J Clin Aesthet Dermatol. 2021;14(8):E69–E75.
Download a PDF of this guideline here
by Gillian Murray, MPharm, PG Dip Clin Pharm, INP; Cormac Convery, MB ChB, MSc, MASLMS; Lee Walker, BDS, MFDS, RCPSG, MJDF, RCS, ENG; and Emma Davies, RN INP
Ms. Murray is with Clinical Academic Kings College in London, England. Dr. Convery is with The Ever Clinic in Glasgow, Scotland. Dr. Walker is with B City Clinic in Liverpool, England. Ms. Davies is Clinical Director of Save Face in Cardiff, United Kingdom. All authors are founding board members of the Complications in Medical Aesthetics Collaborative (CMAC).
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Vascular occlusions can occur with injection of dermal fillers, causing devastating outcomes for the patient. Hyaluronidase is an enzyme that was first used in general medicine in 1949, but has gained widespread use in aesthetic medicine to dissolve hyaluronic acid (HA)-based dermal fillers. Knowledge of this drug and its use for other aesthetic indications is evolving, and there is often anxiety attached to the administration of animal-derived product due to fears of an allergic reaction.This paper provides guidance on the indications for use when dissolving HA filler with hyaluronidase. It discusses formulation differences, adverse events, allergy to wasp and bee stings, and how this increases risk of allergy to hyaluronidase. It also discusses incidence of allergy, which includes a discussion of types of allergic response and how this correlates to skin tests and a discussion on skin testing for allergy.
KEYWORDS: Hyaluronidase, hylenex, vascular occlusion, elective dissolution, cross-linked hyaluronic acid, dermal filler, hyaluronic acid, high-dose protocol, delayed-onset nodule, delayed inflammatory reaction, DON
Cross-linked hyaluronic acid (HA) is the most commonly used filler for volume replacement and soft tissue augmentation. Endogenous hyaluronic acid is ubiquitous through the body and is a major structural component of the extracellular matrix. It is found within the skin and acts to support tissue architecture and maintain hydration due to its hygroscopic nature.1
Hyaluronidase is a soluble protein that functions as an enzyme. It is used within aesthetic medicine for both elective and emergency procedures; however, it has been used within the sphere of medical practice since 1949. It is commonly used in various fields of medicine, including anesthesia and pain, cardiology, radiography, oncology, ophthalmology, and plastic surgery. Within the past 15 years, hyaluronidase has been used in aesthetic medicine to dissolve cross-linked hyaluronic acid. It has a broad spectrum of clinical applications due to its unique ability to facilitate the dispersal and/or absorption of fluids and a variety of medicines. It has also been used for specific indications, such as treating keloids scars as an alternative to steroids, dissolution of hematomas and treatment of lymphedema.1,2 Hyaluronidase breaks down complex hyaluronan glycosaminoglycan polysaccharides by a hydrolysis reaction. Hyaluronidase targets the breakdown of the C1 and C4 bond between the glucosamine and glucuronic acid components causing the complex molecule to unfold and break down.3 Its primary function within aesthetic medicine is to dissolve cross-linked HA dermal fillers; however, it can also be used to improve resistant edema, given its ability to increase capillary and tissue permeability.4
It is important that in medical aesthetics, the practitioner understands the clinical application of hyaluronidase in practice, risks associated with its use (e.g., allergy), storage, and directions for administration.
Practical Application of Hyaluronidase in Aesthetic Medicine
Vascular occlusion. It might be necessary to dissolve cross-linked HA in the event of a vascular occlusion (VO). A VO resulting from an accidental intravascular injection is a time-sensitive but not a time-critical event, unless there is visual or neurological disturbance. Failure or delay in dissolution could lead to tissue necrosis, scarring, blindness, and/or cerebrovascular accident. Refer to the Complications in Medical Aesthetics Collaborative (CMAC) guideline for the management of hyaluronic acid-filler induced vascular occlusion for more information.
Tyndall effect. Tyndall effect is a phenomenon seen when particulate fillers are injected into a superficial plane. It has been postulated that this effect is due to shorter wavelengths (i.e., blue) being preferentially scattered by the filler particles, creating a blue hue which is visible to the naked eye. Historically, the Tyndall effect has been attributed to the scattering of light when it is passes through particles with a smaller wavelength than light itself. HA molecules are much larger than the wavelength of light, making this theory seem unlikely. Recent evidence has suggested that the HA alters the tissue physiology, allowing deeper absorption of red light. The effect of this makes the tissue appear more blue.5
Delayed-onset nodules. Delayed nodules can appear weeks or months after injection of the dermal filler and can be caused by any soft-tissue filler, including cross-linked HA. Understanding the underlying pathophysiology of these nodules is challenging due to the limited access to investigations and the reluctance of patients to permit tissue sampling.
Delayed (Type IV) hypersensitivity reactions, granulomas, and biofilms are possible causes of delayed-onset nodules, with a possibly mixed pathology. When the filling agent is HA, there is a significant advantage in being able to dissolve the nidus of the problem. When there is a significant infective component, hyaluronidase should be administered only under broad-spectrum antibiotic cover.6
Poor aesthetic outcome. Incorrect placement of filler, excess filler, and migration/redistribution can lead to a poor aesthetic outcome and patient distress. Good product and rheological knowledge, a deep appreciation of three-dimensional anatomy, and correct technique are vital for an optimal aesthetic outcome.
