumarasiri is with the Faculty of Medicine at the University of Peradeniya in Sri Lanka.
FUNDING: This study was funded by the University Grants Commission, Ministry of Higher Education, Sri Lanka.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. The effectiveness of different combined oral contraceptive pills and metformin in reducing hirsutism in patients with polycystic ovary syndrome (PCOS) remains unclear.
Objective. We sought to determine the effects of ethinylestradiol (35ug)/cyproterone acetate (2mg) (EE/CPA) and ethinylestradiol (20ug)/desogestrel (0.15mg) (EE/DES), alone or with metformin, on hirsutism in PCOS.
Methods. A randomized, double-blind, triple-dummy study was conducted on women with PCOS and hirsutism (N=107) who received one of four drug combinations (Arm A: EE/CPA; Arm B: EE/DES; Arm C: EE/CPA plus metformin; or Arm D: EE/DES plus metformin). Hirsutism was assessed at baseline, six months, and 12 months by using five outcomes variables.
Results. No outcomes variable showed a significant difference between the four arms at 12 months. There was a significant reduction in both hair density and modified Ferriman-Gallwey score (mFGS) in Arm A, mFGS in Arm B, hair density in Arm C, and diameter of sideburn hair in Arm D, respectively. Separately, there was a significant increase noted in the hair growth rate of chin and an improvement in patients’ perceptions of hirsutism in all four study arms.
Conclusion. EE/CPA and EE/DES were equally effective in improving hirsutism in PCOS, with no added benefit from low-dose metformin. Sri Lanka Clinical Trials Registry (http://www.slctr.lk) registration no. SLCTR/2015/007.
Keywords: Hirsutism, polycystic ovary syndrome, cyproterone acetate, desogestrel, metformin, Ferriman-Gallwey score, hair growth rate, hair density, hair diameter
Polycystic ovary syndrome (PCOS) is a common disorder affecting women with diverse hormonal and metabolic abnormalities. It is characterized by irregular menstruation, hyperandrogenism, and polycystic ovaries. Hirsutism, the presence of male-pattern terminal hair growth in women, is an important clinical feature of hyperandrogenism with a major psychological impact on the quality of life in women.1 Pharmacological means of treating hirsutism include combined oral contraceptive pills (COCPs), antiandrogens (e.g., spironolactone, flutamide, finasteride), and insulin-sensitizing agents, such as metformin.2–7 Only a few studies to date have included hirsutism as the primary outcome in assessing the effectiveness of these therapies.
COCPs are considered the first-line treatment in managing hirsutism in women with PCOS who do not wish to become pregnant. Among the COCPs, progestins with less androgenic properties are considered superior to other contraceptives. However, there is a paucity of evidence to show the definitive superiority of one progestin over another in treating hirsutism, particularly among South Asians.8–11 Recent recommendations by the International PCOS Network suggest that all COCPs have equal efficacy in the treatment of hirsutism.12
Although the beneficial effects of metformin on the metabolic and reproductive derangements of PCOS are well-established, the impacts on hirsutism have not been adequately studied.8,13–15 However, one study has observed metformin to be as effective as COCPs in reducing the features of hirsutism.16 As patients with PCOS in the South Asian region commonly show features of metabolic syndrome, we decided to investigate the effects of COCPs with and without metformin on hirsutism.
The objective was to determine the effectiveness of ethinylestradiol/cyproterone (EE/CPA) and ethinylestradiol/desogestrel (EE/DES) both alone and in combination with low-dose metformin on hirsutism in women with PCOS.
