J Clin Aesthet Dermatol. 2024;17(7):50–51.
J Clin Aesthet Dermatol. 2024;17(7):50–51.
by Robyn Siperstein, MD; Younghoon Cho, MD, PhD; and Jessica Hicks, PhD
Dr. Siperstein is with the Siperstein Dermatology Group in Boynton Beach, Florida. Dr. Cho is with Integrated Aesthetics in Spring, Texas. Dr. Hicks is with Galderma Medical Affairs in Dallas, Texas.
FUNDING: This study was funded by Galderma Laboratories, L.P.
DISCLOSURES: Dr. Siperstein has served as a primary investigator, consultant, and trainer for Allergan and Galderma, and as a primary investigator for Cutera. Additionally, Dr. Siperstein received a research grant and speaking fees from Allergan/Galderma and Sciton. Dr. Cho has served as a trainer, consultant, and investigator for Allergan and Galderma. Dr. Hicks is an employee of Galderma Laboratories, L.P.
ABSTRACT: HARES is a supportive (high G’) HA filler with a low degree of water affinity (gel swelling) and modification (<1% BDDE) that has a well-established safety and efficacy profile in the literature, especially for infraorbital hollow (IOH) rejuvenation. To further support the safety of this product, a long-term review of delayed-onset adverse events of interest (DAEIs) related to HARES was conducted using reports from a global post-marketing safety surveillance database over the past 23 years. This review demonstrated low reporting frequencies of delayed-onset nodules and inflammatory events, establishing a long-term safety profile for HARES that supports its continued use in clinical practice, especially for IOH rejuvenation. Keywords: Delayed onset complications, nodules, swelling, HA filler, infraorbital hollows
Introduction
Hyaluronic acid (HA) fillers have been used for soft tissue augmentation for over 26 years due to their effectiveness and favorable safety profiles. Generally, the overall incidence of complications is low in clinical practice and most are mild in severity.1 Rare events, such as delayed-onset (≥14 days) nodules and inflammatory reactions (e.g., hypersensitivity and granulomas) have gained recent attention and prompted updates to the Federal and Drug Administration (FDA) dermal filler warnings.1,2 Due to this heightened awareness, there is a need to review how often these delayed-onset adverse events of interest (DAEIs) are reported.
An HA filler formulated with non-animal stabilized hyaluronic acid (NASHA)™ technology was the first available non-animal HA filler (EU approval in 1996) and has over 26 years of clinical experience (Restylane® [HARES], Galderma, Uppsala, Sweden). HARES is a supportive, high G’ gel with a low degree of water affinity (i.e., gel swelling) and modification (<1% BDDE).3 These properties make HARES suitable for injection across several anatomical regions, but it is especially ideal for infraorbital hollow (IOH) rejuvenation, as this area is prone to swelling.1 Moreover, a systematic review identified HARES as the most used HA filler for IOH rejuvenation.4
While HARES safety is well-established in the literature, we present here a review of the long-term, global post-marketing safety experience for this product. Herein, we report frequencies of DAEIs related to HARES, including delayed-onset nodules, hypersensitivity reactions, and granulomas, collected over 23 years from 1999 to 2022. Of particular note, DAEIs related to IOH rejuvenation were reported separately.
Methodology
This safety review was conducted by collecting adverse events related to HARES from a global manufacturer post-marketing safety database between 1999 and 2022. Medical experts assessed the relationship to product and coded events using Medical Dictionary for Regulatory Activities Preferred Terms (MedDRA PT) based on collated case information. Reports with early-onset (<14 days) events or undetermined product causality (e.g., layering of different products) were excluded from analyses.
DAEIs (≥14 days) were grouped into their respective categories based on these MedDRA PT codes: nodules (mass, nodule, papule, induration), granulomas (granuloma, foreign body), and hypersensitivity reactions (hypersensitivity, inflammation, swelling, edema). Event descriptions were thoroughly examined to confirm categorizations, product causality, and time to onset after keyword categorizations. Inflammatory nodules were differentiated from non-inflammatory nodules based on whether they were accompanied by pain, swelling, tenderness, erythema, or irritation.1 Hypersensitivity reactions were confirmed by diffuse, local, or persistent swelling. Granulomas were confirmed with positive histologic analyses or re-categorized based on the description. The total number of syringes sold worldwide from 2010 (earliest available data) to 2022 was used for calculating DAEI reporting frequencies.
