J Clin Aesthet Dermatol. 2025;18(9):22–25.
by Sarah Romanelli, BS; Gretchen D. Ball, BS; Hassan Hamade, MD; Mark Taliercio, MD; Zachary Levy, BS; Lourdes Perez-Chada, MD, MMSc; Joseph F. Merola, MD, MMSc; and Alice B. Gottlieb, MD, PhD
Ms. Romanelli, Ms. Ball, Dr. Hamade, Dr. Taliercio, Mr. Levy, and Dr. Gottlieb are with the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Perez-Chada is with the Department of Dermatology at Brigham and Women’s Hospital at Harvard Medical School in Boston, Massachusetts. Dr. Merola is with the Department of Dermatology and the Department of Medicine, Division of Rheumatology at the University of Texas Southwestern Medical Center in Dallas, Texas.
FUNDING: Funding for this study was provided by International Dermatology Outcome Measures (IDEOM) nonprofit organization.
DISCLOSURES: Dr. Perez-Chada has received grant funding from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and honoraria from IDEOM. Dr. Merola is a consultant and/or investigator for AbbVie, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Incyte, Janssen, LEO Pharma, Lilly, MoonLake, Novartis, Pfizer, Sanofi-Regeneron, Sun Pharma, and UCB. Dr. Gottlieb has received honoraria as an advisory board member and consultant for Amgen, Highlights Therapeutics, Janssen, Lilly, Novartis, Sanofi, Sun Pharma, Takeda, Teva, UCB, and Xbiotech (stock options for RA) and research/educational grants from Avalo Therapeutics, Bristol Myers Squibb, Janssen, MoonLake, and UCB (all paid to Mount Sinai School of Medicine). The remaining authors have no conflicts of interest to disclose.
Abstract: Objective: Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with systemic inflammation and reports of increased prevalence and risk of inflammatory arthritis. Despite this, there is a lack of tools to assess musculoskeletal (MSK) symptoms in patients with HS. This quality improvement initiative aims to evaluate MSK symptom severity and impact in patients with HS using the International Dermatology Outcome Measures (IDEOM) Musculoskeletal Questionnaire (MSK-Q). Methods: Over 20 months, the IDEOM MSK-Q was distributed to 115 patients with HS receiving care at a single dermatology clinic. Demographic and clinical data were collected at all visits. The IDEOM MSK-Q is a 9-item tool scored on a 10-point scale, with subscores evaluating MSK symptom severity, impact on quality of life, and fatigue in the past week. Results: At baseline, 79.14 percent of patients reported joint symptoms, with 33.91 percent rating joint pain ≥7/10. Fatigue was also prevalent (47.82% rating ≥7/10). Higher HS severity was significantly correlated with greater MSK symptom burden, with the greatest impact reported in work and/or school activities and daily physical activities. Follow-up assessments suggested symptom improvement with systemic treatment, though statistical significance was not achieved. Limitations: This study has a small sample size and limited follow-up duration. Conclusion: Our analysis shows substantial MSK symptom burden and fatigue among patients with HS. The IDEOM MSK-Q may be a valuable tool for assessing MSK symptoms and impact, supporting further development and validation in the HS population. Keywords: Hidradenitis suppurativa, inflammatory arthritis, musculoskeletal symptoms, spondyloarthritis, patient-reported outcome measures, quality improvement
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease of follicular biology commonly found in the intertriginous regions. It is characterized by painful nodules, suppuration, abscesses, draining sinus tracts, and scarring.1 Although the exact pathogenesis of HS remains unclear, recent studies indicate a complex interplay between pilosebaceous occlusion and dysregulation of the immune system, sex hormones, and microbiome.2 HS has been shown to exhibit high levels of chronic systemic inflammation, thereby linking HS to systemic comorbidities such as cardiovascular disease, chronic pulmonary disease, diabetes, and depression.3
Previous studies report a higher prevalence of inflammatory arthritis among patients with HS as well as an increased risk of developing the condition, particularly spondyloarthritis (SpA).4,5 Additionally, studies suggest there is an increased incidence of rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis in patients with HS when compared with estimates in the general population.6 Therefore, there is a distinct need to develop more robust patient-reported outcome measures to effectively screen for and monitor inflammatory arthritis symptoms in patients with HS in response to treatment.