Methods
Sample size. Based on previous studies, the sample size was calculated using the following formula:
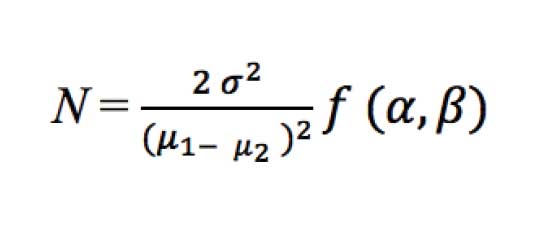 where sigma is the standard deviation and mu is the sample mean. The value of alpha was taken at the 95% confidence level and that of beta was taken at the 80% confidence level.
where sigma is the standard deviation and mu is the sample mean. The value of alpha was taken at the 95% confidence level and that of beta was taken at the 80% confidence level.
According to the calculation, a sample size of 100 patients in each arm was planned. During the trial, an interim analysis was conducted by an independent statistician to ascertain the adequacy of the sample because of logistic reasons using the same formula as above. Accordingly, a sample size of 25 patients in each arm was considered adequate and patient recruitment was stopped when that target was reached.
Study population. A total of 157 patients attending medical and gynecology clinics at teaching hospitals in Kandy and Peradeniya, Sri Lanka were assessed for eligibility and 107 were recruited. Eligible subjects included those aged 18 to 40 years with PCOS diagnosed in accordance with the 2003 Rotterdam Consensus Conference criteria with a modified Ferriman-Gallway score (mFGS) of hirsutism of eight points or more. Patients with secondary causes of hyperandrogenism, significant comorbidities such as diabetes, contraindications to the use of trial drugs, or who were taking any form of hormonal contraceptives or antiandrogens three months prior to starting the study and seeking fertility were excluded. Ethical approval was obtained from the Institutional Ethics Review Committee of the Faculty of Medicine, University of Peradeniya, Sri Lanka (protocol no. 2013/EC/52). The trial was registered at the Sri Lanka Clinical Trials Registry (http://www.slctr.lk) and the registration number was SLCTR/2015/007.
Written informed consent was obtained from all subjects and the study was conducted in accordance with the Declaration of Helsinki. Patient recruitment commenced on April 1, 2015, and each patient was followed up for a period of one year.
Study design. A randomized, double-blind, triple-dummy, parallel-group study was conducted at the Department of Pharmacology, Faculty of Medicine at the University of Peradeniya in Sri Lanka.
Randomization and masking. Simple randomization to four study arms was completed using a computer-generated random number table. According to the list generated by a random number generator, a piece of paper indicating the study arm (A, B, C, or D) was placed inside serially-numbered, sealed, opaque envelopes. When a participant was recruited, the patient’s name, address, and telephone number were written on the envelope. Treatment was started according to the assigned arm (A, B, C, or D). All logistics were overseen by an administrator who was not involved in the rest of the study.
Subjects of all four arms were prescribed three drugs using appropriate placebos. The active tablets were removed from the commercial packings and placed in adhesive polythene bags. All the investigators, the statistician, and the involved patients were blinded to the treatment; the pharmacist who dispensed the drugs was not.
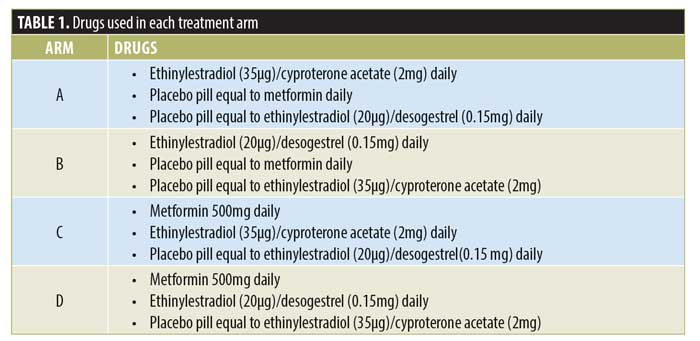
Treatment allocation. Treatment allocation for the four arms is shown in Table 1. Treatments used in the study were EE/CPA (Diane-35, Schering AG, Berlin, Germany), EE/DES (Fermion, Infar (India) Ltd, India), metformin (State Pharmaceuticals Manufacturing Corporation, Sri Lanka), and three placebo pills (State Pharmaceuticals Manufacturing Corporation, Sri Lanka) equal to the above three drugs.
