J Clin Aesthet Dermatol. 2025;18(10):33–39.
by Naiem T. Issa, MD, PhD; Kabir Al-Tariq, MS; and Christopher G. Bunick, MD, PhD
Dr. Issa is with Forefront Dermatology in Vienna, Virginia, Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery at the University of Miami School of Medicine in Miami, Florida, and George Washington University School of Medicine and Health Science in Washington, District of Columbia. Mr. Al-Tariq is with Georgetown University School of Medicine in Washington, District of Columbia. Dr. Bunick is with the Department of Dermatology and Program in Translational Biomedicine, Yale School of Medicine, New Haven, Connecticut.
FUNDING: No funding was provided for this article.
DISCLOSURES: Dr. Issa has received funding from the following entities either as a speaker, consultant, advisor, or investigator: AbbVie, Almirall, Arcutis, Bristol Myers Squibb, Castle Biosciences, Dermavant Sciences, DermTech, Galderma, Incyte, Journey, LEO Pharma, Lilly, National Eczema Association, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, Sun Pharma Industry, Topix, UCB, Verrica Pharmaceuticals. Dr. Bunick has served as an investigator and/or consultant for AbbVie, Almirall, Alumis, Amgen, Apogee, Arcutis, Botanix, Connect BioPharma, Daiichi Sankyo, Dermavant, EPI Health/Novan, Incyte, LEO Pharma, Lilly, Novartis, Ortho Dermatologics, Palvella, Pfizer, Regeneron, Sanofi, Sun Pharma, Takeda, Timber, Teladoc, Triveni, and UCB. Mr. Al-Tariq has no conflicts of interest to declare.
Abstract: Objective: In patients with moderate-to-severe plaque psoriasis, recent meta-analyses compared efficacies among biologics and time to relapse after treatment withdrawal; however, there was notable heterogeneity in the clinical trials and their criteria used to define relapse. Furthermore, while biologics are effectively grouped into treatment classes based on their mechanisms of action (ie, anti-interleukin (IL)-23 class, anti-IL-17 class), not all biologics should necessarily be grouped into a class effect. For example, the anti-IL-17 class is heterogeneous in their mechanisms of action due to the variety of cytokines and receptors antagonized along with their accompanying gene expression changes in psoriatic disease. Therefore, we performed an in-depth assessment of biologics comprising the anti-IL-17 class and their associated pharmacokinetics (PK). We identified differences in PK parameters that may augment our understanding of how these biologics differ in function and explain variations in time to relapse of psoriatic disease after treatment withdrawal. Methods: A PubMed literature search was performed and articles screened to only include double-blind, randomized, placebo or comparator-controlled trials. The remaining articles were screened to ensure inclusion of a treatment withdrawal period and to confirm they investigated and defined relapse. Results: We identified five unique randomized controlled trials that examined time to relapse after treatment discontinuation for anti-IL-17 biologics. Brodalumab, an IL-17RA antagonist, consistently demonstrated the quickest time to relapse, whereas bimekizumab, the first-in-class dual IL-17A and IL-17F antagonist, demonstrated the longest time to relapse. Limitations: There is heterogeneity in both the criteria for treatment success prior to undergoing treatment withdrawal as well as relapse criteria. The only relapse datapoints shared by all four anti-IL-17 class biologics examined were median time to loss of PASI-75 and PASI-90. Conclusion: Differences in time to relapse after treatment discontinuation following treatment success can be attributed to both differences in biologic PK properties and mechanisms of action. These results warrant further investigation into the role of IL-17F in the pathogenesis of plaque psoriasis, as targeting this isoform appears to confer synergistic therapeutic benefit compared to targeting the IL-17A isoform alone. Furthermore, the classic understanding of IL-17RA blockade with brodalumab must be revisited as IL-17F was found to partially bind and signal through IL-17RA despite the presence of brodalumab. Keywords: Psoriasis, biologics, disease remission, disease modification, remittive effect
Introduction
Systemic treatment options for plaque psoriasis (PsO) have significantly evolved and become increasingly more specific in targeting distinct pathways involved in PsO pathogenesis. These therapeutics now include the classes of interleukin (IL)-17 inhibitors and IL-23 inhibitors, among other small molecule inhibitors.1 While there is abundant evidence of their efficacy in placebo-controlled clinical trials, there is a paucity of literature comparing these drugs in randomized head-to-head trials. Additionally, there is even less literature investigating the time to relapse when patients with PsO discontinue treatment after initially achieving treatment success. In the trials that do investigate time to relapse after treatment cessation, they tend to differ in their definition of what constitutes relapse rendering the comparability of drugs across these different trials especially difficult.2–5 Our analysis here addresses the aforementioned differences in biologics specifically within the IL-17 inhibitor class, as this “class” contains therapeutics with heterogeneous mechanisms of action (IL-17A inhibitors, dual IL-17A/F inhibitor, and IL-17 receptor (IL-17RA) inhibitor). This is uniquely different from the IL-23 class in which all p19-specific biologics of this class directly target the IL-23 cytokine and the same isoform of IL-23 albeit via different epitopes.6 The goal of this article is to: (1) establish drug discontinuation as an important aspect in the therapeutic journey of patients with PsO; (2) better guide clinical decision making when considering disease relapse after treatment; and (3) emphasize the need for more stringent relapse criteria to facilitate PsO therapeutic comparisons in the absence of head-to-head randomized controlled trials.