As cross-linked hyaluronic acid breaks down in the tissue, its physicochemical properties alter. These changes can affect all rheological parameters of the filler. As the properties change, by virtue of the tissue condition and being subjected to hydrolytic degradation, the aesthetic outcome can begin to change. This change in physicochemical properties can also contribute to some dermal fillers migrating into areas of high muscle activity.7
Hyaluronidase
In the United Kingdom (UK), hyaluronidase (Wockhardt; Wrexham, United Kingdom) is a prescription-only medicine. It is licensed to enhance the permeation and uptake of subcutaneous or intramuscular injections, local anesthetics and subcutaneous infusions, and to promote the resorption of excess fluids and blood in the tissues. In the UK and the United States, its use in aesthetic medicine to dissolve cross-linked hyaluronic acid is considered an off-license indication.6 As part of the consent process, the patient must be advised of its off-license status.
The manufacturer’s data sheet from Wockhardt states that the product must be stored at temperatures of less than 25°C to ensure formulation stability. The product includes an expiry date and can be used until the last day of the month its due to expire, if correctly stored. When stored at temperatures consistently above 25°C, the product’s expiry date will be affected. Once the ampoule is opened, it must be used immediately, and any unused contents discarded.6
If the clinician resides outside of the UK and has access to other brands of hyaluronidase, the manufacturers data sheet should be followed for specific storage instructions.
Hyaluronidase can be reconstituted with most common infusion fluids; however, for use in medical aesthetics, it is most frequently reconstituted with bacteriostatic sodium chloride (NaCl) 0.9%, which is less painful upon injection. Bacteriostatic and non-preserved NaCl 0.9% have similar pH values (pH 4.5–7) (Alison Stevenson, CEO, Tor generics; email communication, February 2021).8 This is important, as enzyme activity is pH sensitive and bovine/ovine extracted hyaluronidase has a bimodal activity with maximum activity of pH 4.5 and 7.5.9 However, in the event of a VO, where there might be pain due to ischemia, it is recommended that hyaluronidase be reconstituted with a local anesthetic without adrenaline to make the experience more comfortable for the patient. Hyaluronidase is physically compatible with lidocaine in solution, and in ocular and spinal anesthesia, it is commonly used in combination with local anesthetic agents and administered as part of anesthetic blocks.7 Formulations can differ from country to country, so always refer to the manufacturer’s data sheet when checking compatibilities with diluents.
Allergic Risk Associated with Hyaluronidase treatment
Incidence of allergy. There is a growing concern among clinicians that an anaphylactic (Type I hypersensitivity) reaction can occur when injecting hyaluronidase, with many clinicians performing skin tests to screen for these reactions. The British Society of Allergy and Clinical Immunology (BSACI)10 state that skin tests must always be interpreted within the appropriate clinical context and not used to screen for drug allergy.11,12 The BSACI further elaborate that skin tests should not be used to screen for drug allergy in the absence of a clinical history compatible with an immunoglobulin E (IgE)-mediated allergy, otherwise known as a Type I hypersensitivity.10 Skin testing is usually done in specialist centers where there is a clinical history of drug allergy.13 Despite this, skin testing is frequently used in aesthetic medicine when there is no reason to suspect allergy. In such circumstances, it is deployed as a method of reassuring the clinician that the patient will not develop anaphylaxis, which is against BSACI recommendations for appropriate allergy testing.10 Since 1949, there have been four case reports detailing allergic reactions to hyaluronidase requiring adrenaline. The incidence, therefore, is extremely small. The incidence of hypersensitivity is often widely quoted as approximately 0.1 percent, unless large intravenous doses (in excess of 200,000 units) have been given, where treatments yielded a Type I reaction rate of 33 percent.14,15 However, reported rates of allergy include all allergy, not specifically Type I hypersensitivity. When information from the reported incidences of allergies are combined, the incidence of overall allergy is actually very small (Table 1). The allergic responses consist mainly of localized Type IV reactions (defined as reactions occurring at one hour or later) unless large doses are injected intravenously.16,17 It is also important to state that the incidence rates of allergy do not specify rates for first exposure compared to subsequent exposure, and allergy was almost exclusively linked to prior exposure to hyaluronidase or bee/wasp venom.18–31
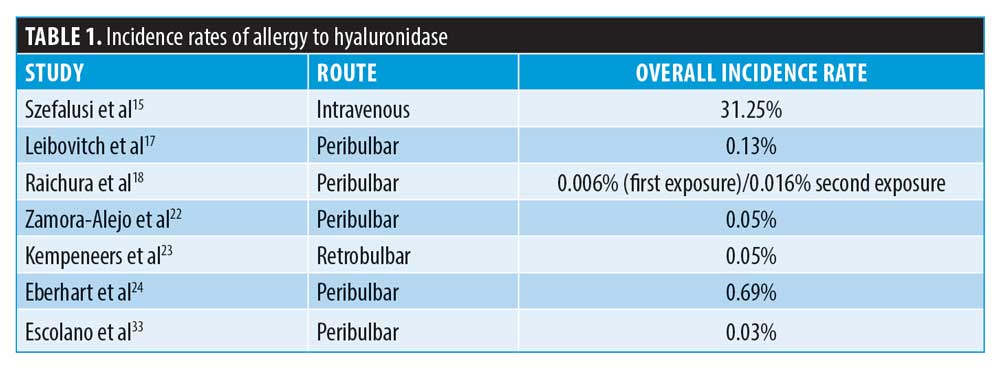
Regarding the risk of allergy in aesthetic medicine, there are only three case reports of allergy after hyaluronidase injection for the purpose of dissolving cross-linked hyaluronic acid filler, totaling four patients in the literature.18,19,20 However, the authors believe this incidence is under-reported, having seen several cases of localized allergy in their own clinical experience.
