 J Clin Aesthet Dermatol. 2022;15(3 Suppl 1):S17–S19
J Clin Aesthet Dermatol. 2022;15(3 Suppl 1):S17–S19
by Archana M. Sangha, MMS, PA-C
Ms. Sangha is a medical science liaison for Incyte in Wilmington, Delaware. Prior to that, she spent over a decade as a dermatology PA specializing in general, surgical, and cosmetic dermatology. She is a fellow of the American Academy of Physician Assistants in Alexandria, Virginia. She is also Immediate Past President of the Society of Dermatology Physician Assistants.
FUNDING: No funding was provided for this article.
DISCLOSURES: Ms. Sangha is an employee of Incyte in Wilmington, Delaware.
Melasma is a common acquired hypermelanosis affecting the face and neck. While the global incidence of melasma is approximately one percent, it disproportionately impacts skin of color populations. For example, Latin American populations have a prevalence of 9 to 30 percent, while South-East Asian and South Asian populations have a prevalence of 40 percent,1 and Arab-American populations have a 13.4- to 15.5-percent prevalence.2 Numerous studies have shown that melasma has a negative impact on patient quality of life. In particular, patients with melasma have reported feeling embarrassed, having decreased confidence, and that it negatively impacts their relationships.3
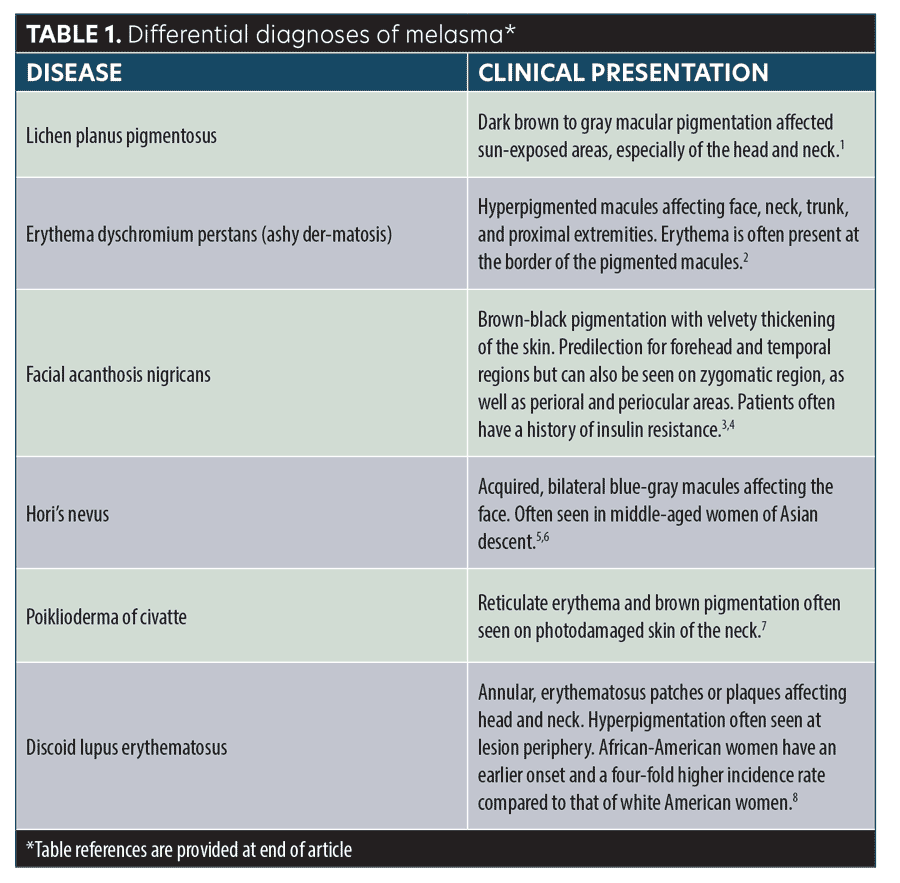
Melasma classically appears as bilateral macular brown patches affecting the cheeks, forehead, upper lip, and/or mandible. The diagnosis is made clinically. If a patient is not improving with treatment as expected, it’s important to reconsider your differential diagnosis (Table 1).
Topical Treatment
Hydroquinone (HQ). HQ is a depigmenting agent that works by inhibiting melanin synthesis. Its use as monotherapy and in triple combination cream (hydroquinone, tretinoin, and corticosteroid) remain the most well-studied and effective treatments. Numerous studies have shown that once-daily triple combination cream has good efficacy. After eight weeks of treatment, one study showed that 29 percent of patients had complete clearance of melasma. The same study also showed that 77 percent of patients were clear or almost clear at the end of eight weeks.5 Patients should be counseled on potential adverse side effects of HQ, the most common of which is irritation. Other adverse side effects include allergic contact dermatitis, erythema, inflammation, xeroderma and stinging. It should be noted that adverse reactions to HQ are often related to its strength and the length of treatment. A rare complication of HQ use is ochronosis, which presents as a blue-black or gray-blue macular pigmentation. It is most often associated with high concentrations of HQ used over long periods of time. Patients should be also counseled on the anticipated duration of treatment with HQ. Improvement often begins 5 to 7 weeks after treatment initiation and treatment should be continued for a minimum of three months.6
Azelaic acid (AzA). AzA is a naturally occurring acid that has antityrosinase activity. It selectively targets abnormal melanocytes and does not depigment normally pigmented skin.7 One study comparing AzA 20% to HQ 4% twice daily for eight weeks showed that those treated with AzA 20% had statistically better Melasma Area Severity Index (MASI) scores than those treated with HQ 4%.8
Cysteamine. Cysteamine is an aminothiol that has tyrosinase inhibition properties. It is also known to be a potent depigmenting agent.9 One study (N=20) comparing cysteamine to HQ in patients with melasma showed no statistically significant difference in modified MASI (mMASI) scores between the two groups at Week 16.10 It should be noted that side effects, although mild and reversible, were more common in the cysteamine group. Another study (N=40) comparing cysteamine cream once daily to placebo for 16 weeks in patients with melasma showed significantly lower MASI scores in the cysteamine group.11 Further studies are needed with larger sample sizes and long-term follow-up to support these findings.