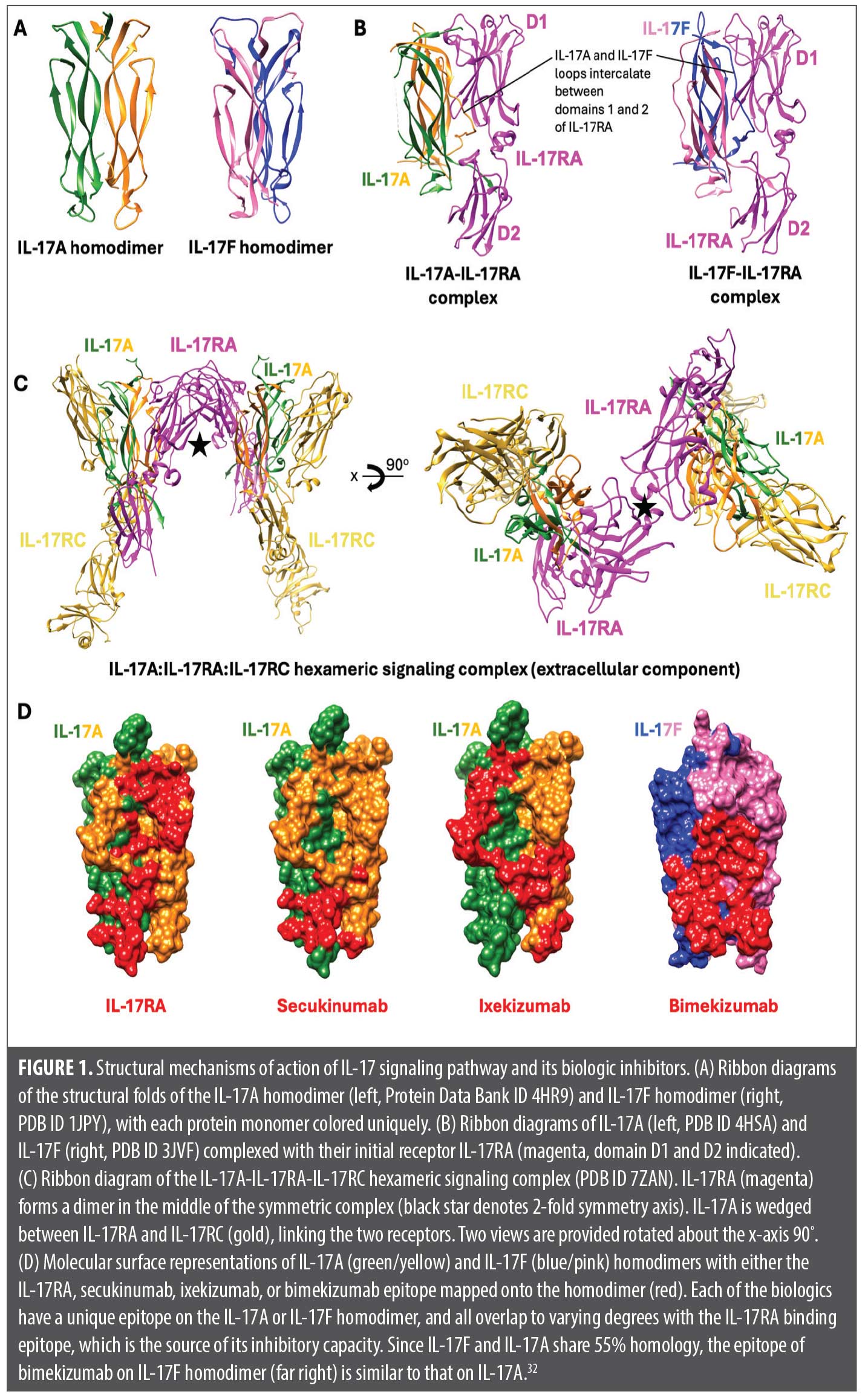
IL-17 is a proinflammatory family of cytokines classically secreted by T helper (Th) 17 lymphocytes after IL-23 stimulation.7 While the classic isoform of the IL-17 subtypes is IL-17A, there are six isoforms in total, denoted IL-17A to IL-17F, with both IL-17A and IL-17F being targets of three biologics currently approved by the United States Food and Drug Administration (FDA) for the treatment of PsO.1 With the exceptions of IL-17A and IL-17F, which can both heterodimerize, the IL-17 isoforms function as homodimers (Figure 1A).8 IL-17A promotes both proliferation and abnormal differentiation of keratinocytes, a hallmark feature in the pathogenesis of PsO. Additionally, IL-17A induces the differentiation of proinflammatory factors and promotes leukocyte migration and the secretion of matrix metalloproteinase-3, which is a key effector of tumor necrosis factor TNF-α-induced collagen degradation in skin and an inducer of apoptosis, further contributing to the development of an inflammatory milieu.8-11 In addition to sharing the highest homology with IL-17A at 55%, IL-17F is also commonly co-expressed with IL-17A and stimulates a similar pattern of genes as IL-17A, albeit with a weaker response.8 Specifically, IL-17A is 100-fold more potent than IL-17F; however, IL-17F is almost 30-fold more abundant in inflamed tissue.12,13 While the other IL-17 isoforms are not as well studied, transcripts of IL-17B and IL-17D are uniquely decreased in psoriatic skin compared to unaffected skin.9,14 Biologics that target the IL-17A cytokine are secukinumab (SEC), ixekizumab (IXE), and bimekizumab (BKZ). While SEC and IXE both target only the IL-17A isoform, BKZ targets both IL-17A and IL-17F isoforms. On the other hand, brodalumab (BROD) targets the IL-17 receptor A (IL-17RA) as opposed to the cytokines.1 Furthermore, each biologic has a different dosing schedule, loading dose, binding affinity, and half-life, which may be relevant when deciding between a biologic that is best in line with a patient’s preference or clinical need.