Formulation and allergy. Hyaluronidase is used globally, with most formulations being derived from ovine or bovine testes. In the UK, for example, the Wockhardt product is ovine derived. It is important to note that some countries use compounded (i.e., pharmacy formulated) hyaluronidases, containing more protein impurities than the ovine- or bovine-derived products, while many practitioners in the United States use recombinant hyaluronidase (Hylenex®; Halozyme Therapeutics, Inc., San Diego, California) which is known to be the purest formulation.2 Much of the literature, based around case reports, assumes that the problematic protein in the hyaluronidase formulation is the enzyme itself. The presence of protein impurities within the formulation is also a contributing factor to the allergic response, but this is difficult to quantify.2 Thimerosal, a preservative used in some hyaluronidase preparations, has long been known to cause allergic reactions. Hyaluronidase produced by Wockhardt in the UK does not contain a preservative, or any other excipients, but will contain impurities by virtue of the formulation process. There has never been a study which has isolated and tested protein impurities within all the hyaluronidase products and established links to allergy, or whether the allergy is potentiated by the combination of the hyaluronidase and the preservative/ protein impurities. Of note, there have been no documented cases of allergy with Hylenex® (human recombinant rHuPH20).33 It was also found that upon comparison to animal-derived hyaluronidases, Hylenex® had 100 times more activity per milligram of total enzyme protein;2 however, the clinical significance of this fact is not yet understood. Another factor to consider is the use of bacteriostatic NaCl 0.9% when reconstituting hyaluronidase. NaCl 0.9% is preserved with benzyl alcohol, with an allergy rate of 1.3 percent.34 This is greater than the average allergy risk to hyaluronidase. Therefore, it remains unknown whether the hyaluronidase enzyme has been the direct cause of allergy in all cases of hyaluronidase allergy reported in the literature.
Dose/route and allergy. The small number of case reports pertaining to allergy in the literature suggest that the route of administration and dosage might be a factor in the severity of allergic reaction. In general, when dose ranges of less than 1,500 units are injected at a local site, allergic reactions to hyaluronidase are confined to localized responses (e.g., edema, erythema, urticaria) in the injection area without generalized symptoms.22 There appears to be a dose-dependant effect on administering hyaluronidase. When doses range from 1,500 to 200,000 IU and /or are injected intravenously, most allergic patients described in the literature present with more generalized symptoms.18–31
The rates of allergy after ocular administration dominate the literature. Residence time in ocular tissue has been reported to be longer than in other tissues that have a denser vascular network. It is thought that the half-life in ocular tissue is 60 to 112 minutes, meaning the tissues might have a greater exposure to the drug before it reaches the plasma, where the serum half-life is around two minutes.35 However, when investigating this proposed half-life of 60 to 112 minutes, the paper citing this figure35 did not cite a reference for the statement. Greater exposure to the drug might allow for a greater degree of primary sensitization, and different tissues exhibit different half-lives. In addition, due to sensitzation or coadministration with other drugs, there is a relatively higher risk of apparent Type IV hypersensitivity to hyaluronidase when administered as part of an ocular block.
Side effects. Hyaluronidase at concentrations greater than 1:10 (1500:10mL) can be irritant. Erythema at the injection site is a commonly known side effect. Side effects are Type A adverse reactions, meaning they are predictable by virtue of the drugs pharmacology, but they are not allergies, which are classed as Type B adverse reactions. High-dose hyaluronidase can provoke hypersensitivity-like responses, such as non-infectious swelling and inflammation, while skin allergy tests remain normal.13,19 This injection-site reaction would typically include erythema and some swelling that subsides within 24 hours, and in the authors’ experience, can occur when injecting hyaluronidase. This would indicate a Type A adverse reaction, and not a Type B reaction, as seen in an allergy presentation.
Intradermal Testing
Skin allergy testing is prevalent in the practice of medical aesthetics; however, the most valuable information to the clinician is the patient’s medical history, drug history, allergy history, and any reaction on prior exposure to the hyaluronidase. There are certain groups of patients who, upon having a Type I reaction to any drug or allergen, will have a more significant anaphylactic response. Examples of these patients include those on an ACE inhibitor, those with mast cell disorder and high trypsin levels, allergic atopic individuals, C1 esterase deficiency, hereditary angioedema, and those on a beta blocker,10,13 where it will be more difficult to restore hemodynamic stability during anaphylaxis. It is therefore important to ensure the medical and drug history of each patient is accurately taken in the first instance of a reaction.