Metformin or placebo was commenced on the day of recruitment and continued daily. The other two tablets or placebos were started on the first day of the menstruation and continued for 21 days, then resumed after a seven-day interval. Likewise, treatment was continued for 12 months and subjects were assessed according to the protocol detailed below.
Outcomes measures. Hair density, hair diameter, and hair growth rate were assessed at the beginning and at the end of six and 12 months. Patients were requested to abstain from shaving the area for a minimum period of five days before each assessment. Patients who had undergone other long-lasting depilatory maneuvers, such as electrolysis and laser hair removal, were not recruited into the study until at least three months after their procedure. During the study, they were instructed not to undergo either procedure; only shaving was allowed. Interobserver variability was minimized by having assessments performed by a single examiner who was blinded to the therapy.
Hair density was assessed using photographs of sideburns taken with a Nikon digital camera with a Nikon 60-mm f1.2.8 micro lens (Nikon Inc., Melville, New York). An area measuring 2×2 cm was marked just in front of the tragus of the ear. The photograph was opened with Microsoft Paint (Microsoft Corporation, Redmond, Washington) with 25% magnification and the number of hairs arising within the quadrangle was counted. Then, the hair density (number of hairs per cm2) was calculated.
Hair diameter was measured using plucked hair from sideburns and chin. Five hair strands from each site were mounted on a glass slide and fixed using a cover slip. The diameter of hair was obtained using an Olympus B53 microscope (Olympus Corporation, Tokyo, Japan) with a magnification power of 10. Images were projected onto a computer monitor screen and the hair diameter was measured using the software Cell Sense Entry (Olympus Corporation, Tokyo, Japan). Three diameters of the hair shaft were taken 100um apart, with the starting point being where the hair emerged from the skin. Those three diameters were added up and divided by three to get the mean diameter of a hair. Likewise, five hairs from each site were taken and the mean diameter of those five hairs were added and divided by five to get the final average diameter of a hair strand at a given anatomical site.
Hair growth rate was measured using five plucked hair strands (from the left and right sideburns and chin of each subject), which were fixed to a glass slide using transparent adhesive tape. The length of each hair was measured using digital Vernier calipers, which had the capacity to measure from 0 to 150mm. Hair lengths in millimeters were obtained and their results were averaged to produce one mean length. This value was divided by the shaving interval (in days) to calculate the hair growth rate (mm/day) of the left and right sideburns and chin.
Subjective assessment. The distribution and severity of hirsutism were assessed using mFGS. The inter-observer variability was minimized by having a single examiner who was blinded to the therapy conduct the assessments and intra-observer variability was minimized by using the same photographic scale of mFGS to score the hirsutism.
Patient’s perceptions were also assessed by a self-assessment of their status of hirsutism at baseline and the third, sixth, ninth, and 12th months of the study. This was estimated in a quantitative manner using a graded, linear, horizontal visual analog scale (VAS).
Statistical analysis. Statistical analysis was completed using the Statistical Package for the Social Sciences version 22 software program (IBM Corporation, Armonk, New York). Per-protocol analysis was perfornmed to find out the baseline group differences in completed subjects, within-group differences, and group differences from baseline to 12 months.
A two-way mixed analysis of variance (ANOVA) model for repeated measures was used during the analysis. Additionally, a one-way ANOVA and categorical data analytical methods were used wherever appropriate.
Before applying the two-way mixed ANOVA model, significant outliers in each variable were excluded. Leven’s and Mauchly’s tests were used to ensure the homogeneity and sphericity of the outcome variables, respectively. Where sphericity was not assured, the Greenhouse-Geisser or Huynh-Feldt test was used to calculate the p-values. Statistical significance was defined as p<0.05.