Although these drugs are developed to target the same, crucial pathway in the pathogenesis of PsO (IL-17 signaling), intraclass differences reported in time to relapse suggest that there are differences either in the pharmacokinetics (PK) and/or in the targets of these drugs. To clarify this knowledge gap, we performed a detailed PK and molecular analysis of the IL-17 inhibitor class to help elucidate a mechanistic hypothesis as to why there are differences in disease relapse rates after drug withdrawal.
Methods
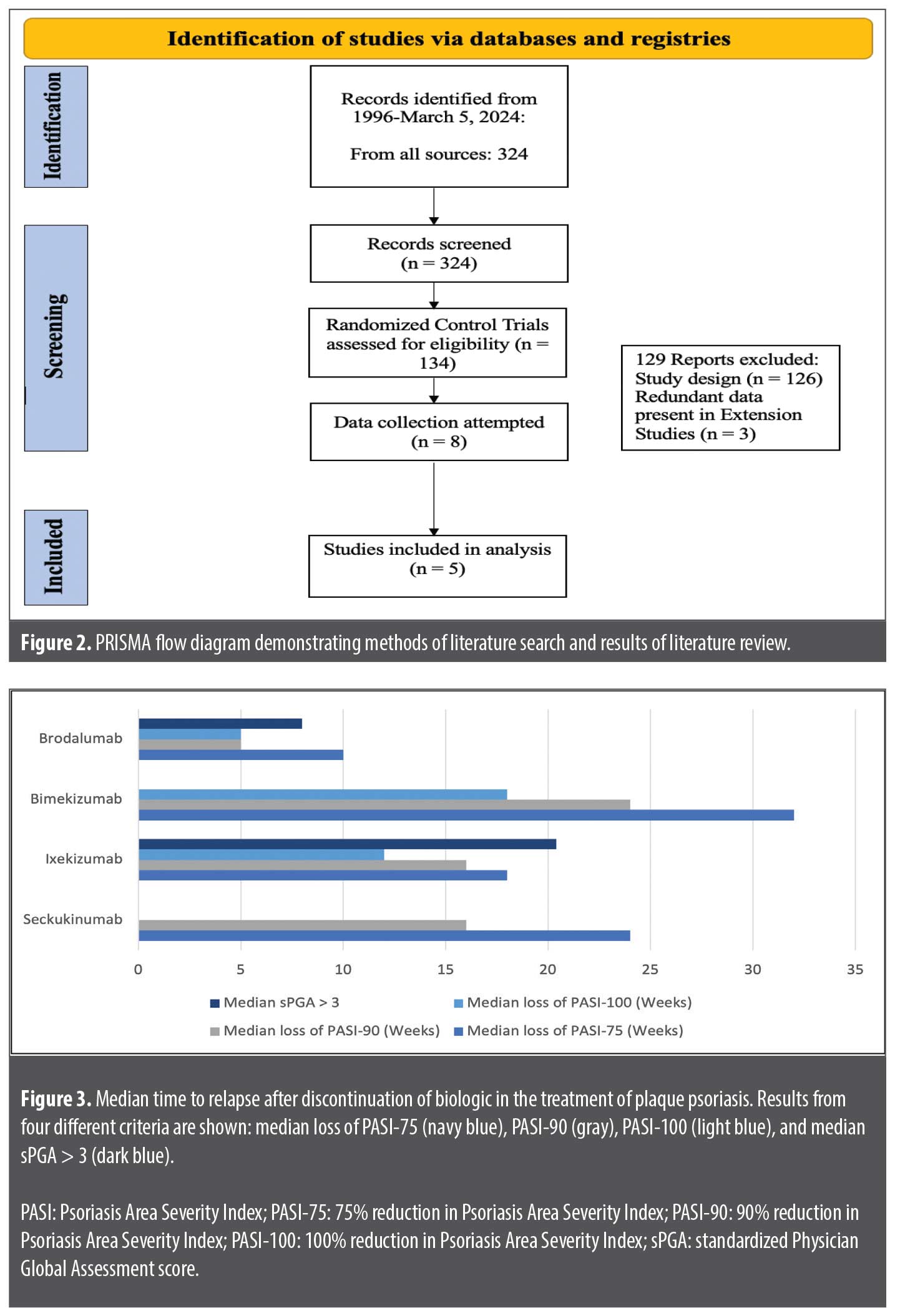
A PubMed literature search was performed using the terms “secukinumab,” “ixekizumab,” “brodalumab,” “bimekizumab,” “plaque psoriasis,” and “relapse” from database inception to March 5, 2025. Articles were screened to only include double-blind, randomized, placebo, or comparator-controlled trials (Figure 2). The remaining articles were further screened to ensure they investigated and defined disease relapse after treatment withdrawal. If there were multiple extension trials all containing the same data, only the most recently published extension trial was included in our analysis. Subsequent Google Scholar and Web of Science searches were performed to identify articles that included information about the PK properties of IL-17 biologics.