Given the absence of a validated tool to assess musculoskeletal (MSK) symptoms and their burden in patients with HS, the International Dermatology Outcome Measures (IDEOM) group strives to address this gap. IDEOM, a nonprofit organization dedicated to developing free, accessible, evidence-based, and consensus-driven outcome measures in dermatology, has created the musculoskeletal questionnaire (MSK-Q), a patient-reported outcome measure originally designed and validated to evaluate MSK symptom severity and its impact on health-related quality of life (QOL) in psoriatic disease.7 Given the lack of a known rheumatologic diagnosis in its stem, the IDEOM MSK-Q has potential for use across various dermatologic conditions. While the questionnaire is already validated in psoriasis,8 its role in HS remains exploratory. This clinic-based quality improvement initiative aims to assess MSK symptom burden in a cohort of patients with HS using the IDEOM MSK-Q.
Methods
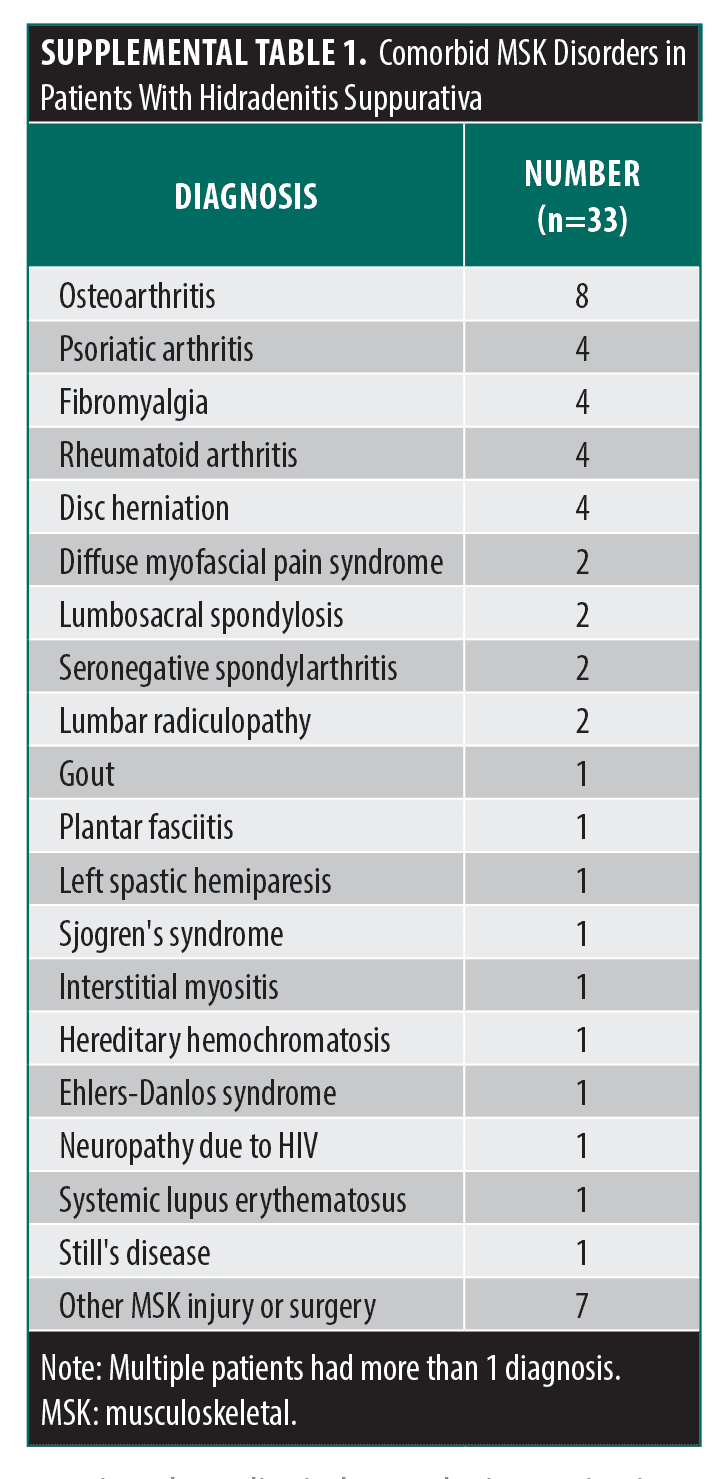
Our quality improvement initiative included a convenience sample of patients who presented to 1 dermatology clinic in a tertiary hospital between July 2023 and March 2025. All patients had dermatologist-diagnosed HS, regardless of MSK symptoms, and completed the IDEOM MSK-Q at least once. The IDEOM MSK-Q is a 9-item tool scored on a 10-point scale that assesses a patient’s MSK symptoms and impact during the past week. The questionnaire is structured into 3 subscores: intensity of MSK symptoms (3 items), impact of MSK symptoms on QOL (5 items), and intensity of fatigue (1 item) (Figure 1). Responses to the IDEOM MSK-Q along with demographic and clinical data were collected at each patient visit. All patients consented with the understanding that questionnaire results could be de-identified and made publicly available.