Results
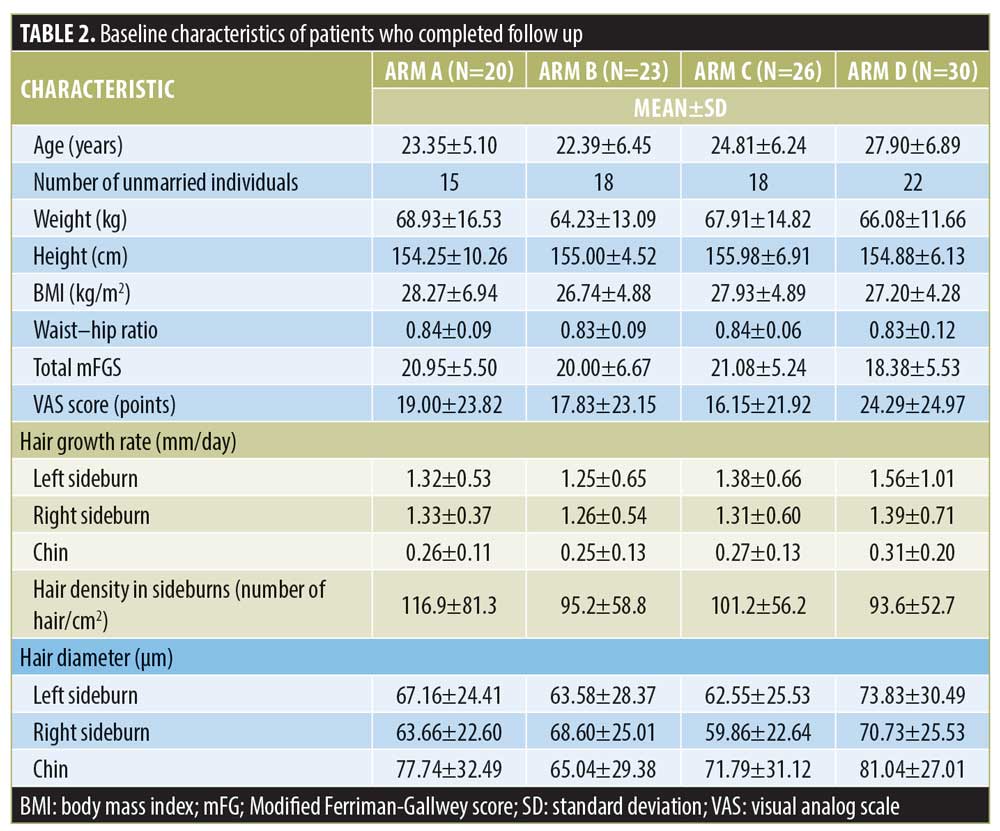
Baseline characteristics and follow-up. The summary of patient recruitment, follow-up, and analysis is shown in Figure 1. Baseline characteristics (Table 2) did not differ significantly except for age, which was higher in Arm D.