Results
Our search strategy identified five unique randomized controlled trials that examined time to relapse for biologics in the IL-17 class of inhibitors. Across these five trials, 4 unique definitions were used to define relapse (Table 1). Additionally, across the five trials, there were three unique eligibility criteria allowing individuals to enter the withdrawal phase of these trials after conclusion of the treatment phase: reduction of 90% from baseline Psoriasis Area Severity Index (PASI) score (PASI-90), reduction of 75% from baseline PASI (PASI-75), and sPGA score of 0/1.2–5,14,15 In the withdrawal phases across all studies, patients were rerandomized to either receive the treatment drug or receive placebo injections. For patients who were rerandomized to placebo and then relapsed, rescue medication consisted of the trial-specific drug with the same induction dosing across all trials without concomitant use of high- potency topical steroids.2-5
There was additional heterogeneity regarding the use of topical treatments during the trials. In the BE READY and UNCOVER trials investigating time to relapse for BKZ and IXE, respectively, participants were allowed to use weak topical corticosteroids (Class VI or VII) to the face, axilla, and genitalia, but these could not be used 24 hours before study visits.3,5 In the FIXTURE and ERASURE trials investigating time to relapse for SEC, participants were not allowed to use any topical treatments likely to impact signs and symptoms of PsO including corticosteroids, vitamin D analogues, calcineurin inhibitors, or salicylic acid. These trials enforced a two-week washout period for eligible participants with current use of topical medications prior to randomization.4 In the AMAGINE trials investigating BROD, patients were not allowed to use topical therapy and were also subject to a two-week washout period prior to their first dose of BROD or placebo.14
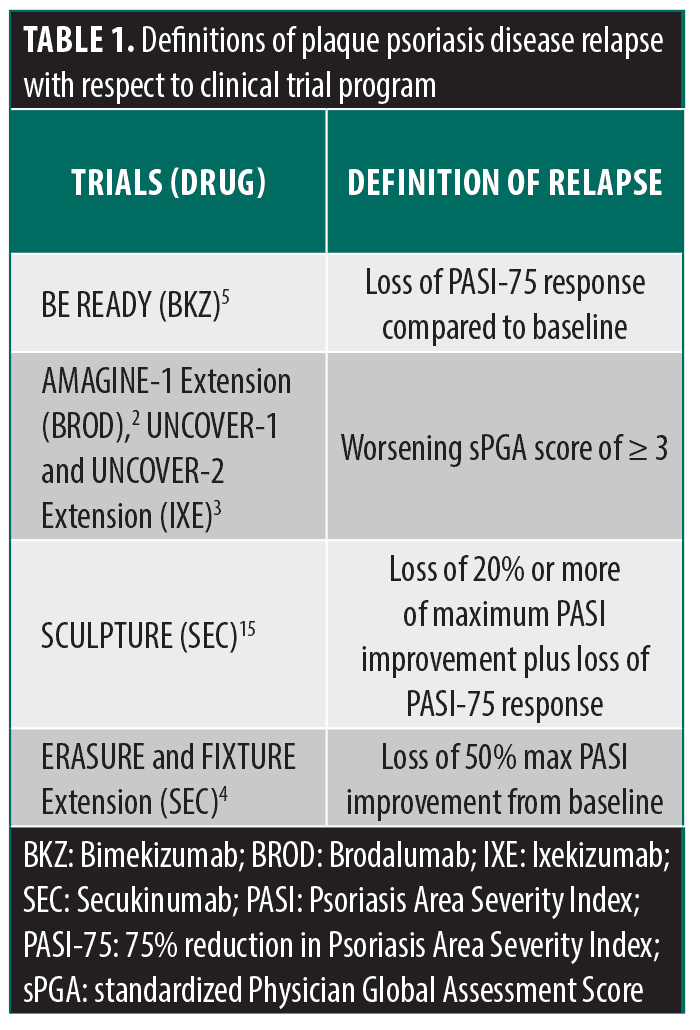
Due to the heterogeneity of relapse criteria, the only relapse data point shared by all four drugs was median time to loss of PASI-90 and median time to loss of PASI-75 (Figure 3). The median time to loss of PASI-90 for SEC, IXE, BKZ, and BROD was 16 weeks, 16 weeks, 24 weeks, and 5 weeks, respectively. The median time to loss of PASI-75 for SEC, IXE, BKZ, and BROD was 24 weeks, 18 weeks, 32 weeks and 10 weeks, respectively. Median time to loss of PASI-100 was available only for IXE, BKZ, and BROD which were 12 weeks, 18 weeks, and 5 weeks, respectively. Median time to a worsening sPGA score of ≥3 was only available for IXE and BROD, which were 20.4 weeks and 8 weeks, respectively.
