 J Clin Aesthet Dermatol. 2022;15(3 Suppl 1):S25–S28
J Clin Aesthet Dermatol. 2022;15(3 Suppl 1):S25–S28
by Sara M. Wilchowski, MS, PA-C
Ms. Wilchowski is with Forefront Dermatology in East Lansing, Michigan.
FUNDING: No funding was provided for the preparation of this article.
DISCLOSURES: The author reports no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. The objective of this article is to bring awareness to the emerging frontier of the correlation of the gut microbiome and its impact on psoriasis.
Methods. A Google Scholar and PubMed literature search was conducted utilizing key words “gut microbiome,” “psoriasis,” “diet,” and “inflammation,” yielding several articles for review and classification.
Results. Randomized, controlled trials have revealed that gut microbial imbalances contribute to inflammatory cytokines as well as to the progression and development of psoriasis. Perhaps more importantly, perturbations in the gut microbiome have been correlated to elevated plasma levels of claudin-3, zonulin, and intestinal fatty acid-binding protein, contributing to intestinal barrier dysfunction and permeability. This translocation results in systemic immune activation leading to phenotypic expression of psoriasis in genetically susceptible individuals.
Limitations. Numerous limitations were found during the research on this topic, including lack of standardization of diets, coverage of stool testing by insurance, and personalized interactions of microbes on the host. Further studies are needed with longer follow-up and increased number of patients.
Conclusion. A healthy diet positively impacts the gut microbiome, which can dampen inflammatory cytokines and lessen the severity of psoriasis. The use of probiotics can also influence this dynamic.
Keywords: psoriasis, gut microbiome, inflammation
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting approximately 2 to 4 percent of the population worldwide.1 Thought to once be limited to the skin, the implications of the inflammation associated with psoriasis are now known to be systemic with numerous different phenotypic expressions. Psoriasis is associated with several comorbidities, including metabolic syndrome, inflammatory arthritis, depression, inflammatory bowel disease, cardiovascular disease, and nonalcoholic fatty liver.2-4 The exact mechanism of this complex disease has yet to be fully elucidated. The cytokine profile is primarily a Type 3 mediated disease with the attention on interleukins (IL) 17 and 23 and overproduction of interferon-gamma and tumor necrosis factor-alpha.3 Current effective therapies consist of topical medications that oppose these cytokines that are being overproduced. However, our current understanding of the pathophysiology suggests there are several triggering factors, such as intestinal dysbiosis, stress, alcohol consumption, proinflammatory diet, and infections.4,5
With the recent advances in technology, evidence shows that the intestinal microbiome plays a crucial role in the pathogenesis of psoriasis, and restoring the microbiome is a promising preventative and therapeutic strategy.2 Understanding that 70 percent of the immune system is found in the gastrointestinal tract reveals the clinical significance of immune modulation at this site.6 Perturbation of the gut microbiome poses a risk of a pro-inflammatory environment by innate and adaptive immune cells penetrating the lamina propria, signaling the release of interferon-gamma, tumor necrosis alpha, IL 17, and IL-1-beta, triggering epithelial cell damage and extraintestinal symptoms.7 Alterations in the microbial composition may result in increased permeability of the gut lining as a trigger to immune activation by translocation of microbial antigens and their metabolites into systemic circulation.8 More recently, bacterial deoxyribonucleic acid (DNA) translocation originating from the intestinal lumen of patients with psoriasis has been described, indicating the crucial role of intestinal microbe composition on flares of disease.9 Gut microbes are responsible for adaptive and innate immune responses by inducing immune activation and have a regulatory effect on systemic immunity related to function or dysfunction of distant organ systems.2
This emerging information gives rise to the growing hypothesis that psoriasis may be a disease of the gut lumen that causes systemic immune activation. With the abundance of information available via the internet and delays in care due to the COVID-19 pandemic, in our experience, many patients are seeking alternative therapies and numerous different diets to improve disease on their own. While there are several modifying factors involved in the development and progression of psoriasis, recent attention has focused on nutrition and restoration of the gut microbiome, suggesting that psoriasis may actually be more of an internal disease than previously thought. This article will review the recent literature regarding the influence of the gut microbiome on the development and progression of psoriasis.