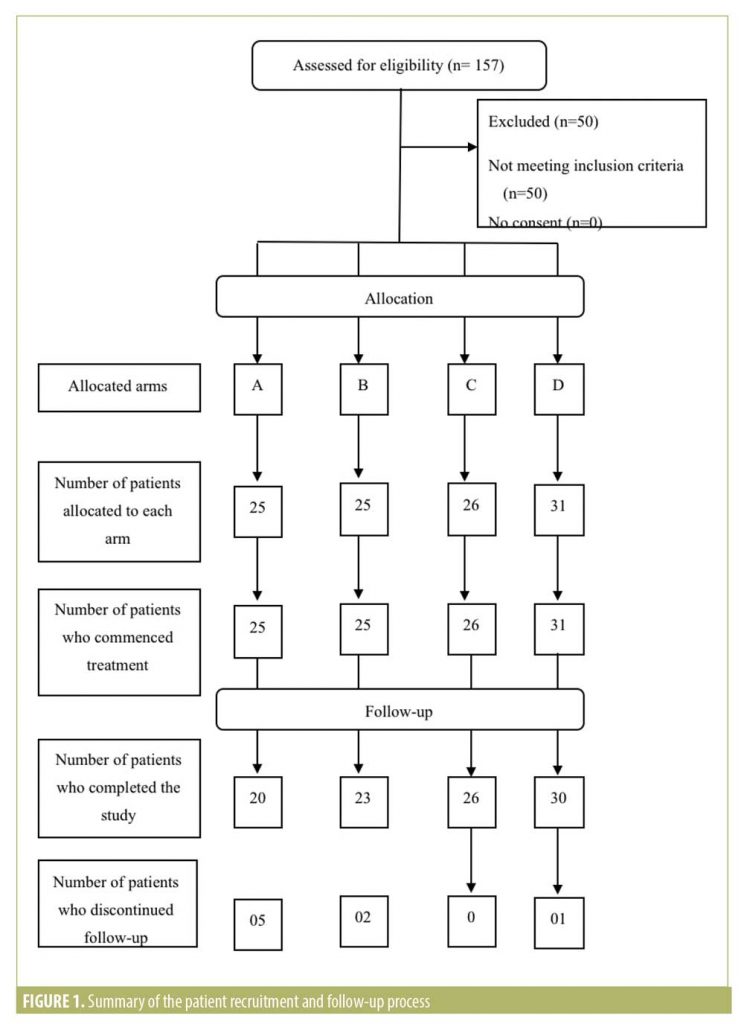
 Group A had five dropouts. All five patients had treatment-related adverse effects. Each patient had different adverse effects, which included migraine-type headache, joint stiffness, severe breast tenderness, vomiting, and a feeling of faintness. Group B had two dropouts. One patient was diagnosed with papillary carcinoma of the thyroid and the other wished to conceive. Group C had no dropouts. Group D had one dropout due to recurrent feeling of faintness.
Group A had five dropouts. All five patients had treatment-related adverse effects. Each patient had different adverse effects, which included migraine-type headache, joint stiffness, severe breast tenderness, vomiting, and a feeling of faintness. Group B had two dropouts. One patient was diagnosed with papillary carcinoma of the thyroid and the other wished to conceive. Group C had no dropouts. Group D had one dropout due to recurrent feeling of faintness.
Total mFGS. There was a reduction in mFGS in all four treatment arms but the difference was not statistically significant at the end of 12 months between the arms (p=0.479). However, a significant reduction in mFGS was observed in patients in treatment arms A (p=0.001) and B (p=0.010).
Changes of mFGS according to anatomical site. There were no statistically significant differences between the four treatment arms concerning anatomical sites at the end of 12 months. However, there were changes seen within the treatment arms at the end of 12 months (Table 3).