Wasp/bee allergy. Prior to injecting hyaluronidase, it is important to ascertain a possible or confirmed allergy to bee and/or wasp stings. Allergies to bee and wasp venom, part of the Hymenoptera order, pose a significant risk of cross reactivity. When assessing allergies to wasp and bee stings it is important to assess the type of reactions, and any information known about allergy status to any of the Hymenoptera order. Small local reactions around the site of stings are a normal occurrence, and not allergy. The clinician should assess the history and the description of the reaction, if consulting a patient who has self-diagnosed their own allergy. Allergy to these stings take two forms:
1) A large, localized reaction, which is defined as painful swelling and erythema limited to the skin and subcutaneous tissue surrounding the sting. The affected area is large and can exceed 10cm and peaks at Days 1 to 2, taking up to 10 days to resolve. The risk of anaphylaxis from a subsequent sting if a patient has had a large local reaction (LLR) is greater than five percent. If the patient has had an anaphylactic reaction to bees or vespidae wasps, then the risk of anaphylaxis to a subsequent sting is at least 60 percent.36
2) Wasp and bee stings contain more than one allergen (i.e., protein), including hyaluronidase. If no specialist allergy testing has been performed on a patient with reported anaphylaxis to these stings, the clinician would have to assume it could be the hyaluronidase, based on worst-case scenario. Given this, the risk of anaphylaxis on administering hyaluronidase on a background of a previous LLR, or anaphylaxis to stings, is up to five percent and 60 percent, respectively.36 If the patient has had an allergy to both bee and wasp stings, it is more likely hyaluronidase is the allergen.36
Performing an intradermal test on patients with a history of LLR or anaphylaxis to stings from the Hymenoptera order is an anaphylaxis risk. It is important that allergy tests in these individuals are done at an allergy clinic where full allergy testing can be undertaken to ascertain the specific allergenic substance with anaphylaxis support available.
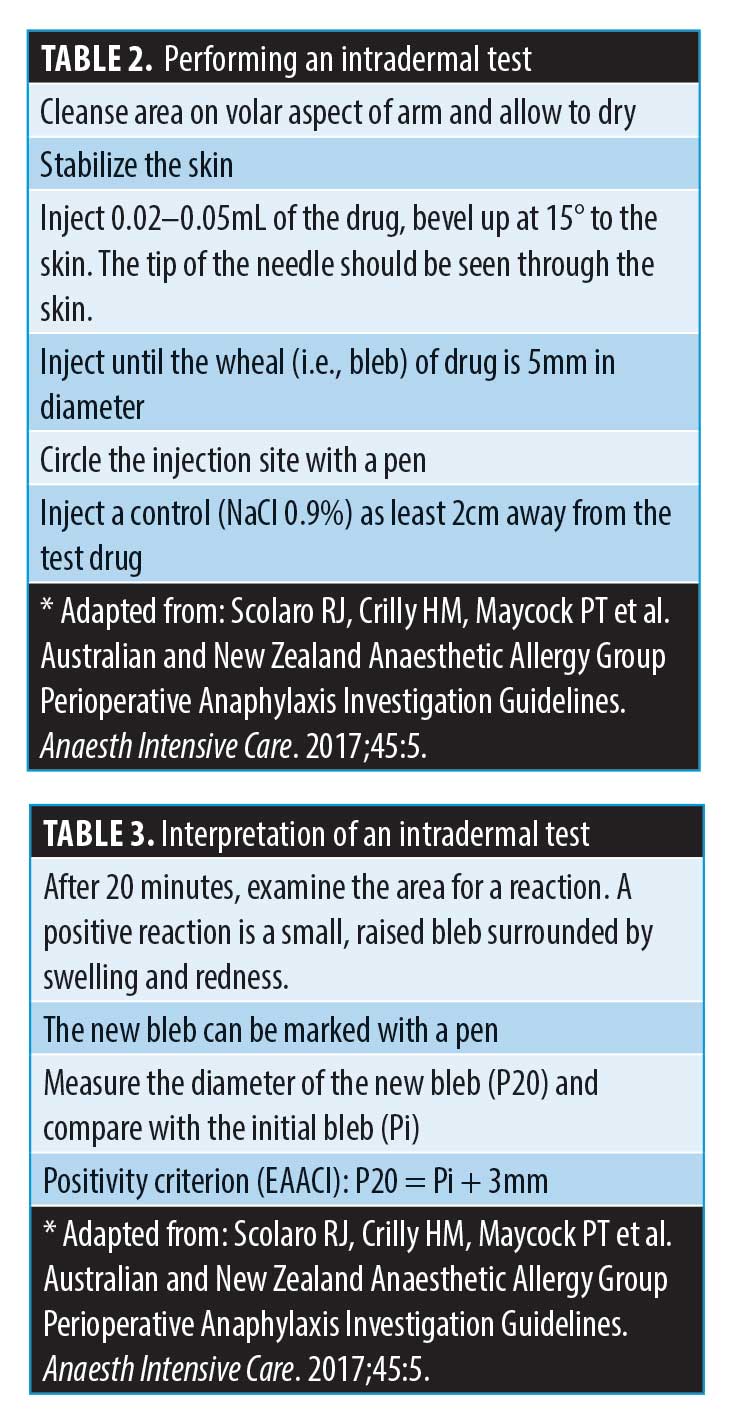
If the decision is taken to perform an intradermal test (IDT), please note that there is no validated concentration that is used to assess hyaluronidase allergy in the UK (M. Shayegi, MD, Chief Scientific Officer, BSACI; email communication, July 2020). In the literature, case reports of hyaluronidase allergy have cited doses of 15units/mL to 150 units/mL to verify the allergy.26,37 This is the concentration, and not total volume injected. The volume injected to assess a Type I hypersensitivity using an intradermal test, according to allergy guidelines, is around 0.02–0.05mL.13 This is the volume needed to achieve a 5-mm bleb. If best practice was followed in these studies, the number of units used in total, despite not being stated in most of the studies, might have been a fraction of this.38 Kim et al19 and Rajalakshmi et al41 suggested that 15 units might be used to verify the allergy, resulting in a positive IDT. Borchard et al26 reported having to increase the concentration from 1.5u/mL to 150u/mL to achieve a positive IDT, where 1.5u/mL and 15u/mL gave negative reactions. There was a lack of consistency in concentration used to verify allergies in these case reports. A publication by Vartanian et al40 described a local reaction when 10, 20, and 30 units of hyaluronidase was injected to dissolve HA (Restylane®; Galderma, Lausanne, Switzerland) once injected into the volar aspect of the arm, to assess degradation. The authors found a dose-dependant local reaction occurred in some of the test studies. However, as discussed, it is common for hyaluronidase to give rise to local irritation when injected, as a predictable type A reaction.39 It remains to be established whether in the cases reported by Vartanian et al40 these were allergic Type IV reactions, or predictable irritation on injecting the drug.