Results
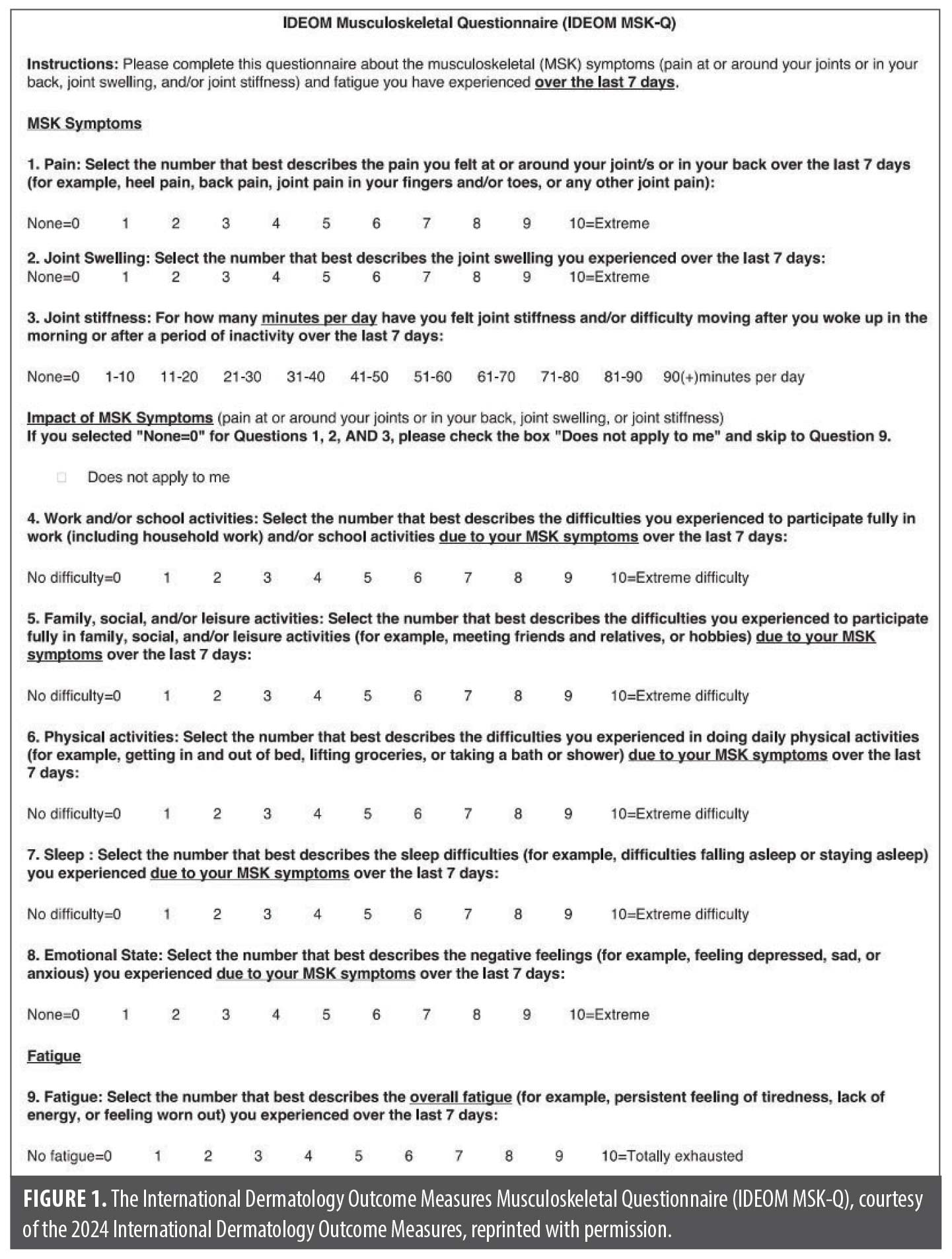
Over 20 months, the IDEOM MSK-Q was distributed to 115 patients with HS. At initial visit, the mean age of our patient cohort was 35.44 years, with a predominance of female participants (74.78%). Among all patients, 39.13 percent identified as Black or African American, 31.30 percent identified as other, 21.74 percent identified as White, and 7.83 percent identified as Asian. The average body mass index (BMI) of our cohort was 31.47 (Table 1).