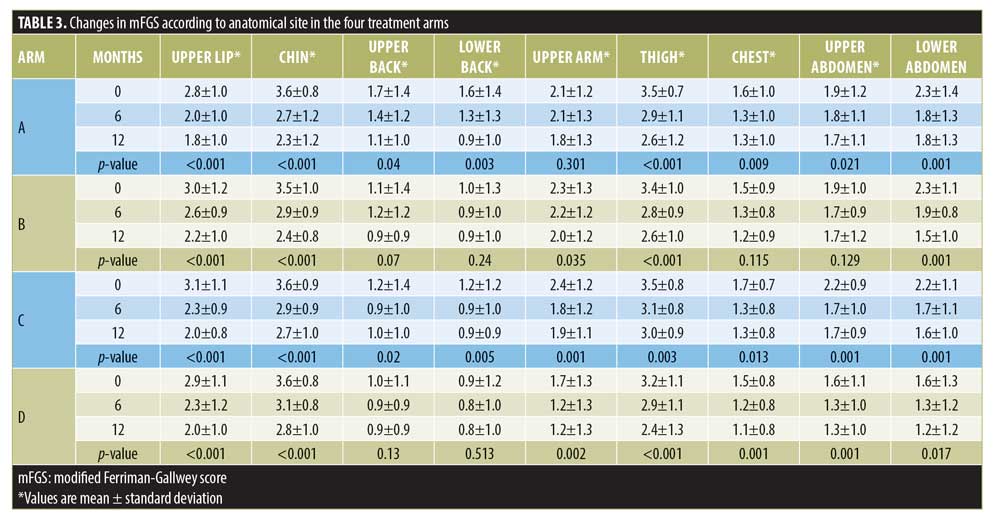
According to body areas, Arm A patients showed a persistent and significant reduction in mFGS in all body areas except the upper arms. In Arm B, the reduction of mFGS was significant over all body areas except the upper back, lower back, chest, and upper abdomen. In Arm C, the reduction of mFGS in all body areas was significant. In Arm D, the reduction of mFGS was significant over all the body areas except the upper and lower back.
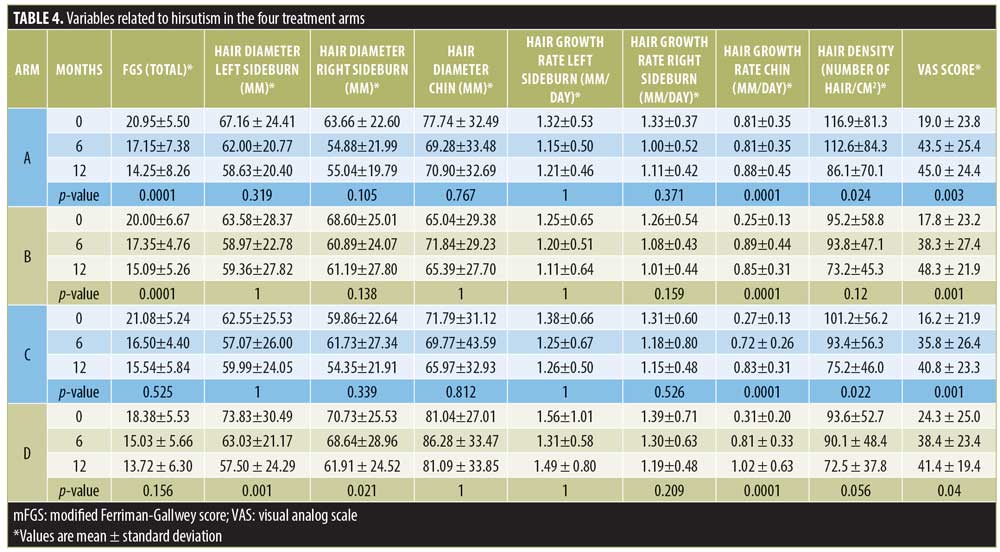
Hair density of sideburns. The hair density of the sideburns decreased in all four treatment arms with no significant difference found between four treatment arms (p=0.650). However, in treatment Arm A, there was a statistically significant reduction seen between the sixth- and 12th-month measurements (p=0.024). Treatment Arm C showed a statistically significant reduction over 12 months (p=0.022).
Hair diameter. There was a reduction in the hair diameter of hair from the left sideburn (p=0.249), right sideburn (p=0.452), and chin (p=0.553) in all four treatment arms, but the difference was not statistically significant at the end of 12 months between the different arms. However, a significant reduction in the hair diameter of the sideburns was seen among the patients of treatment Arm D (p=0.001).
Hair growth rate. The hair growth rate of the left and right sideburns in all four treatment arms did not show a significant difference (p=0.229 and p=0.229, respectively).
The hair growth rate of the chin did not exhibit a significant difference between treatment arms (p=0.417). However, within all four individual treatment arms, the hair growth rate of the chin was increased significantly (p<0.05).
