 J Clin Aesthet Dermatol. 2021;14(3):38–41.
J Clin Aesthet Dermatol. 2021;14(3):38–41.
by Sumir Kumar, MD; Simranjeet Singh Dhillon, PG REsident; BK Brar, MD; and Amarbir Singh, MD
All authors are with the Department of Dermatology, Venereology, and Leprology at Guru Gobind Singh Medical College and Hospital in Faridkot, Punjab, India,
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Urticaria affects 0.5 to 1 percent of the population at any given time. Treatments include nonsedative antihistamines, autologous serum therapy, and injected histaglobulin.
Objective. This study sought to compare the therapeutic efficacy and safety of injected histaglobulin with autologous serum therapy in chronic urticaria.
Methods. This was a hospital-based prospective study performed in the Department of Dermatology, Venereology, and Leprology at Guru Gobind Singh Medical College and Hospital in Faridkot, India. A total of 96 patients with chronic idiopathic urticaria were enrolled after applying inclusion and exclusion criteria and were divided into two groups of 48 patients each using an envelope method. Autologous serum skin tests were performed in each patient irrespective of their group assignment. Group A then received injected histaglobulin and Group B received autologous serum therapy (AST). Patient were evaluated using the Urticaria Activity Score (UAS) every week for six weeks, with follow-up conducted at three and six weeks after the completion of treatment. The Chronic Urticaria Quality of Life questionnaire was used to assess the quality of life of the study participants.
Results. Out of the 96 initially enrolled patients, 62 completed the six weeks of treatment and two follow-up visits. Twenty patients dropped out due to remission and 14 patients left the study for other reasons. Reductions in UAS values occurred in both the groups by the end of follow-up but were more significant in Group A. Improvement in quality of life scores was also greater in Group A. Recurrence occurred in both groups after treatment cessation but was less common in Group A.
Conclusion. Both treatments were validated for treating chronic urticaria; however, injected histaglobulin showed statistically more consequential results than AST.
Key words: Autologous serum therapy, chronic urticaria, injected histaglobulin
Urticaria, from urtica (“to burn”) in Latin, also known as hives, affects 0.5 to 1 percent of the population at any given time. Chronic urticaria (CU) is defined as widespread, short-lived (<24 hours) wheals occurring daily or almost daily for at least six weeks.1 CU is further divided into chronic idiopathic urticaria (CIU) or chronic spontaneous urticaria (CSU) and urticaria secondary to physical causes. Approximately 70 percent of patients with CU are diagnosed with CIU, as no cause can be identified.1 Some cases of CIU were reported to have histamine-releasing immunoglobulin (Ig)G autoantibodies in their blood.1 This disease subgroup is referred to as autoimmune or autoreactive urticaria. Mast cell degranulation is of central importance in the pathogenesis of CIU.2 There is now strong evidence for an autoimmune basis to the disease in some patients, and circulating functional histamine releasing autoantibodies reactive against either the alpha subunit of the high-affinity IgE receptor or IgE have been identified in about one-third of patients with CIU.3–7 Various therapeutic options are available for the treatment of CU. First in the treatment ladder are nonsedative antihistamines, which are initially started at low doses. Escalation of the dosage of these agents and the addition of a leukotriene antagonist are the next strategies typically attempted. The use of immunomodulators, such as cyclosporin, methotrexate, omalizumab, and dapsone, is a last resort for treating this condition. Autologous serum therapy (AST) and injected histaglobulin have also been studied individually in the past in CU. Both AST and injected histaglobulin act via slowly modulating the immune system of the body. The patient’s own serum is prepared from patient’s blood for AST, while injected histaglobulin is available commercially. This current study was conducted to compare the therapeutic efficacy and safety of injected histaglobulin and AST in patients with CIU. Specifically, this study sought to:
- Evaluate the therapeutic efficacy and safety of injected histaglobulin in patients with CU.
- Evaluate the therapeutic efficacy and safety of AST in patients with CU.
- Perform a comparative evaluation of the therapeutic efficacy and safety of injected histaglobulin and AST in patients with CU.
Methods
This hospital-based prospective study was conducted in the Department of Dermatology, Venereology, and Leprology at Guru Gobind Singh Medical College and Hospital in Faridkot, India. It was initiated following approval from the institutional ethics committee in May 2019 and was continued until the desired sample size was obtained.