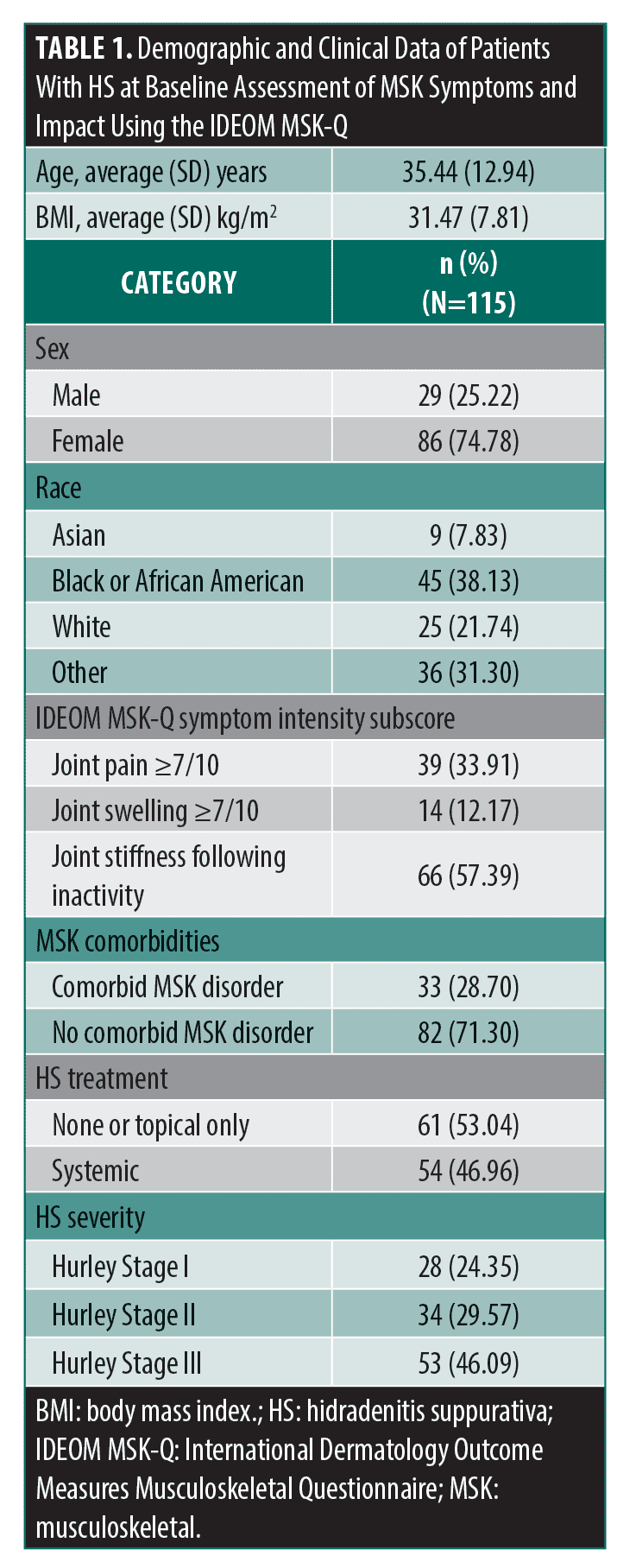
Responses from the MSK symptom intensity subscore revealed 91 patients (79.14%) reported joint pain, swelling, and/or stiffness in the past 7 days. Baseline MSK disorders were seen in 33 patients (28.70%), most commonly osteoarthritis (n=8) (Table 1; Supplemental Table S1). For questions regarding intensity of MSK symptoms, 33.91 percent of patients ranked joint pain ≥7/10 (mean