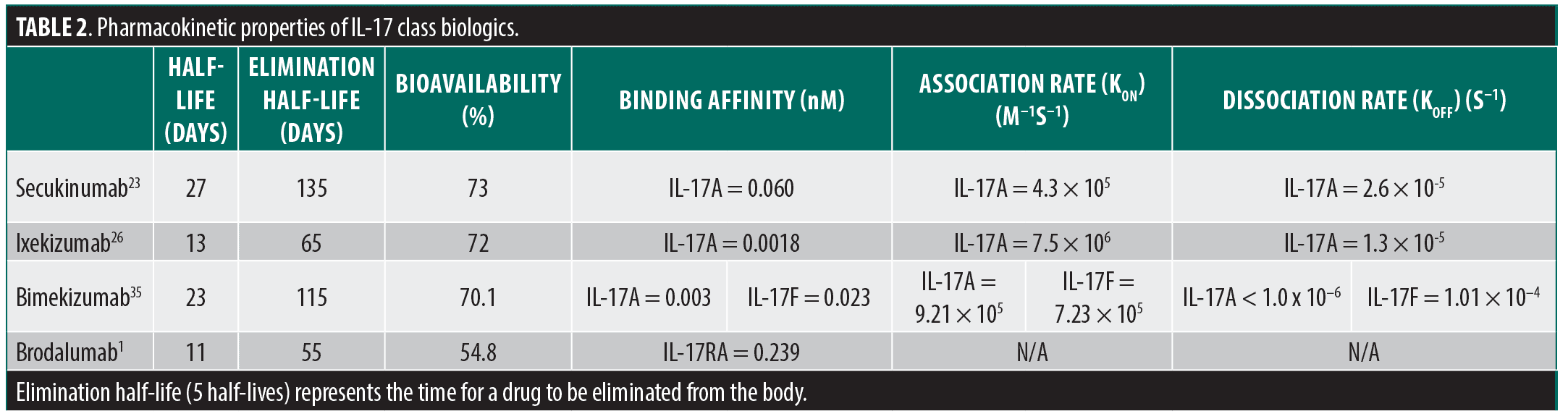
The relevant PK properties for each drug include half-life, bioavailability, and equilibrium dissociation constant (Kd, which is inversely proportional to binding affinity), the latter being a composite of the association rate constant (kon) and the dissociation rate constant (koff) (Table 2).6 Of note, kon and koff for BROD binding to IL-17RA are not readily available in public literature. The half-lives for SEC, IXE, BKZ, and BROD are 27 days, 13 days, 23 days, and 11 days, respectively. The bioavailability for SEC, IXE, BKZ, and BROD are 73%, 72%, 70.1%, and 54.8%, respectively.
Discussion
While SEC, IXE, BROD, and BKZ were developed to target the same IL-17 pathway, there are notable differences observed in the time to relapse between them. Due to differences in relapse criteria used across the five included trials (Table 1), only two metrics allowed for comparison: loss of PASI-90 and loss of PASI-75. Nonetheless, clear patterns emerged with BKZ and BROD consistently demonstrating the longest and shortest time to relapse, respectively.