Role of the Gut Microbes in Immunity and Inflammation
The human microbiome is established during the first few years of life, and route of infant delivery affects colonization. Specifically, infants born via Cesarean section were observed to have depleted levels of Bacteroides.10 Early colonization comprises bacteria, eukaryotes, viruses, and archaea, encompassing the total diversity of microbes; the diversity varies per anatomic area.1 The colon harbors the highest distribution of micro-organisms, at an estimated 1014 bacterial cells, followed by the skin, which is two orders of magnitude less.2 Influencers on the formation of the microbiota are the use of medications, particularly antibiotics, mode of infant delivery, method of infant feeding, genetics, and lifestyle. The first 2 to 3 years of life are critical for shaping the gut microbiome.11 Disruptions during this period have led to an increased risk of metabolic and autoimmune disease development later in life.11 The change in the gut microbiome also influences the progress and maturation of host immunity, and which goes far beyond digestion.
Colonic microbes have the potential to hasten or dampen inflammation. Mucosal integrity, driven by a healthy diverse microbiota, plays a crucial role in epithelial permeability via secretory IgA and short-chain fatty acids (SCFA) production.8,12 SCFA, such as acetate, propionate, and butyrate, are metabolites produced as bacteria consume nondigestible dietary fibers; butyrate, the primary fuel for colonocytes, is an essential factor in intestinal homeostasis and anti-inflammatory actions.7 If intestinal permeability occurs, translocation of lipopolysaccharides into the blood stream can result in systemic bacterial endotoxemia.13 While every patient is different, the pathophysiology is illustrated in Figure 1.2 Interestingly, there is also an emergence of elevated plasma levels of claudin-3, zonulin, and intestinal fatty acid-binding protein supporting intestinal barrier dysfunction and permeability.2,9,13
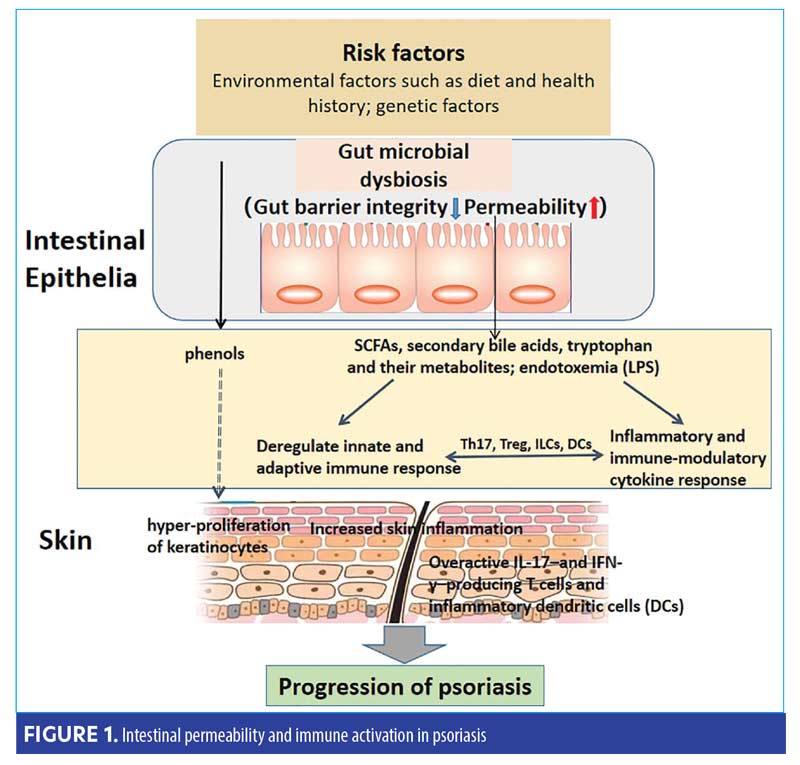
Intestinal Dysbiosis and Psoriasis
An association between flares of psoriasis, alterations of microbiome diversity and composition, and blooms of opportunist pathogens have recently been established.1,2 This alteration in microbial diversity drives subclinical gut inflammation (including passage of lipopolysaccharides and toxins [more commonly recognized as intestinal permeability]), trigger systemic immune responses, and appear to be a hallmark of psoriasis and its known comorbidities.13,14
To date, there are a limited number of studies examining the microbial composition of patients with psoriasis, but an association between alterations in gut diversity, compared to healthy controls, and the development of psoriasis has been shown.2 The two dominant phyla, Firmicutes and Bacteroidetes, represent 90 percent of the microbiota and five out of eight studies reported imbalances within these two phyla in patients with psoriasis.1
A study conducted by Chen et al2 revealed that the ratio of Firmicutes and Bacteroidetes were altered in patients with psoriasis when compared to healthy controls, classified by a decrease in Bacteroidetes (B) and an increase in Firmicutes (F). Other studies conducted by Huang et al15 and Chen et al16 also confirmed a significant difference in the relative abundance of Firmicutes and Bacteroidetes, further confirming microbial dysbiosis in psoriasis patients.15,16 Sequencing of microbial composition also demonstrated decreased abundance of Akkermansia and Prevotella, especially those on biologic therapy.16 Another study confirmed distinct differences in the beta diversity of psoriasis patients and healthy controls.14 Moreover, the repeated increased ratio of F/B seen in patients with psoriasis was linked to a Western diet and obesity.16
Role of Diet in Psoriasis