In patients with a history of anaphylaxis to bee and wasp stings, performing intradermal testing with unvalidated test concentration could result in anaphylaxis. Reliable testing must be valid and sensitive. Current skin test practice conducted by medical aesthetic clinicians is neither valid nor reliably sensitive, given there is no validated test concentration. The clinician must consider this when interpreting results.

Performing an IDT. If the decision is made to perform an IDT, please follow the instructions outlined on Tables 2 and 3. CMAC recommends using 15 units of hyaluronidase (if the formulation is ovine/bovine or compounded enzyme). Table 2 describes the steps taken to perform an intradermal test, and Table 3 how to interpret the test. Often, erythema can develop at the site either within 20 minutes or some hours later. Neither of these are positive results for Type 1 hypersensitivity (i.e., anaphylaxis).
Elective and Emergency Use of Hyaluronidase
Doses. For guidance on the use of hyaluronidase in the event of a vascular occlusion, please refer to the CMAC guideline for the management of hyaluronic acid filler-induced vascular occlusion.
Elective reversal. In the event of elective reversal of hyaluronic acid fillers, there are no standard concentrations or doses described in the literature. Different brands of cross-linked HA can dissolve differently, some requiring more hyaluronidase and some less. Cross-linked HAs demonstrate different physicochemical properties and rheological properties that may change as residence time in the tissue increases. The volume of filler and how densely it appears in the tissue can also require the need for more or less enzyme. Various studies in the literature have compared how some of the well-known filler brands degrade in response to hyaluronidase,41 however the findings are inconsistent across studies and do not reflect the complete set of fillers used frequently within the UK or global market. More research in this area is required.
As the literature describes a variety of doses used to electively dissolve cross-linked HA, CMAC suggests that treating to effect is more reliable than specific doses. Simplistically, we recommend using enough hyaluronidase to treat the problem area (i.e., treat to effect). Complications data obtained by the CMAC board indicate that the doses in the literature are very conservative. CMAC recommends not using concentrations of less than 1,500 units in 5mL. Although hyaluronidase removes native hyaluronic acid, given the rapid turnover time, the body will restore native HA in 15 to 20 hours2 and the clinician should not feel reluctant to administer what is needed on this basis.
After elective treatment with hyaluronidase, the patient can be assessed after 48 hours and the treatment repeated if necessary. It might take longer than 48 hours for the postprocedural swelling to subside;42 however, no enzyme activity remains at this time and cross-linked HA may be administered without risk of being dissolved. However, CMAC recommends waiting a minimum of two weeks until the swelling has settled, longer in the event of significant postprocedural swelling, to ensure a more predictable aesthetic outcome.
Emergency reversal. CMAC has previously published guidance on the identification, pathophysiology, and management of a hyaluronic acid-based vascular occlusion.
CMAC recommends following the steps outlined in Table 4 for the effective management of a vascular occlusion without the aid of ultrasound guidance.