Systemic Treatment
Tranexamic acid (TA). TA is an antifibrinolytic agent used to treat menorrhagia. Several studies have reported its efficacy in the treatment of melasma. A study comparing TA 250mg twice daily to placebo twice daily for 12 weeks in patients with melasma showed that those in the active treatment group had statistically better results.12 Fifty percent of those in the active treatment group had improvement of their melasma compared to 5.9 percent of those in the placebo group. Another study comparing different modalities of treatment in patients with melasma showed that patients treated with HQ 4% and TA 250mg twice daily for 3 months had lower mean mMASI scores than those treated with TA alone and those treated with TA and two sessions of Q-switched ND:YAG laser.13 As a procoagulant, patients should be appropriately screened and counseled on potential serious side effects of TA, such as deep vein thrombosis, pulmonary embolism, and myocardial infarction.14
Practice Pearls
It’s important to explain the chronicity of melasma to patients. Many believe that once their melasma is cleared, it will remain clear with no further maintenance treatment. Thus, patients are often frustrated and disappointed when the melasma “returns.” During the initial visit, take time to thoroughly explain your diagnosis and treatment plan. Emphasize the need to use a broad spectrum sunscreen daily. Often SOC patients believe that they do not need to use sunscreen because their skin doesn’t burn. Take the time to explain that melasma is a photosensitive condition and that the use of daily sunscreen is essential for treatment success.
References
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89(5):771–82.
- Kaleem S, Ghafoor R, Khan S. Comparison of efficacy of tranexamic acid mesotherapy versus 0.9% normal saline for melasma: a split face study in a tertiary care hospital of Karachi. Pak J Med Sci. 2020;36(5):930-934.
- Zhu Y, Zeng X, Ying J, et al. Evaluating the quality of life among melasma patients using the MELASQoL scale: a systematic review and meta-analysis. PLoS One. 2022;17(1):e0262833. Published 2022 Jan 27.
- Schwartz C, Jan A, Zito PM. Hydroquinone. [Updated 2021 Nov 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK539693/. Accessed 14 Feb 2022.
- McKesey J, Tovar-Garza A, Pandya AG. Melasma treatment: an evidence-based review. Am J Clin Dermatol. 2020;21(2):173-225.
- Torok HM. A comprehensive review of the long-term and short-term treatment of melasma with a triple combination cream. Am J Clin Dermatol. 2006;7(4):223-230.
- Bandyopadhyay D. Topical treatment of melasma. Indian J Dermatol. 2009;54(4):303-309.
- Farshi S. Comparative study of therapeutic effects of 20% azelaic acid and hydroquinone 4% cream in the treatment of melasma. J Cosmet Dermatol. 2011;10(4):282-287.
- Prignano F, Ortonne JP, Buggiani G, Lotti T. Therapeutic approaches to melasma. Dermatol Clin. 2007;25:337–42.
- Nguyen J, Remyn L, Chung IY, et al. Evaluation of the efficacy of cysteamine cream compared to hydroquinone in the treatment of melasma: A randomised, double-blinded trial. Australas J Dermatol. 2021;62(1):e41-e46.
- Farshi S, Mansouri P, Kasraee B. Efficacy of cysteamine cream in the treatment of epidermal melasma, evaluating by Dermacatch as a new measurement method: a randomized double blind placebo controlled study. J Dermatolog Treat. 2018;29(2):182-189. Erratum in J Dermatolog Treat. 2020 Feb;31(1):104].
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101(2):E7-E8.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma [published online ahead of print, 2018 Dec 9]. J Cosmet Dermatol. 2018;10.1111/jocd.12830.
- Elkamshoushi AM, Romisy D, Omar SS. Oral tranexamic acid, hydroquinone 4% and low-fluence 1064 nm Q-switched Nd:YAG laser for mixed melasma: clinical and dermoscopic evaluation. J Cosmet Dermatol. 2022;21(2):657-668.
*Table references
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57(5):505-514.
- Schwartz RA. Erythema dyschromicum perstans: the continuing enigma of cinderella or ashy dermatosis. Int J Dermatol. 2004;43:230-232.
- Panda S, Das A, Lahiri K, et al. Facial acanthosis nigricans: a morphological marker of metabolic syndrome. Indian J Dermatol. 2017;62(6):
591-597. - Verma S, Vasani R, Joshi R, et al. A descriptive study of facial acanthosis nigricans and its association with body mass index, waist circumference and insulin resistance using HOMA2 IR. Indian Dermatol Online J. 2016;7(6):498-503.
- Polnikorn N, Tanrattanakorn S, Goldberg DJ. Treatment of Hori’s nevus with the Q-switched Nd:YAG laser. Dermatol Surg. 2000;26(5):477-480.
- Bhat RM, Pinto HP, Dandekeri S, Ambil SM. Acquired bilateral nevus of ota-like macules with mucosal involvement: a new variant of Hori’s nevus. Indian J Dermatol. 2014;59(3):293-296.
- Bernstein EF, Schomacker K, Paranjape A, Jones CJ. Treatment of poikiloderma of Civatte using a redesigned pulsed dye laser with a 15mm diameter treatment spot. Lasers Surg Med. 2019;51(1):54-58.
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. [Updated 2021 Dec 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. https://www.ncbi.nlm.nih.gov/books/NBK493145/. Accessed 145 Feb 2022.