Study population. Patients with CIU who visited the Department of Dermatology, Venereology, and Leprology of Guru Gobind Singh Medical College and Hospital as outpatients were considered the target study population. Patients with clinically diagnosed CIU who fit the inclusion and exclusion criteria and who were willing to participate were considered eligible for inclusion. Patients of either sex were included if they were 18 to 45 years of age and completed a written consent form. Patients were excluded if they had an active infection or a history of physical urticarias, were pregnant or lactating, aged younger than 15 years or older than 45 years, or were using another immunosuppressant for CU.
Sampling. Taking the mean Urticaria Activity Score (UAS) values from a study conducted by Rajesh et al,8 using the web-based, open-source OpenEpi software program (Version 3), our ideal sample size for each group was calculated as 48 patients. Thus, a total of 96 patients were included in this study. Study participants were then divided equally into two groups (Group A and Group B) of 48 patients, each using an envelope method of selection. Group A was treated with histaglobulin and Group B was treated with AST.
Diagnosis. The diagnosis of CU was made on the basis of history-taking and clinical examination of the patient, followed by autologous serum skin test (ASST). For the ASST, venous blood drawn from the patient was placed in sterile plastic tubes and allowed to clot at room temperature for 30 minutes. Then, the serum was separated by centrifugation at 2,000rpm for four minutes. Five units of the autologous serum and 0.9% sterile saline (for negative control) were separately injected intradermally into the volar aspect of the patient’s forearm skin with 22-gauge needles, leaving gaps of at least 3cm. Areas involved in spontaneous wheals in the last 24 hours were avoided. Then, wheals and flare responses were measured after 30 minutes. A positive ASST result was defined as a serum-induced wheal that was both red in color and had a diameter of 1.5mm or more relative to the saline-induced response after 30 minutes.
In addition to ASST, baseline measurements, including complete blood count with PBF for abnormal cells, liver function tests, renal function tests, serum IgE level, and absolute eosinophilic count, were performed for all participants at the start of the treatment.