Results
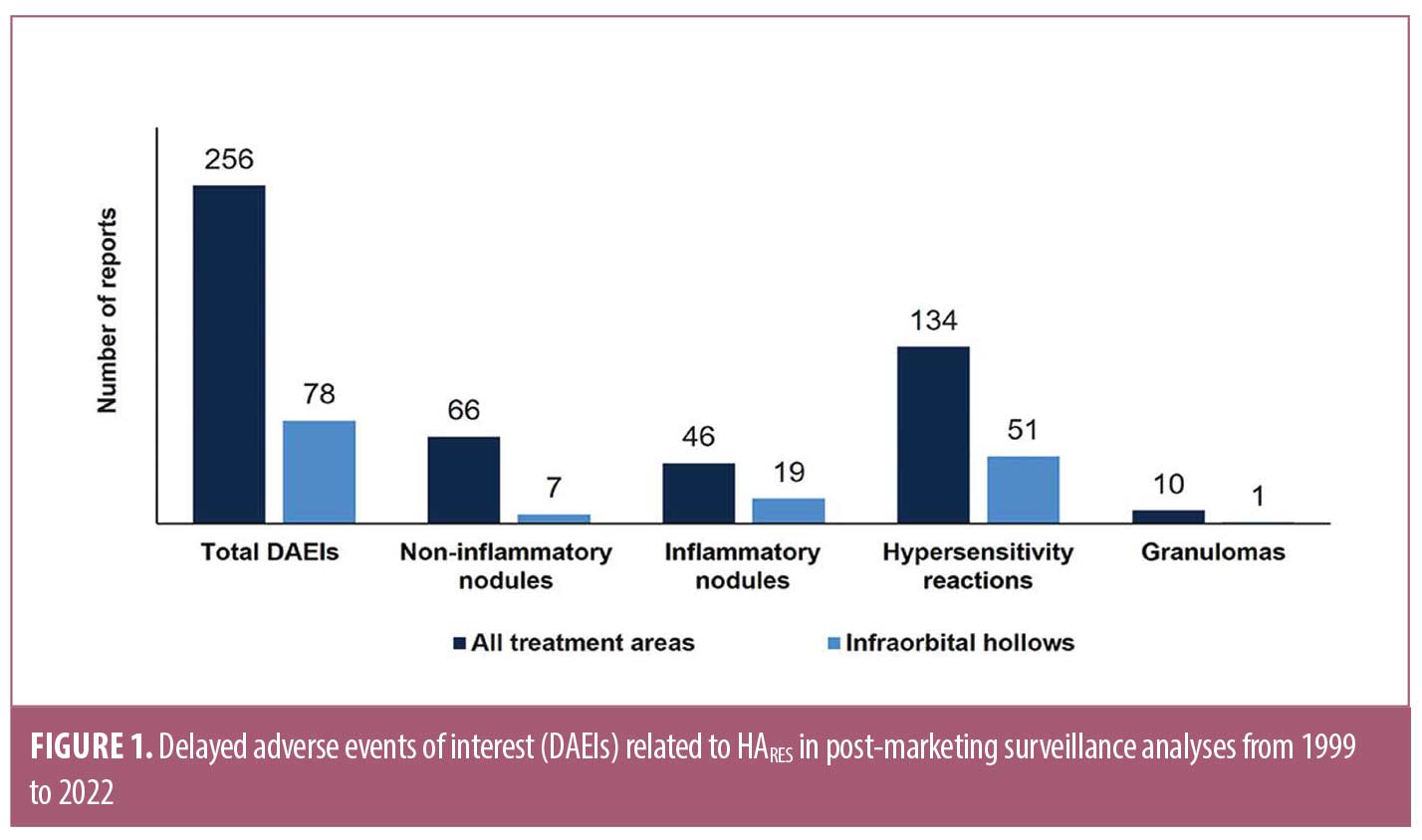
Of the 7,972 reports collected, 421 were identified with keywords and 227 (2.8%) reports contained 256 DAEIs that met the search criteria. The total numbers of DAEIs identified for HARES are displayed in Figure 1. Approximately half (52%, n=134) were hypersensitivity reactions and 26% (n=112) were non-inflammatory nodules, with reporting frequencies of 0.0009% and 0.0005%, respectively. Sequentially, 18% (n=46) were inflammatory nodules and the remaining 4% (n=10) were histologically confirmed granulomas, with reporting frequencies of 0.0003% and <0.0001%, respectively. In some cases, hypersensitivity reactions were reported concurrently with either non-inflammatory nodules (n=12) or inflammatory nodules (n=20) and counted in both categories.