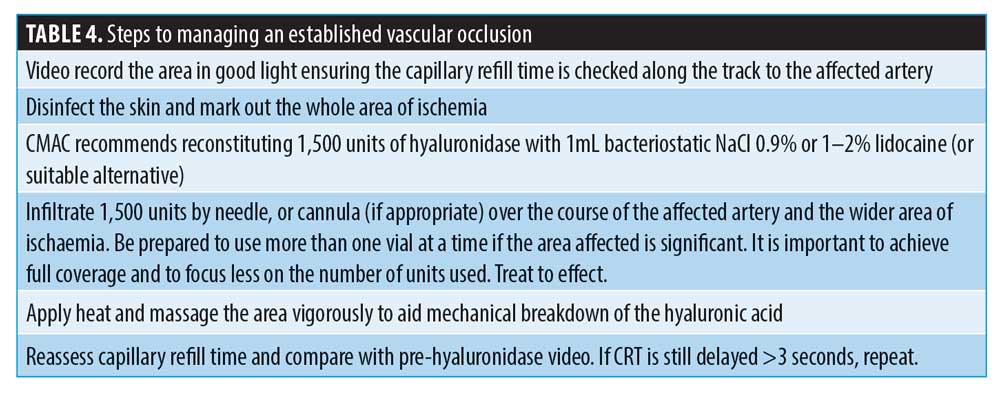
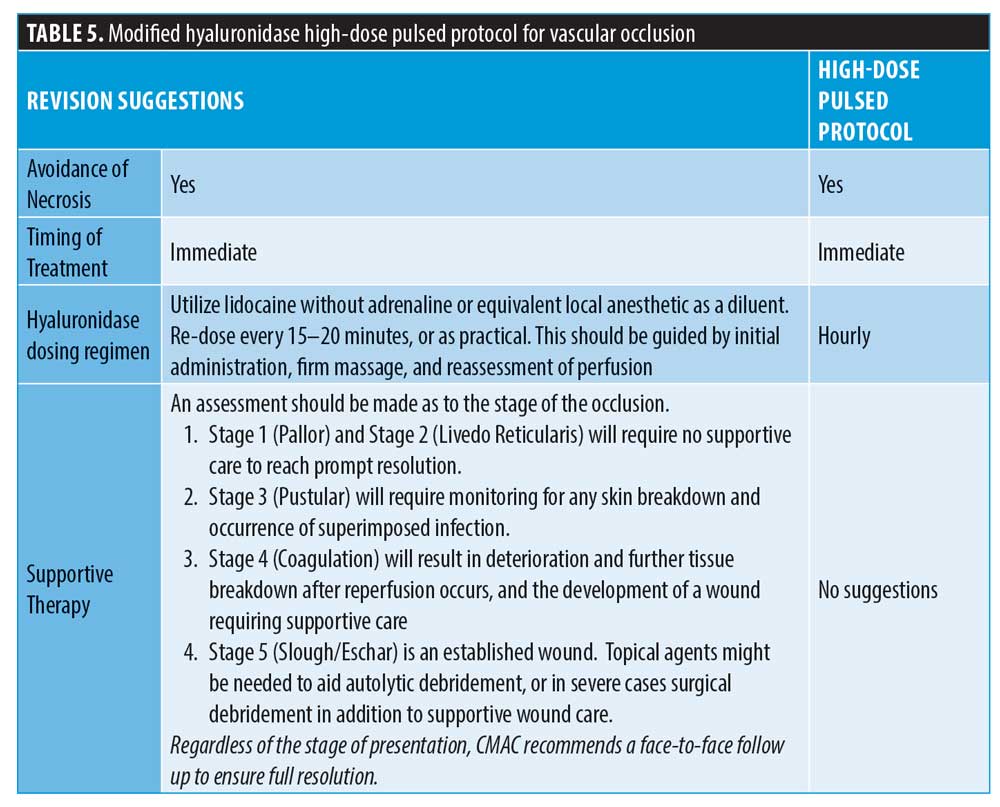
Following on from the previously published protocol on high-dosed, pulsed infusion,43 CMAC would like to propose a change to the frequency of administration of hyaluronidase. While the plasma half-life of hyaluronidase is around two minutes,35 this is not relevant for the application of use in aesthetic medicine, where the injection site is subcutaneous or intramuscular.45 Once it reaches the systemic blood supply, it will rapidly degrade. The pharmacokinetic modeling of hyaluronidase after subcutaneous and intramuscular administration has been documented in rodent studies. It was found that the half-life after subcutaneous and intramuscular administration was 5.1 minutes and 7.5 minutes, respectively.45 Hyaluronidase is a dispersal agent, moving the diluent away from the point of injection.6 Based on the pharmacology of hyaluronidase and the fact that the half-life in both the subcutaneous and muscular tissues is so brief, CMAC advises administering the initial dose and applying firm massage before reassessing the vascular flow. The hyaluronidase should be readministered at this point if flow has not been established. Re-dosing is estimated to be at around 15 to 20 minutes, if required, after the initial dose following reassessment.