Differences in time to relapse when comparing BROD to the IL-17 cytokine blockers can be attributed to multiple reasons, including PK differences or differences pertaining to the actual target (receptor versus cytokine). BROD not only has the shortest half-life and lowest bioavailability, but it also has the lowest binding affinity for its target, IL-17RA.1 When comparing the efficacy of BROD to its sibling drugs according to the placebo-controlled pivotal trials over 16 weeks (with the exception of BKZ), however, there is no significant difference in the likelihood of achieving PASI-75, PASI-90, or PASI-100 as per network meta-analysis.16 This suggests that the stark contrast in time to relapse between BROD and the other IL-17 biologics is not due to a lack of targeting the IL-17 pathway, but rather, more likely attributable to its comparatively short half-life, reduced bioavailability, and potential downstream effects (or lack thereof) in targeting a receptor versus a cytokine. It is also important to consider that IL-17RA blockade still allows for IL-17A and IL-17F signaling through the IL-17RC receptor homodimer.17 This is interesting, considering the evidence demonstrating the reversal of disease-relevant gene expression signatures by BROD, as well as the ability of BROD to rescue treatment failures to TNF-α, IL-23, and IL-17A blockers.18-21 Blocking the IL-17RA receptor isoform has been shown to increase the signaling threshold for IL-17A and IL-17F for the induction IL-36G and chemokine ligand CXCL1 mRNA in human keratinocytes, which are key downstream effector cytokines in the Th17 pathway.22 This occurs due to the role of the IL-17RA dimer, which forms in response to binding IL-17A or IL-17F. The IL-17RA dimer coordinates the formation of a 2:2:2 hexameric signaling assembly with IL-17A homodimers (or IL-17F homodimers or IL-17A/F heterodimers) and IL-17RC, ultimately leading to activation of the nuclear factor NF-κB and mitogen-activated protein (MAP) kinase pathways and subsequent synthesis of IL-36G and CXCL1 (Figures 1B and 1C).22 Thus, the short time to relapse observed in patients after stopping BROD is unlikely due to inadequate targeting of the IL-17 pathway. Rather, it is likely due to the PK properties of BROD, which cause its effects to diminish rapidly after drug cessation. It is important to note that while BROD has the lowest binding affinity for its target compared to the other drugs in the IL-17 family, without knowledge of its association and dissociation constants, it remains uncertain which binding characteristic most significantly influences the time to relapse.
Both IXE and SEC specifically target the IL-17A cytokine isoform. Although there is no difference in time to loss of PASI-90 (both 16 weeks), there is a 6-week difference in time to median loss of PASI-75 (24 weeks for SEC vs. 18 weeks for IXE). When looking at their PK properties, IXE has a nearly 40-fold increase in binding affinity for IL-17A compared to SEC, but the half-life of IXE is more than twice as short.22-26 IXE also has a lower koff of 1.3 ± 0.8 × 10−5 s−1 than SEC (2.6 ± 0.8 × 10−5 s−1).25 Additionally, while IXE may have a higher binding affinity, both IXE and SEC bind to different epitopes on the IL-17A molecule. While almost all of the SEC epitope overlaps with the IL-17RA binding surface, a much larger portion of the IXE epitope lies outside of the receptor binding region (Figure 1D). Additionally, once bound, the SEC epitope interacts with both molecules in the IL-17A dimer, compared to IXE which only binds one molecule in the homodimer.27,28 However, the overall surface area for the SEC epitope (1830 Å2) is smaller compared to the surface area covered by the IXE epitope (3330 Å2).28 In the IL-23 class of biologics, drugs with larger epitope surface areas and lower koff were found to be more clinically efficacious in the treatment of PsO.6 Indeed, in a network meta-analysis, IXE was shown to be more efficacious than SEC at achieving PASI-90 and PASI-100 at both 16 weeks and 52 weeks after starting treatment.28 Thus, the difference in binding epitope may partially explain the longer time to relapse seen with SEC given its lower binding affinity for IL-17A as well as smaller epitope surface area. The difference in half-life likely explains the difference in dosing between these two drugs, with IXE requiring biweekly dosing of 80mg after a starting dose of 160mg, and SEC requiring monthly doses of 300mg after weekly loading doses of 300mg for four weeks.1 As such, given the near identical bioavailability (SEC 73% and IXE 72%), the difference in median time to loss of PASI-75 is likely primarily due to the difference in elimination half-life and less due to differences in the target epitopes of the IL-17A cytokine. It takes approximately 5 half-lives for a drug to be eliminated from the body (technically, about 97%); this would be 135 days and 65 days after withdrawal for SEC and IXE, respectively (Table 2). Therefore, following IL-17A inhibitor withdrawal, SEC persists in the patient for twice as long as IXE and will have some level of PsO therapeutic effect as drug concentrations decrease.