DAEIs occurred between 2006–2018 with most occurring after 2013. Time to onset ranged from 15 days to 3 years, with a median of 2 months. A majority of DAEIs were reported in either the lips, periorbital, or perioral areas, with IOH treatment being the most common. Of those that reported event outcome (n=247) and severity (n=133), about half were resolved or resolving at the time of reporting and moderate in severity.
Out of the 256 DAEIs identified, 78 (30%) occurred after IOH rejuvenation. A majority of those were hypersensitivity reactions (n=51, 65%), followed by inflammatory nodules (n=19, 24%), non-inflammatory nodules (n=7, 9%), and histologically confirmed granulomas (n=1, 1%) (Figure 1). Time to onset for IOH DAEIs ranged from 15 days to 14 months with a median of 2.2 months.
Discussion
This global post-marketing safety review captures delayed-onset nodules and inflammatory events related to HARES reported during the past 23 years (1999-2022). Hypersensitivity reactions were most reported (0.0009%), followed by non-inflammatory nodules (0.0005%), inflammatory nodules (0.0003%), and granulomas (0.0001%) with a median time to onset of 2 months. DAEIs occurring after IOH rejuvenation comprised 30% of all DAEIs identified, and a majority of those were hypersensitivity reactions (65%). While there is an unknown number of syringes sold prior to 2010, we can assume reporting frequencies are overestimated by using the numbers sold from 2010 -2023 as a base for calculations. Regardless, we still recognize that DAEIs are under-reported due to their voluntary nature.
This long-term safety review confirms a favorable safety profile for HARES in clinical practice over the past 23 years. This aligns with previous articles demonstrating relatively low reporting frequencies for HARES compared to other HA fillers.5 Additionally, given that HARES is the most used product to treat the IOH4, the low reporting frequencies of DAEIs after IOH treatment should help ensure injectors of the product’s legacy of safety. This is especially insightful since delayed malar edema is frequently reported after injections in this region.1
Conclusion
This 23-year global post-marketing safety review establishes a long-term safety profile for HARES, with low reporting frequencies of delayed-onset nodules and inflammatory events. Notably, these data support its continued use in clinical practice as the preferred product for IOH rejuvenation.
References
- Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018 Apr;42(2):498–510.
- Dermal Fillers (Soft Tissue Fillers). Risks of Using Dermal Fillers. Accessed 2023 Sep. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/dermal-fillers-soft-tissue-fillers
- Fagien S, Bertucci V, von Grote E, et al. Rheologic and Physicochemical Properties Used to Differentiate Injectable Hyaluronic Acid Filler Products. Plast Reconstr Surg. 2019 Apr;143(4):707e–720e
- Trinh LN, Grond SE, Gupta A. Dermal fillers for IOH rejuvenation: a systematic review. Facial Plast Surg. 2022 Jun;38(3):228–239.
- Povolotskiy R, Oleck NC, Hatzis CM, et al. Adverse events associated with aesthetic dermal fillers: a 10-year retrospective study of FDA data. Am J Cosmet Surg. 2018;3:143–151.