VAS score for the perception of hirsutism. There was no variation between treatment arms in the VAS score for hirsutism (p=0.949). However, there was a significant improvement in hirsutism according to the VAS score at the end of 12 months in all four treatment arms (p<0.05).
Table 4 shows the summary of data on hirsutism.
Discussion
We assessed hirsutism using mFGS, hair density, hair growth rate, hair diameter, and VAS score for the perception of hirsutism. There was no significant difference between EE/CPA and EE/DES with or without metformin in relation to all parameters of hirsutism.
However, both EE/CPA and EE/DES, when given without metformin, triggered a significant reduction in the total mFGS at the end of 12 months within the respective treatment arms, indicating that metformin has had a surprisingly negative effect on the total mFGS.
Meanwhile, when EE/CPA or EE/DES were given alone, a significant reduction in mFGS was observed uniformly in the upper lip, chin, thighs, and lower abdomen, which are considered to be more responsive to androgens. The reduction in hair growth with EE/CPA alone and EE/CPA with metformin showed an almost identical distribution, suggesting that metformin has not had an additional benefit.
The reduction in the hair diameter of the sideburns was significant only when EE/DES plus metformin were given in combination with one another. It is noteworthy that the sideburns are not included in the formal mFG scoring and our findings suggest that hair growth in the sideburns of women may be related to insulin resistance.
The increase in the hair growth rate of the chin in all treatment arms is an unexpected finding. Our study confirms that there is no extra benefit by using COCPs containing CPA, which presents troublesome side effects including a higher cost.
Patients’ own perceptions of changes in hirsutism as assessed by VAS score experienced a significant improvement in all treatment arms, indicating that the interventions in the study led to a perceived improvement in hirsutism.
In summary, the effects of treatment on hirsutism parameters did not show a uniform change in any particular treatment arm. The total mFGS presented a significant reduction with EE/CPA and EE/DES alone, whereas the hair diameter of sideburns was reduced with EE/DES plus metformin. The hair density of sideburns was reduced with EE/CPA alone and with metformin. Interestingly, the hair growth rate of the chin was significantly increased in all the treatment arms.
Our findings in this study are consistent with the results of other studies in adult patients with PCOS and hirsutism showing that EE/DES and EE/CPA are equally effective in treating hirsutism.17–19 The reduction in hirsutism varies according to the duration of therapy but hirsutism can persist even after 60 cycles of treatment with EE/CPA.20
Certain studies have found EE/CPA to be superior to EE/DES in lowering hirsutism when used for 12 months.21 The reason could be that cyproterone acetate has more anti-androgenic properties than desogestrel. Of note, in our study, we found that both have similar effects on hirsutism. Meanwhile, recent guidelines discourage the use of EE/CPA as first-line treatment for hyperandrogenism in PCOS because of its relatively higher risk of causing venous thromboembolism.12
A moderate reduction in hirsutism has been shown with metformin therapy in certain studies but only a few to date have adopted hirsutism as a primary outcome measure.22 One such report observed a reduction in mFGS with metformin at the end of 14 months but the sample size was just 16 patients, which is not large enough to come to a definite conclusion.23
Another study comparing metformin 500mg three times per day with EE/CPA daily for 12 months showed that metformin is equally or more effective relative to EE/CPA in bringing down the quantitative and qualitative markers of hirsutism.2
In our study, adding metformin to both hormonal therapies did not yield any added benefit. One possible reason for this is the low dose of metformin used in this study.
The response to metformin therapy varies according to the BMI of patients with PCOS.24 A limitation of our study is that we did not analyze the effects of metformin according to the body mass index.
According to our findings, both EE/CPA and EE/DES were equally effective in reducing hirsutism as assessed by mFGS. Adding metformin 500 mg per day did not trigger a significant added advantage. A higher dose of metformin may be required to achieve a benefit.