Of all IL-17 inhibitors, BKZ consistently demonstrates the longest time to relapse, regardless of the criterion used to define relapse. In addition to having the longest time to relapse, BKZ has been estimated to have the highest probability of patients achieving PASI-100 after 16 weeks of treatment compared to all other biologics used to treat PsO.16 Furthermore, of all the IL-17 biologics, BKZ is the only drug that does not require a loading phase and can be administered at 320 mg every 4 weeks from week 0, with some patients even being eligible to switch to 320 mg every 8 weeks after 16 weeks of treatment.1 BKZ is the only biologic in the IL-17 class that targets the IL-17F isoform in addition to the IL-17A isoform. BKZ possesses a long half-life at 23 days and a high bioavailability at 70.1%.29,30 However, it possesses neither the longest half-life nor the largest bioavailability. This insinuates that there are other downstream properties of BKZ that allow its effects to be more sustained compared to the other biologics, namely SEC and IXE, which possess a longer half-life and greater binding affinity for IL-17A, respectively. In fact, the median time to flare for BKZ for any PASI category surpasses 16 weeks, which is the time required to achieve drug elimination (five half-lives, or approximately 115 days/16.4 weeks). This suggests that the longer time to relapse may be attributed to the effects of simultaneously targeting the IL-17F isoform. Both IL-17A and IL-17F are often co-expressed, and while IL-17F has been shown to have a lower proinflammatory effect than IL-17A, IL-17F is found at up to 30-fold higher levels in both lesional skin and the serum of patients with PsO.12 Additionally, both IL-17A and IL-17F are the only two IL-17 isoforms that are able to homodimerize and induce the dimerization of the IL-17RA receptors, which coordinates the downstream effects of IL-17 seen in PsO.8 However, depending on which IL-17 isoform binds to the IL-17RA receptor, the D2 domain of the IL-17RA receptor will undergo differential rotations. The binding of IL-17A, IL-17F, and IL-17A/F results in a rotation of 23º, 19º, and 30º of the D2 domain, respectively.22 Thus, it is plausible that these different binding-induced receptor conformational changes may impact downstream signal activation/signal potency, as is the case for erythropoietin and downstream JAK/STAT signaling.22 Consequently, although the binding of different IL-17 isoforms to the IL-17RA receptor does not disrupt the formation of the 2:2:2 hexameric complex with IL-17RA and IL-17RC, IL-17F binding appears to result in higher absolute IL-36G mRNA expression compared to IL-17A when the IL-17RA receptor is saturated with either isoform.22 IL-36G itself plays a large role in the pathogenesis of PsO by inducing the secretion of IL-23 and recruiting immune cells such as neutrophils and dendritic cells to psoriatic lesions.31 Therefore, the sustained efficacy of BKZ may be attributed to its targeting of the more abundant isoform present in affected skin, as well as the differential rotation of the IL-17RA D2 domain and the expression of IL-36G mRNA in response to IL-17F binding to IL-17RA. Consistent with its potent neutralization of IL-17A and IL-17F, the BKZ fragment antigen-binding (Fab) was shown to bind 45.5% and 41.5% of the residues recognized by IL-17RC and IL-17RA, respectively.32 Thus, the increased efficacy seen with targeting both IL-17A and IL-17F can be reasonably extrapolated; however, the longer time to relapse remains less clear. Prior studies showed that, unlike IL-17A, IL-17F can uniquely synergize with IL-23 in human eosinophils to promote the production of IL-1B and IL-6, both of which are required for the differentiation of Th17 cells.33,34 Therefore, the sustained efficacy seen with BKZ may be explained by its ability to further dampen the Th17 pathway via the unique suppression of IL-17F as a negative feedback event.
We integrated mechanism of action, available molecular modeling data, and PK properties to analyze differences in time to relapse among the IL-17 class of biologics for the treatment of PsO. Noteworthy limitations include the lack of head-to-head trials among biologics within this class and the heterogeneity in relapse criteria used across the 52-week randomized withdrawal extension trials analyzed. The paucity of relapse data likely stems from biologics being a relatively novel therapeutic option for the treatment of autoimmune diseases and the understandable need for studies to preferentially demonstrate their safety and efficacy. With recent advances in biologics and their ability to achieve PASI-100 clearance in some patients, there needs to be a paradigm shift from focusing on biologic efficacy and treating disease, to investigating disease remission and relapse (Table 1).
It is especially crucial in future trials to establish a standard definition of relapse via a joint consensus effort between clinicians, patients, and industry stakeholders. One example of a standard definition used to define relapse in future clinical trials could be loss of 50% maximum PASI improvement compared to baseline prior to target drug initiation. Not only is time to relapse especially important as patients often express interest in medications that allow for treatment “holidays,” but it is also an important point to consider when deciding which biologic would maximize treatment success.
Conclusion
The variation in time to relapse among the IL-17 class of biologics is largely attributed to differences in PK properties in addition to mechanisms of action, as evidenced by distinctions between SEC and IXE. This is further evidenced by BROD exhibiting the quickest time to relapse of all IL-17 biologics and having the shortest half-life, the lowest bioavailability, and the lowest affinity for its target. Of note, BROD is the only drug in this class that targets the IL-17RA receptor as opposed to individual cytokines; this may also affect the relapse time seen with BROD given the potential for residual IL-17F signaling through IL-17RC. On the other hand, BKZ proves a notable exception to this trend: while it does not have the best PK properties in the class, it still demonstrates the longest time to relapse. This is likely attributed to the fact that BKZ also targets IL-17F, which is found at higher concentrations in lesional skin in patients with PsO and that IL-17F can uniquely produce cytokines responsible for Th17 differentiation.12,31,33,34 Further research should analyze the role of both IL-17F and cells that release IL-17F, as these could prove to be viable therapeutic targets for the treatment and possible remittance of PsO.
References
- Regnault MM, Shourick J, Jendoubi F, Tauber M, Paul C. Time to relapse after discontinuing systemic treatment for psoriasis: a systematic review. Am J Clin Dermatol. 2022;23(4):433-447.