The ever-growing importance of nutritional influences on disease and microbial composition have led to numerous publications recently correlating this relationship.17 Processed food, including additives highly prevalent in the Western diet, have been associated with microbial dysbiosis, including inflammatory potential.12 Additionally, poor dietary intake for patients with psoriasis indicates lack of awareness of the importance of nutrition and disease severity.5,18 Not only can a pro-inflammatory diet potentiate psoriasis, the immune system in the gastrointestinal tract is directly influenced by food, which can lead to inappropriate immune activation via alterations in microbiota.5 While there may be a need and use for probiotics, the best and most effective way to alter biodiversity is through dietary modification. Dramatic changes in diet, even if for a short term, alter biodiversity within 24 to 48 hours; however, it is important to understand these are transient alterations that do not last after host resumes their old lifestyle.11
A recent study in mice revealed that the standard Western diet decreased microbial diversity leading to pronounced dysbiosis, while switching to a nutrient-dense diet restored biodiversity and improved skin and joint inflammation.19 Not only can food induce microbiome changes, but many foods also contain bacteria, which can transiently affect the microbiome and comprises 5 to 20 percent of the microbial diversity.20
A study conducted by Afifi et al21 found that 86 percent of patients with psoriasis reported that consuming food containing certain ingredients, such as sugar, alcohol, nightshades, or gluten, triggered or exasperated their psoriasis. Previous studies demonstrated that these foods cause alterations in the microbial composition, irritation of the intestinal lining, upregulation of the inflammatory cytokines, and dysbiosis. The researchers also concluded that foods high in fiber, such as fruits, vegetables, and complex carbohydrates, reduce proinflammatory cytokines and rebalance microbial composition.
Western diets are typically high in animal protein, saturated fats, and added sugars, while being low in fiber. However, patients who incorporate a wide variety of fruits, vegetables, and complex carbohydrates into their diets have been shown to have lower levels of circulating tumor necrosis alpha, C-reactive protein, and IL-6.22 Additionally, diets containing fermented foods, such as kombucha and kimchi, have higher levels of bacterial diversity in their guts. However, there are a number of other factors that contribute to this variation, including environment, early life exposures, and immune status.20 It is important to understand this relationship, as positive, long-term changes in dietary habits can reshape the microbiome and, therefore, dampen inflammatory cytokines and lessen severity of disease.
Limitations. There are several limitations to our review that are worth noting. Food-microbe interactions vary per individual. Within the reviewed studies, the studied diets varied widely, posing challenges for comparison. Preselecting diets for participants to strictly adhere to would help mitigate these variations during clinical trials. Reporting errors in diet questionnaires can also impact validity. There are limited randomized, controlled trials available and many have inadequate sample size of participants. Microbial diversity and composition vary; among other factors, they can be affected by birth, use of antibiotics, and lifestyle, making it difficult to study the ideal composition. These findings also give rise to yet another number of complex variables, such as the role of stress, genetics, medications, and lifestyle on the gut microbes. Lack of insurance coverage for stool testing will limit its usefulness in clinical practice. Moreover, there are no long-term studies examining the relationship between psoriasis and gut microbiome nor studies specifically examining specific probiotics.