Acknowledgments
We thank Jeevani Gunawardana, Indira Athauda, Sashika Dassanayake, and Tharanga Bandara for technical and logistic assistance.
References
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47.
- Harborne L, Fleming R, Lyall H, et al. Metformin or antiandrogen in the treatment of hirsutism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(9):4116–4123.
- Legro RS. Polycystic ovary syndrome: current and future treatment paradigms. Am J Obstet Gynecol. 1998;179(6 Pt 2):S101–S108.
- Bhattacharya SM, Jha A. Comparative study of the therapeutic effects of oral contraceptive pills containing desogestrel, cyproterone acetate, and drospirenone in patients with polycystic ovary syndrome. Fertil Steril. 2012;98(4):1053–1059.
- Venturoli S, Porcu E, Gammi L, et al. The effects of desogestrel and ethinylestradiol combination in normal and hyperandrogenic young girls: speculations on contraception in adolescence. Acta Eur Fertil. 1988;19(3):129–134.
- Falsetti L, Galbignani E. Long-term treatment with the combination ethinylestradiol and cyproterone acetate in polycystic ovary syndrome. Contraception. 1990;42(6):611–619.
- Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
- Martin KA, Chang RJ, Ehrmann DA, et al. Evaluation and treatment of hirsutism in premenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(4): 1105–1120.
- Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21(11):1291–1300.
- Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84(1):165–169.
- Teede HJ, Misso ML, Deeks AA, et al. Assessment and management of polycystic ovary syndrome: summary of an evidence-based guideline. Med J Aust. 2011;195(6):S65–S112.
- Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110(3):364–379.
- Diamanti-Kandarakis E, Economou F, Palimeri S, Christakou C. Metformin in polycystic ovary syndrome. Ann N Y Acad Sci. 2010;1205:192–198.
- Diamanti-Kandarakis E, Kouli C, Tsianateli T, Bergiele A. Therapeutic effects of metformin on insulin resistance and hyperandrogenism in polycystic ovary syndrome. Eur J Endocrinol. 1998;138(3):269–274.
- Cosma M, Swiglo BA, Flynn DN, et al. Clinical review: Insulin sensitizers for the treatment of hirsutism: a systematic review and metaanalyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1135–1142.
- Wu J, Zhu Y, Jiang Y, Cao Y. Effects of metformin and ethinyl estradiol-cyproterone acetate on clinical, endocrine and metabolic factors in women with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24(7):392–398.
- Porcile A, Gallardo E. Long-term treatment of hirsutism: desogestrel compared with cyproterone acetate in oral contraceptives. Fertil Steril. 1991;55(5):877–881.
- Charoenvisal C, Thaipisuttikul Y, Pinjaroen S, et al. Effects on acne of two oral contraceptives containing desogestrel and cyproterone acetate. Int J Fertil Menopausal Stud. 1996;41(4):423–429.
- van der Vange N, Blankenstein MA, Kloosterboer HJ, et al. Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception. 1990;41(4):345–352.
- Falsetti L, Gambera A, Tisi G. Efficacy of the combination ethinyl oestradiol and cyproterone acetate on endocrine, clinical and ultrasonographic profile in polycystic ovarian syndrome. Hum Reprod. 2001;16(1):36–42.
- Bhattacharya SM, Jha A. Comparative study of the therapeutic effects of oral contraceptive pills containing desogestrel, cyproterone acetate, and drospirenone in patients with polycystic ovary syndrome. Fertil Steril. 2012;98(4):1053–1059.
- Harborne L, Fleming R, Lyall H, et al. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet. 2003;361(9372):1894–901.
- Kelly CJ, Gordon D. The effect of metformin on hirsutism in polycystic ovary syndrome. Eur J Endocrinol. 2002;147(2):217–221.
- Fleming R, Hopkinson ZE, Wallace AM, et al. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J Clin Endocrinol Metab. 2002;87(2):569–574.