CMAC further advises the co-administration of hyaluronidase with lidocaine without adrenaline (or equivalent local anesthetic agent) if available. Lidocaine results in a more tolerable experience for the patient, which is important to reduce patient fatigue. Additionally, it is known to cause vasodilation in the dermal tissues, which is advantageous in the event of an ischemic injury.44
CMAC introduced the concept of stages of a vascular occlusion in the guideline for the management of hyaluronic acid filler-induced vascular occlusion. While the concept does not allow a system of assessing depth of ischemic insult, it provides some guide as to whether necrosis is present. CMAC agrees that supportive treatment and ancillary medicines are not required if the ischemia is managed early; however, a patient who presents late with some areas of established necrosis will need wound management. Supportive care should be given to ensure there is no infection and optimal conditions for healing. Utilizing a hyperbaric chamber alone is not optimal for the management of established necrosis.46 Table 5 details the proposed changes.
Administration. Prior to injection, the area should be clean and thoroughly disinfected with an antibacterial skin solution. Palpate and mark out the area to be injected. The injections should be performed using an aseptic technique and either a cannula or needle that will reach the appropriate depth. It is important to consider the depth and site of injection of the hyaluronidase, and palpating and targeting nodules individually if present. Treat the area containing the cross-linked HA only, unless there is generalized hyaluronic acid-related swelling, in which case, treat the whole area. Once injected subcutaneously, the hyaluronidase will spread through the tissues and across vessel walls. In the event of a vascular occlusion, follow the steps outlined in Table 4.
In the event of a nodule, inject directly into the area—there may be resistance to the injection; however, it is important to try to penetrate the nodule to remove the filler successfully. While the doses for elective dissolution following a poor aesthetic outcome are subject to response, the amounts required for an individual nodule in the literature are variable.47 In relation to clinical practice, the authors note that larger and sometimes repeated doses are required to resolve the presenting issue fully. The patient must be made aware that repeat treatment might be necessary. Through experience with ultrasound, the authors also note it can be difficult to penetrate a pocket of filler with a blunt cannula. Isolation of problematic filler can be difficult without access to ultrasound, resulting in incomplete resolution of the issue even if a needle is used.
If there is refractory swelling around the orbit that is not amenable to treatment with hyaluronidase, or the nodule is not responding to repeat treatment, consider referring for ultrasound or magnetic resonance imaging to offer an accurate diagnosis and guide any further treatment that might be required.
Ultrasound-guided dissolution is a useful tool to dissolve arterial occlusions and to improve accuracy of injection into nodules, or areas of unwanted residual cross-linked HA.
After injection, firmly massage the area to aid in the breakdown of the cross-linked HA.
Do not inject hyaluronidase in an area where botulinum toxin has been given in the past 48 hours, and do not inject into an area where there is infective sequelae unless there is thought to be a biofilm and the patient is stabilized on antimicrobial treatment.
Adverse events. Aside from the routine issues of postprocedural pain, swelling, and bruising, the CMAC board have experienced reports of local swelling on injection of the hyaluronidase. This is a known side effect alongside erythema and itch. It is important to note that the anaphylaxis risk is extremely small and is lower than the quoted 0.1-percent incidence, which included non-serious local and delayed reactions and secondary exposure.14,16,17,21 It is important that the clinician, when administering any medicines, not just hyaluronidase, can identify and support an anaphylactic response by administering adrenaline until emergency services arrive. It is important to remain calm and assess the patient for signs of actual anaphylaxis. In the absence of any systemic symptoms or impaired swallowing or breathing, facial swelling alone is not an indication of anaphylaxis and can be expected to settle spontaneously within 12 to 24 hours.
References
- Lee A, Grumer S, Kriegel D, Marmur E. Hyaluronidase. Dermatologic Surgery. 2010; 36(7):1071–1077.
- Silverstein SM, Greenbaum S, Stern R. Hyaluronidase in ophthalmology. J Appl Res. 2012;(1):12–13.
- Andre P, Fléchet ML. Angioedema after ovine hyaluronidase injection for treating hyaluronic acid overcorrection. J Cosmet Dermatol. 2008;7(1):36–138.
- Houck JC, Chang CM. Permeability factor contaminating hyaluronidase preparations. Inflammation. 1979;3(4):447–451
- Rootman DB, Lin JL, Goldberg JL. Does the Tyndall effect describe the blue hue periodically observed in subdermal hyaluronic acid gel placement. Opthalmic Plastic and Recon Surg. 2014;30(6):524–527
- Hylase®( Hyaluronidase injection)[package insert].Wrexham UK: Wockhardt UK LTD;1993 [updated2015 May;cited 2020 Aug]. Available from: http//www.medicines.org.uk/emc/product/1505/smpc
- Michaud T. Rheology of hyaluronic acid and dynamic facial rejuvenation: topographical specificities. J Cosmet. Dermatol. 2018; 17(5): 736–743
- Sodium Chloride 0.9% intravenous infusion BP [package insert].Norfolk UK: Baxter Healthcare Limited.[updated 2018 Dec]. Available from: http//www.medicines.org.uk/emc/product/1871
- Csoka TB, Frost GI, Wong T, Stern R. Purification and microsequences of hyaluronidase isozymes from human urine. FEBS letters. 1997;417(3): 307–310.
- Mirakian R, Ewan P, Durham SR et al. BSACI guidelines for the management of drug allergy. Clin and Exp Allergy. 2008;39:43–61.