joint pain rating, 4.37 ± 3.32), 12.17% ranked joint swelling ≥7/10 (mean joint swelling rating, 1.91 ± 2.96), and 57.39 percent of patients reported some degree of joint stiffness related to inactivity. Additionally, 55 patients (47.82%) ranked fatigue ≥7/10 (mean fatigue rating, 5.98 ± 3.08), suggesting a high inflammatory burden of the skin and/or joints (Figure 2).
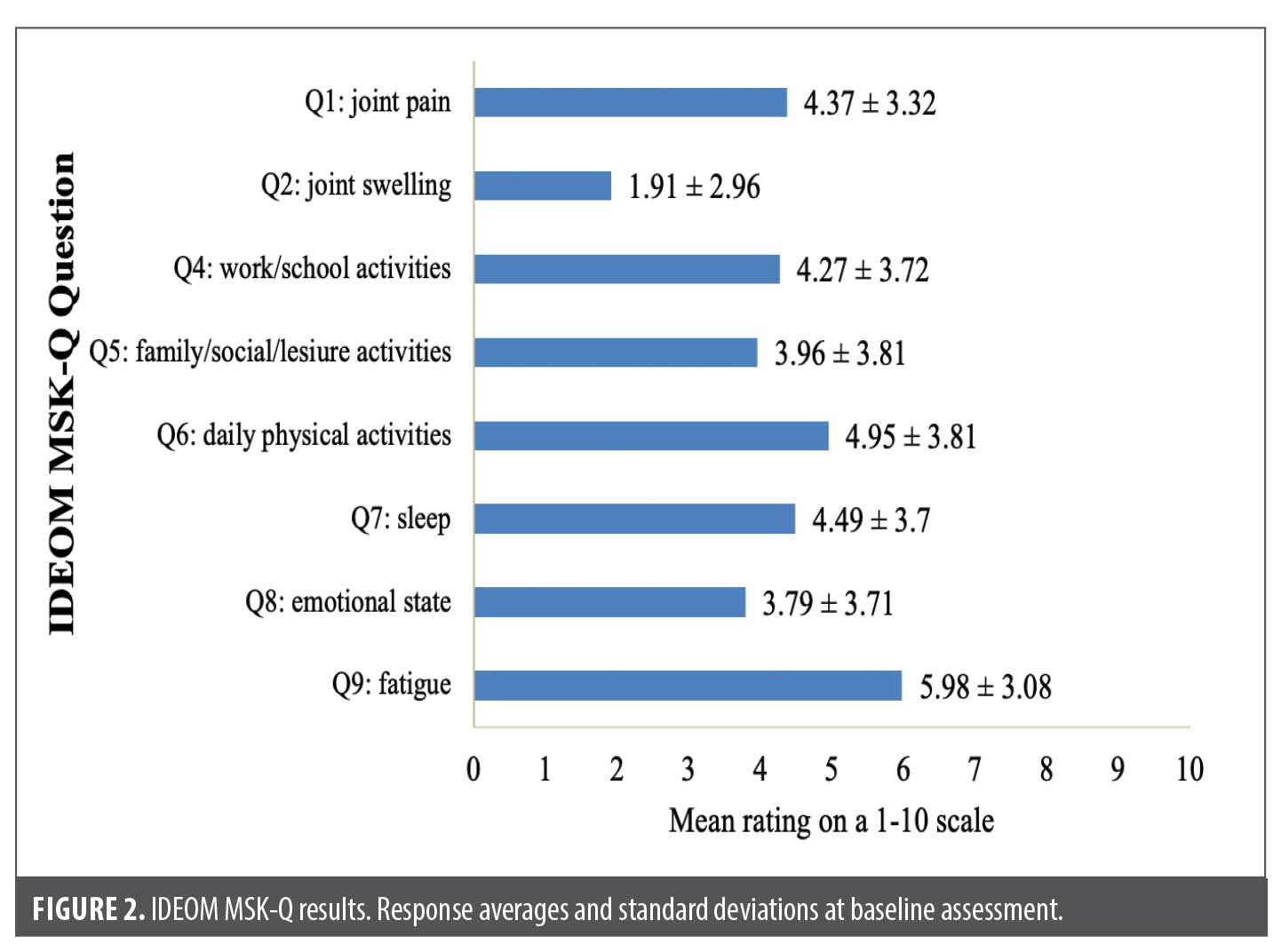
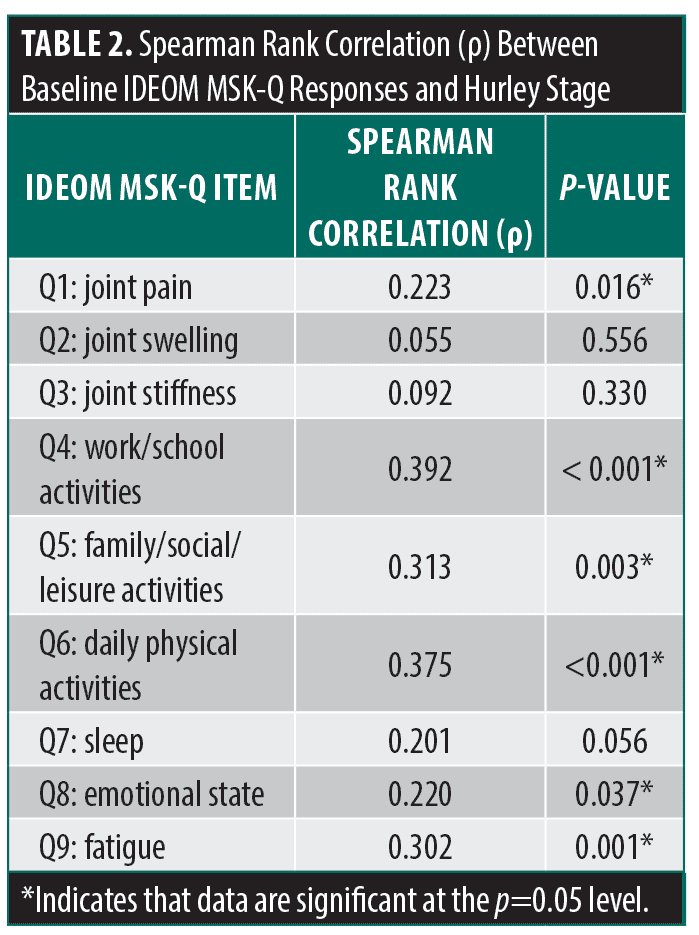
IDEOM MSK-Q responses showed MSK symptoms had the greatest impact on daily physical activities (mean impact rating, 4.95 ± 3.81). Spearman rank correlation analysis showed a significant positive relationship between Hurley stage and most questions listed in the IDEOM MSK-Q; the strongest correlations were seen between Hurley stage and work and/or school activities (ρ = 0.392; p<0.001) and Hurley stage and daily physical activities (ρ = 0.375; p<0.001) (Table 2).