Treatment protocols. Group A patients were administered a subcutaneous injection of 1mL of histaglobulin, which is a combination of human normal immunoglobulin (12mg) and histamine dihydrochloride (0.15mcg),9 in the shoulder. Group B patients were given a 1-mL injection of autologous serum intramuscularly in the buttocks. Ebastine 20mg was taken as the rescue medicine in between treatments if required. Treatments were given weekly for six weeks, followed by evaluation at three and six weeks after treatment.
Calculation of the UAS was performed as per standard guidelines10 and the quality of life was assessed using the Chronic Urticaria Quality of Life (CU-Q2oL) questionnaire11 completed by each patient at every visit. Patients with intolerable urticaria in between treatment visits were instructed to contact the investigator immediately and were instructed to take the rescue medicine.
Statistical evaluation. The results achieved were statistically analyzed at the end of the study using Microsoft Excel (2016 version) (Microsoft Corporation, Redmond, Washington). The demographic profile of the patients was presented in terms of percentages and mean±standard deviation (SD) age, UAS value, and CU-Q2oL values were analyzed using the t-test to discern significant associations.
Results
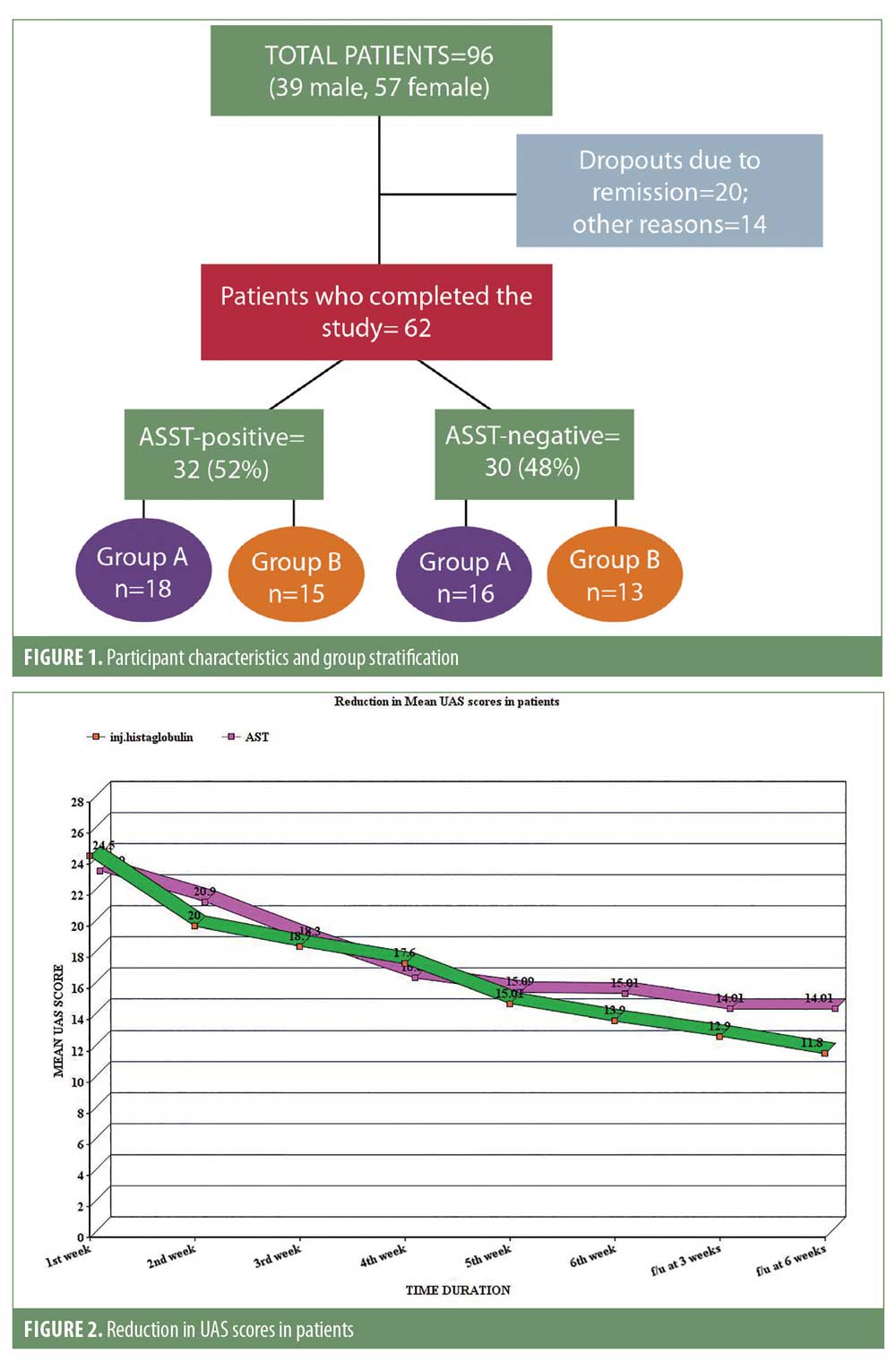
In our study, a total of 96 patients were initially included after applying the inclusion and exclusion criteria, but only 62 patients completed the six weeks of treatment and two follow-up visits. Twenty patients dropped out due to remission and 14 patients left the study for other reasons. Of the remaining 62 patients, 32 (52%) were ASST-positive and 30 (48%) were ASST-negative. Of the 32 patients who were ASST-positive, 18 were included in Group A and 15 were included in Group B, while the ASST-negative patients were stratified as 16 patients in Group A and 14 patients in Group B (Figure 1).
The mean±SD age of the study population was 30.04±2.26 years with a male to female ratio of 1 to 2.4.
The mean±SD UAS values in our study were 23.7±6.3 points. Using a paired t-test, the statistical association was calculated. The last scores of patients lost to follow up were adopted for any subsequent visits missed. Results were statistically significant in both groups, but better results were obtained with injected histaglobulin, with a reduction in UAS values by up to 69 percent compared to up to 60 percent with AST (Table 1, Figure 2). In Group A, more ASST-positive patients were present (n=22 patients), suggesting that ASST-positive patients had better results than ASST-negative patients.