Conclusion
Studies on the intricate interplay between psoriasis and gut microbiome may be limited, and there is much to learn about the relationship these microbes have on the host. As the healthcare paradigm shifts focus on the importance of the gut microbiome and its relation to systemic disease, it is essential to understand this relationship further. Moreover, a better understanding of the systemic and comorbid disease of psoriasis allows for more comprehensive interventions and patient care. Unlocking the unique dynamic that the gut microbiome plays on systemic immune activation can pave the way for alternative therapeutic interventions with dietary and lifestyle modification, probiotics, and the use of pharmaceuticals. This may also unlock the significant role the patient plays in their wellness journey. This interplay may give rise to increased understanding that psoriasis is more than a disease of hyperproliferation of keratinocytes—it is a systemic disease—as well as increase awareness of the role nutrition plays in overall health. These discoveries will allow us to offer precision medicine and open the door for numerous novel therapeutic interventions.
References
- Myers B, Brownstone N, Reddy V, et al. The gut microbiome in psoriasis and psoriatic arthritis. Best Pract Res Clin Rheumatol. 2019;33(6):101494.
- Chen L, Li J, Zhu W, et al. Skin and gut microbiome in psoriasis: gaining insight into the pathophysiology of it and finding novel therapeutic strategies. Front Microbiol. 2020;0.
- Yamanaka K, Yamamoto O, Honda T. Pathophysiology of psoriasis: a review. J Dermatol. 2021;48(6):722-731.
- Polak K, Bergler-Czop B, et al. Psoriasis and gut microbiome-current state of art. Int J Mol Sci. 2021;22(9).
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between mediterranean anti-inflammatory dietary profile and severity of psoriasis: fesults from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154(9):1017-1024.
- Vighi G, Marcucci F, Sensi L, et al. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008;153(s1):3-6.
- Parada Venegas D, De la Fuente MK, Landskron G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277.
- Wells JM, Brummer RJ, Derrien M, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol – Gastrointest Liver Physiol. 2017;312(3):G171-G193.
- Codoñer FM, Ramírez-Bosca A, Climent E, et al. Gut microbial composition in patients with psoriasis. Sci Rep. 2018;8(1):3812.
- Arboleya S, Suarez M, Fernandez N, et al. C-section and the neonatal gut microbiome acquisition: consequences for future health. Ann Nutr Metab. 2018;73(S3):17-24.
- Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of eiet on the gut microbiota: rethinking intervention duration. Nutrients. 2019;11(12):2862.
- Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The gut microbiota and inflammation: an overview. Int J Environ Res Public Health. 2020;17(20):E7618.
- Sikora M, Stec A, Chrabaszcz M, et al. Clinical implications of intestinal barrier damage in psoriasis. J Inflamm Res. 2021;14:237-243.
- Zhang X, Shi L, Sun T, et al. Dysbiosis of gut microbiota and its correlation with dysregulation of cytokines in psoriasis patients. BMC Microbiol. 2021;21(1):78.
- Huang L, Gao R, Yu N, Zhu Y, Ding Y, Qin H. Dysbiosis of gut microbiota was closely associated with psoriasis. Sci China Life Sci. 2019;62(6):807-815.
- Chen Y-J, Ho HJ, Tseng C-H, et al. Intestinal microbiota profiling and predicted metabolic dysregulation in psoriasis patients. Exp Dermatol. 2018;27(12):1336-1343.
- Pham T, Sokol H, Halioua B, et al. Immune-mediated inflammatory diseases and nutrition: results from an online survey on patients’ practices and perceptions. BMC Nutr. 2021;7(1):38.
- Deniz F, Altunay IK, Ozkur E, et al. Evaluation of healthy lifestyle behaviors in psoriasis patients. Med Bull Sisli Etfal Hosp. 2021;55(2):197-202.
- Shi Z, Wu X, Rocha CS, et al. Short-term Western diet intake promotes IL-23-mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141(7):1780-1791.
- Johnson AJ, Zheng JJ, Kang JW, et al. A guide to diet-microbiome study design. Front Nutr. 2020;7:79.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7(2):227-242.
- Jaceldo-Siegl K, Haddad E, Knutsen S, et al. Lower C-reactive protein and IL-6 associated with vegetarian diets are mediated by BMI. Nutr Metab Cardiovasc Dis NMCD. 2018;28(8):787-94.