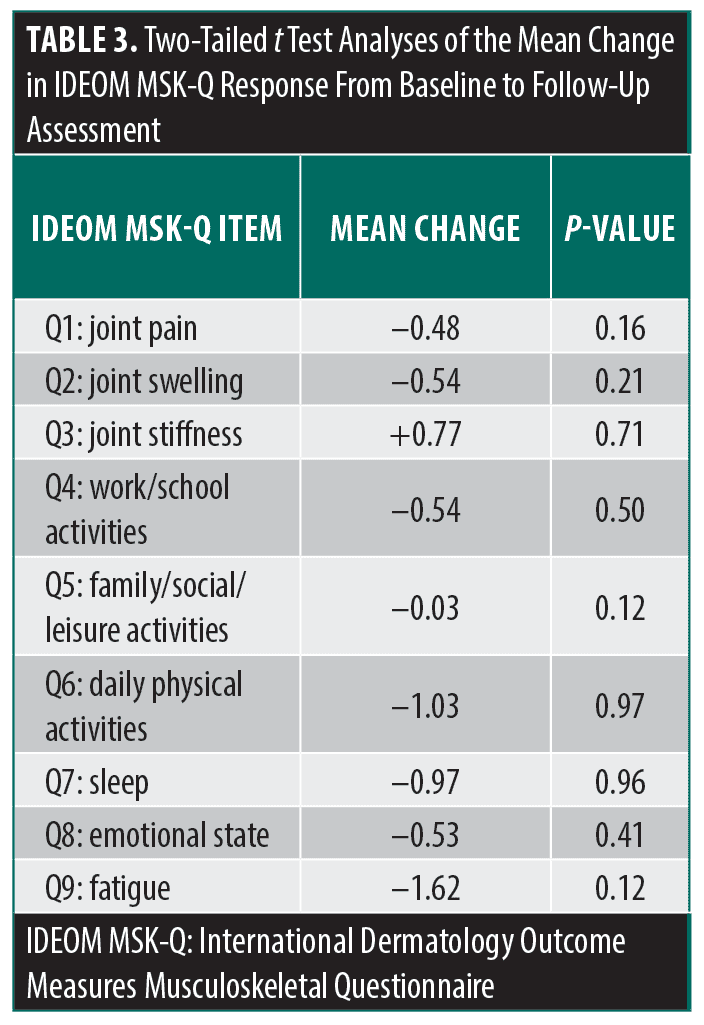
When evaluating changes in questionnaire responses over time, 52 patients (45.22%) completed the IDEOM MSK-Q at 2 distinct points, with a mean follow-up time of 167.96 ± 126.05 days from baseline to most recent follow-up visit. Of this cohort, 18 patients (34.62%) had received no treatment or topical treatment only for HS at the time of initial presentation, with 44 patients (84.62%) reporting joint pain, swelling, and/or stiffness. Between baseline and follow-up visits, 11 patients initiated systemic treatment. By the follow-up assessment, the number of patients reporting MSK symptoms had decreased to 37 (71.15%). While the majority of IDEOM MSK-Q response scores decreased over time, 2-tailed t test analyses revealed no significant differences in the mean changes for questionnaire responses between baseline and follow-up assessments (Table 3).
Discussion
Despite the association of HS with inflammatory arthritis, dermatologists have reported limited comfortability in assessing joint symptoms.9 Therefore, it is crucial to develop patient-reported outcome measures that can capture symptoms and impact related to HS-MSK involvement. Our exploratory analyses indicate that the IDEOM MSK-Q may serve as a useful instrument for measuring MSK symptoms and their impact, warranting further development and validation in the HS population.
Our findings highlight a significant MSK symptom burden in HS, consistent with emerging literature on inflammatory arthritis in patients with HS. In a previous cross-sectional study involving 78 patients with HS, 24 percent of patients reported prolonged morning stiffness and 84 percent reported arthralgia.10 In another cross-sectional study of 294 patients with HS, 50 percent of patients endorsed joint pain without any comorbid musculoskeletal diagnoses.11 Our findings, in concert with previous research, emphasize the need for enhanced screening and early intervention strategies for MSK involvement in this population.