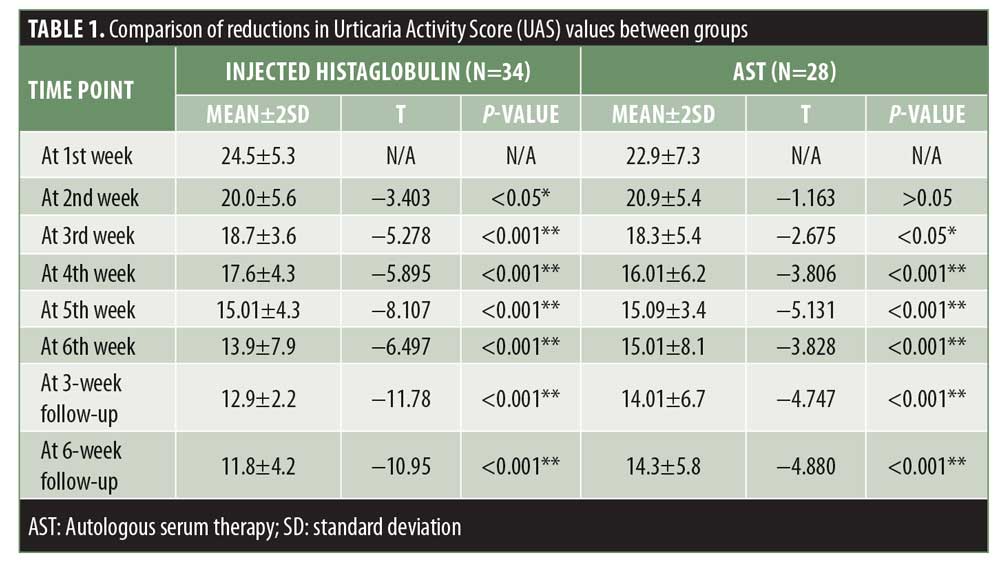
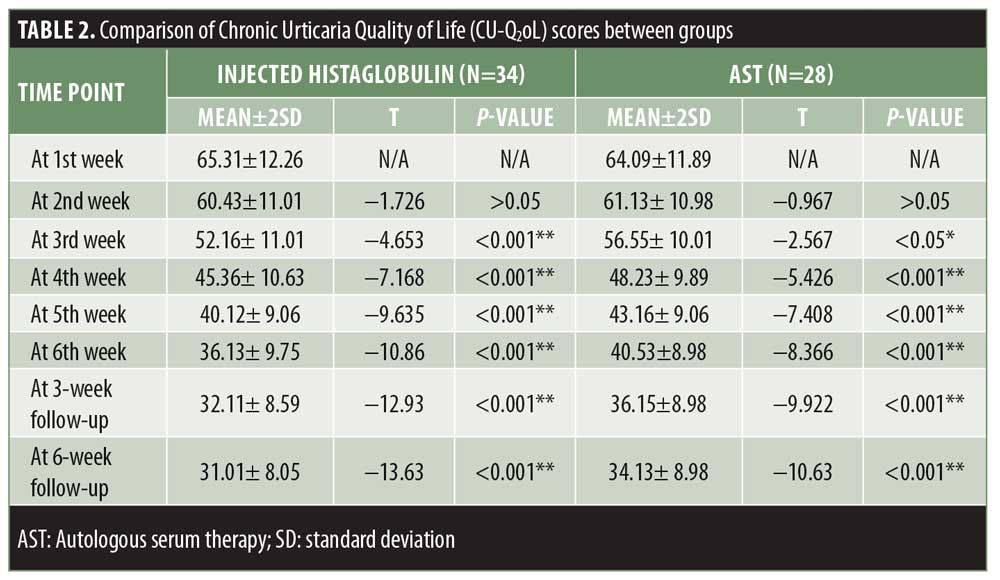
The maximum CU-Q2oL scores in our study ranged from 30 to 100 points, with mean±SD values of 64.70±12.07 points. Using a paired t-test, statistically significant results were obtained in both the study groups. The results in Group A were more significant, with an overall improvement in the quality of life by 52.51 percent, whereas the improvement in Group B was only up to 46.74 percent (Table 2, Figure 3).
Use of antihistamines was noted in 30 percent of the study participants. Greater improvement was observed in ASST-positive patients. No major side effects or reactions were observed in any patients during the study.
Discussion
CU is a perennial health problem that affects the quality of life of patients. Nonsedating antihistamines remain the mainstay of treatment for CU,10,12–14 providing immediate relief from symptoms. Yet, these medications do not alter the immunopathogenesis or the natural course of the disease, so there remains a need to find a drug that provides long-term benefits by acting early in the immunological cascade, thereby preventing more severe urticaria onset.
In our study, 96 patients were included and their mean±SD age was 30.04±12.26 years, with a male to female ratio of 1 to 2.4. These values were comparable to those in research performed by Rajesh et al8 in 2016 which had a sample size of 51 patients with a mean±SD age of 37.2±13.1 years and a male to female ratio of 1 to 2.6.8 An explanation of these concordant findings may be related to the fact that urticaria is more common among women, while younger individuals between the ages of 20 and 30 years might be prone to disease onset.