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183(6):1037-1048.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group; UNCOVER-2 Study Group; UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345-356.
- Blauvelt A, Reich K, Warren RB, et al. Secukinumab re-initiation achieves regain of high response levels in patients who interrupt treatment for moderate to severe plaque psoriasis. Br J Dermatol. 2017;177(3):879-881.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475-486.
- Daniele SG, Eldirany SA, Damiani G, Ho M, Bunick CG. Structural basis for p19 targeting by anti-IL-23 biologics: correlations with short- and long-term efficacy in psoriasis. JID Innov. 2024;4(2):100261.
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517.
- Brembilla NC, Senra L, Boehncke WH. The IL-17 family of cytokines in psoriasis: IL- 17A and beyond. Front Immunol. 2018;9:1682.
- Singh S, Maniakis-Grivas G, Singh UK, et al. Interleukin-17 regulates matrix metalloproteinase activity in human pulmonary tuberculosis. J Pathol. 2018;244(3):311-322.
- Xie Y, Mustaga A, Yerzhan A, et al. Nuclear matrix metalloproteinases: functions resemble the evolution from the intracellular to the extracellular compartment. Cell Death Discov. 2017;3:17036.
- Mirastschijski U, Lupše B, Maedler K, et al. Matrix metalloproteinase-3 is key effector of TNF-α-induced collagen degradation in skin. Int J Mol Sci. 2019;20(20):5234.
- Kolbinger F, Loesche C, Valentin MA, et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139(3):923-932.e8.
- Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160(2):319-324.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273-286.
- Mrowietz U, Leonardi CL, Girolomoni G, et al; SCULPTURE Study Group. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73(1):27-36.e1.
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12(8):1777-1792.
- Goepfert A, Lehmann S, Blank J, Kolbinger F, Rondeau JM. Structural analysis reveals that the cytokine IL-17F forms a homodimeric complex with receptor IL-17RC to drive IL- 17RA-independent signaling. Immunity. 2020;52(3):499-512.e5.
- Russell CB, Rand H, Bigler J, et al. Gene expression profiles normalized in psoriatic skin by treatment with brodalumab, a human anti-IL-17 receptor monoclonal antibody. J Immunol. 2014;192(8):3828-3836.
- Papp KA, Gordon KB, Langley RB, et al. Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate-to-severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE-2 and AMAGINE-3. Br J Dermatol. 2018;179(2):320-328. 179(2):320–328.
- Montaudié H. Is an anti-interleukin-17 receptor able to do the job when an anti-interleukin-12/23 has failed? Br J Dermatol. 2019;180(2):255-256.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81(3):857-859.
- Goepfert A, Barske C, Lehmann S, et al. IL-17-induced dimerization of IL-17RA drives the formation of the IL-17 signalosome to potentiate signaling. Cell Rep. 2022;41(3):111489.
- Kolbinger F, Di Padova F, Deodhar A, et al. Secukinumab for the treatment of psoriasis, psoriatic arthritis, and axial spondyloarthritis: physical and pharmacological properties underlie the observed clinical efficacy and safety. Pharmacol Ther. 2022;229:107925.
- Bruin G, Loesche C, Nyirady J, Sander O. Population pharmacokinetic modeling of secukinumab in patients with moderate to severe psoriasis. J Clin Pharmacol. 2017;57(7):876-885.
- Liu L, Lu J, Allan BW, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A. J Inflamm Res. 2016;9:39-50.
- European Medicines Agency. Taltz (Ixekizumab) – Public Assessment Report. Published online May 28, 2020.
- Eldirany SA, Ho M, Bunick CG. Structural basis for how biologic medicines bind their targets in psoriasis therapy. Yale J Biol Med. 2020;93(1):19-27.
- Armstrong AW, Soliman AM, Betts KA, et al. Comparative efficacy and relative ranking of biologics and oral therapies for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther (Heidelb). 2021;11(3):885-905.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- European Medicines Agency. Bimzelx : EPAR – Product information. Published online July 8, 2024.
- Bridgewood C, Fearnley GW, Berekmeri A, et al. IL-36γ is a strong inducer of IL-23 in psoriatic cells and activates angiogenesis. Front Immunol. 2018;9:200.
- Adams R, Bunick CG, Lawson ADG, Gomez B, Shaw S. Crystal structure of bimekizumab fab fragment in complex with IL-17F provides molecular basis for dual IL-17A and IL-17F inhibition. J Invest Dermatol. 2024;144(11):2581-2583.e2.
- Cheung PHY, Wong CK, Lam CWK. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J Immunol. 2008;180(8):5625-5635.
- Navarro-Compán V, Puig L, Vidal S, et al. The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases. Front Immunol. 2023;14:1191782.
- Ma H, Zhang W, Lui K, et al. Generation and characterization of QLS22001, a humanized monoclonal antibody that neutralizes IL-17A and IL-17F with an extended half-life. Int Immunopharmacol. 2023;117:109947.