The observed positive correlation of Hurley stage with MSK symptom severity and impact suggests that HS disease severity influences MSK involvement and points to the high systemic inflammatory burden patients with HS face. In a previous multicenter study, 43 patients with HS with arthritis, inflammatory back pain, or enthesitis were identified, with 55 percent meeting the European Spondyloarthropathy Study Group (ESSG) criteria for SpA. Notably, 44 percent of these patients were classified as Hurley Stage III, suggesting a link between HS severity and SpA development.12 Our findings underscore that more severe HS may be associated with a higher prevalence of inflammatory arthritis. Additionally, the high prevalence and severity of reported fatigue in our HS cohort suggests a significant systemic inflammatory burden, consistent with prior evidence linking HS to chronic systemic inflammation.3
Despite trends toward improvement in reported MSK symptoms and impact in patients with HS started on systemic therapy, this relationship could not be adequately assessed due to small patient numbers. As our quality improvement project continues, we aim to increase the number of patients returning for follow-up visits, allowing for a more robust assessment of this trend in the future. Nevertheless, our data suggest that systemic treatment may be beneficial for reducing MSK symptom burden in patients with HS, warranting further investigation in larger, controlled studies.
Conclusion
This quality improvement initiative underscores the high prevalence and impact of MSK symptoms in patients with HS as assessed using the IDEOM MSK-Q. The IDEOM MSK-Q can serve as a valuable patient-reported outcome measure for assessing MSK burden in HS, though further validation in this population is needed. Our findings support the integration of systematic MSK symptom assessment into routine HS management to facilitate early detection and treatment for inflammatory arthritis. While our results indicate potential benefits of systemic therapy in alleviating MSK symptoms, further longitudinal studies are needed to better understand the relationship between HS and MSK involvement and to refine treatment strategies. Ultimately, this study contributes to the growing body of evidence advocating for a multidisciplinary approach to HS care, emphasizing the need for dermatologists and rheumatologists to collaborate in optimizing patient outcomes.
References
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82(5):1045-1058.
- Frew JW. Unravelling the complex pathogenesis of hidradenitis suppurativa. Br J Dermatol. 2025;192(Supplement 1):i3–i14.
- Reddy S, Strunk A, Garg A. Comparative overall comorbidity burden among patients with hidradenitis suppurativa. JAMA Dermatol. 2019;155(7):797-802.
- Almuhanna N, Finstad A, Alhusayen R. Association between hidradenitis suppurativa and inflammatory arthritis: a systematic review and meta-analysis. Dermatology. 2021;237(5):740-747.
- Schneeweiss MC, Kim SC, Schneeweiss S, Rosmarin D, Merola JF. Risk of inflammatory arthritis after a new diagnosis of hidradenitis suppurativa. JAMA Dermatol. 2020;156(3):342-345.
- Hanna N, Silverberg OM, Reaume M, et al. Incidence, prevalence, and predictors of inflammatory arthritis in patients with hidradenitis suppurativa: a systematic review and meta-analysis. Int J Dermatol. 2022;61(9):1069-1079.
- Zhang AJ, Ball G, Zundell MP, et al. International Dermatology Outcome Measures (IDEOM): a report from the 2023 annual meeting. J Drugs Dermatol. 2024;23(12):1114-1120.
- Perez-Chada LM, Gondo G, Grant C, et al. Construct validity of the IDEOM Musculoskeletal Questionnaire: an instrument to measure musculoskeletal symptoms in patients with psoriatic disease. J Invest Dermatol. 2025;145(7):1798-1801.
- Rustad AM, Nolan BE, Ollech A, Boctor MJ, Paller As. Incorporating joint pain screening into the pediatric dermatologic examination. Pediatr Dermatol. 2021;38(1):92-97.
- McKee H, Eder L, Jerome D, et al. Prevalence of musculoskeletal symptoms in patients with hidradenitis suppurativa and associated factors: cross-sectional study. JMIR Dermatol. 2024;7:e58989.
- Lu W, Liu WW, Foolad N, Kwock JT, Jaleel T. Clinical characteristics associated with joint pain in hidradenitis suppurativa. Br J Dermatol. 2024;191(5):839-841.
- Richette P, Molto A, Viguier M, et al. Hidradenitis suppurativa associated with spondyloarthritis — results from a multicenter national prospective study. J Rheumatol. 2014;41(3):490-494.