Better results were seen among ASST-positive patients group in our study. Similar finding were reported by Kumar et al15 in 2016. The mean±SD UAS values in our study were recorded as 23.70±6.3 points, with reductions of up to 69 percent among patients treated with injected histaglobulin and 60 percent among patients treated with AST, respectively. In the study conducted by Rajesh et al,8 the mean±SD UAS values were reduced from 18.9±6.3 points to 3.7±5.4 points (80.4% reduction) by eight weeks. A reason for greater reduction in this study might be that the investigators used antihistamines and prednisolone along with injected histaglobulin.8
There were more ASST-positive than ASST-negative patients included in our study and these patients showed greater improvements compared to the ASST-negative patients. Similar results were found in the study conducted by Bajaj et al16 in 2008, where 62 ASST-positive patients (n=32 women) with CU (group 1) and 13 ASST-negative (n=7 women) patients with CU (group 2) were prospectively analyzed to discern the efficacy of nine consecutive weekly autologous serum injections with a postintervention follow-up period of 12 weeks. In the ASST-positive group, 35.5 percent of patients were completely asymptomatic by the end of the follow-up period, while an additional 24.2 percent showed marked improvement. In the ASST-negative group, the corresponding percentages were 23 percent and 23 percent of patients, respectively.16
Debbarman et al17 in 2014, conducted a double-blind, parallel-group, randomized controlled study to find out the efficacy of AST in urticaria patients. Fifty-four patients were given AST and 57 patients were given injections of normal saline (placebo), together with cetirizine administered on an on-demand basis in both groups. UAS values experienced significant improvement from baseline.17 These results are comparable with our study findings of AST-treated patients.
Limitations. Our study had several limitations. Our study sample size was not that large and patients were lost to follow-up, affecting the statistical analysis in the study. Moreover, scores of CU-Q2ol scale varied from individual to individual and were mostly subjective, depending in part on their state of mind at the time of assessment.
Conclusion
In our study, both drugs yielded significant improvement in patients with CIU. However, more consequential results were seen with injected histaglobulin than with AST, with reductions in pill burden and remission rates in the former treatment group. From this, we can speculate that injected histaglobulin is a new emerging modality to consider for the treatment of CU in addition to existing modalities and can be compared with AST.
References
- Greaves MW. Chronic urticaria. N Engl J Med. 1995;332(26):1767–1772.
- Kaplan AP, Hora kova Z, Katz SI. Assessment of tissue fluid histamine levels in patients with urticaria. J Allergy Clin Immunol. 1978;61(6):350–354.
- Hide M, Francis DM, Grattan CEH, et al. Autoantibodies against the high affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328(22):1599–1604.
- Niimi N, Francis DM, Kermani F, et al. Dermal mast cell activation by autoantibodies against the high affinity IgE receptor in chronic urticaria. J Invest Dermatol. 1996;106(5):1001–1006.
- Fiebiger E, Maurer D, Holub H, et al. Serum IgG autoantibodies directed against the a chain of FceRI. a selective marker and pathogenetic factor for a distinct subset of chronic urticarial patients?. J Clin Invest. 1995;96(6):2606–2612.
- Tong LJ, Balakrishnan G, Kochan JP, et al. Assessment of autoimmunity in patients with chronic urticaria. J Allergy Clin Immunol. 1997;99(4):461–465.
- Zweiman B, Valenzano M, Atkins PC, et al. Characteristics of histamine releasing activity in the sera of patients with chronic idiopathic urticaria. J Allergy Clin Immunol. 1996;98(1):89–98.
- Rajesh G, Keerthi S, Karthikeyan K, Venkatesan M. Weekly injection of histaglobulin produces long-term remission in chronic urticaria: a prospective clinical study. Indian J Pharmacol. 2016;48(3):292–297.
- HISTOGLOB (histaglobulin). Ambernath, India: Bharat Serums and Vaccines Limited; 2012.
- Zuberbier T, Asero R, Bindslev-Jensen C, et al. EAACI/GA2LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64(10):1417–1426.
- Baiardini I, Pasquali M, Braido F, et al. A new tool to evaluate the impact of chronic urticaria on quality of life: chronic urticaria quality of life questionnaire (CU-QoL). Allergy. 2005;60(8):1073–1078.
- Powell RJ, Leech SC, Till S, et al. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015;45(3): 547–565.
- Kaplan AP. Treatment of chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2012;19(4):326–31.
- Jurakic Toncic R, Lipozencic J, Marinovic B. Treatment of chronic urticaria. Acta Dermatovenerol Croat. 2009;17(4)s:305–322.
- Kumar YH, Bhaskar S, Shankar K. Comparative study of positive versus negative autologous serum skin test in chronic spontaneous urticaria and its treatment outcome. N Am J Med Sci. 2016;8(1):25–30.
- Bajaj A K, Saraswat A, Upadhyay A, et al. Autologous serum therapy in chronic urticaria: Old wine in a new bottle. Indian J Dermatol Venereol Leprol. 2008;74(2):109–113.
- Debbarman P, Sil A, Datta PK, et al. Autologous serum therapy in chronic urticaria: a promising complement to antihistamines. Indian J Dermatol. 2014;59(4):375–382.