- Tamayo E, Alvarez FJ, Rodriguez-Ceron G, Gomez-Herreras JI, Castrodeza J. Prevalence of positive prick test to anaesthetic drugs in the surgical population. Allergy. 2006; 61:952–953.
- Markman M, Zanotti K, PetersonG, Kulp B, Webster K, Belinson J. Expanded experience with an intradermal skin test to predict for the presence or absence of carboplatin hypersensitivity. J Clin Oncol. 2003; 21: 4611–4614.
- Scolaro RJ, Crilly HM, Maycock PT et al. Australian and New Zealand Anaesthetic Allergy Group Perioperative Anaphylaxis Investigation Guidelines. Anaesth Intensive Care. 2017;45:5.
- Szepfalusi Z, Nentwich I, Dobner M, Pillwein K, Urbanek R. IgE-mediated allergic reaction to hyaluronidase in paediatric oncological patients. Eur J Pediatr. 1997;156(3):199–203.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase:A biological overview. Life Sci. 2007;80: 1921–1943.
- Leibovitch I, Tamblyn D, Casson R, Selva D. Allergic reaction to hyaluronidase: a rare cause of orbital inflammation after cataract surgery. Graefes Arch Clin Exp Ophthalmol 2006;244(8):944–949.
- Raichura ND, Alam MS, Jaichandran VV, Mistry S, Mukherjee B. Hyaluronidase allergy mimicking orbital cellulitis. Orbit 37(2): 149–153.
- Wu L, Liu X, Jian X, et al. Delayed allergic hypersensitivity to hyaluronidase during the treatment of granulomatous hyaluronic acid reactions. J Cosmet Dermatol. 2017;17(6): 991–995.
- Kim MS, Youn S, Na CH, Shin BS. Allergic reaction to hyaluronidase use after hyaluronic acid filler injection. J Cosmet Laser Ther. 2015;(18):1–3
- Ebo DG, Goossens S, Opsomer F, Bridts CH, Stevens WJ. Flow-assisted diagnosis of anaphylaxis to hyaluronidase. Allergy. 2005;60(10):1333–1334.
- Zamora-Alejo K, Moore S, Leatherbarrow B, et al. Hyaluronidase toxicity: a possible cause of postoperative periorbital inflammation. Clin Exp Opthalmol. 2013;41(2):122–126.
- Kempeneers A, Dralands L, Ceuppens J. Hyaluronidase induced orbital pseudotumor as complication of retrobulbar anesthesia. Bull Soc Belge Ophtalmol. 1992;243:159–166.
- Eberhart AH, Weiler CR, Erie JC. Angioedema related to the use of hyaluronidase in cataract surgery. Am J Opthalmol. 2004;138(1): 142–143.
- Lyall D, McQueen M, Ramaesh et al. A sting in the tail: cross reaction hypersensitivity to hyaluronidase. Eye. 2012;26:1490.
- Kim TW, Lee JH, Yoon KB, Yoon DM. Allergic reactions to hyaluronidase in pain management – A report of three cases. Korean J Anesthesiol. 2011;60:57–59.
- Borchard KLA, Puy R, Nixon R. Hyaluronidase allergy: A rare cause of periorbital inflammation. Australas J Dermatol. 2010;51(1):49–51.
- Ortiz-Perez S, Fernandez E, Molina JJ, et al. Two Cases of Drug-Induced Orbital Inflammatory Disease. Orbit. 2011;30(1):37–39.
- Delaere L, Zeyen T, Foets B, Calster JV, Stalmans I. Allergic reaction to hyaluronidase after retrobulbar anaesthesia: a case series and review. Int Ophtalmol. 2009;29(6):521–528.
- Feighery C, McCoy EP, Johnston PB, Armstrong DK. Delayed hypersensitivity to hyaluronidase (HyalaseTM) used during cataract surgery. Contact Dermatitis. 2007;57(5):343.
- Musa F, Srinivasan S, King CM, Kamal A. Raised intraocular pressure and orbital inflammation: a rare IgE-mediated allergic reaction to sub-Tenons hyaluronidase. J Cataract Refract Surg. 2006;32(1):177–178.
- Youseff Z, Pennefather PM, Watts MT. Orbital cellulitis vs allergic reaction to hyaluronidase as the cause of periorbital oedema. Eye (lond). 2005;19(6):691–692.
- Escolano F, Pares N, Gonzalez I, Castillo J, Valero A, Bartolome B. Allergic reaction to hyaluronidase in cataract surgery. Eur J Anaesthesiol. 2005;22(9):729–730.
- HYLENEX recombinant (hyaluronidase human injection) [package insert]. San Diego (CA): Halozyme Therapeutics, Inc.; 2005 [updated 2012 Jan; cited 2020 Aug]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021859s009lbl.pdf
- Fisher’s. In: Reitschel RL, Fowler JF Jr, eds. Contact Dermatitis. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2001.
- Bailey S, Fagien S, Rohrich R. Changing role of hyaluronidase in plastic surgery. Plast Reconstr Surg. 2014;133(2):127e–32e
- Keller EK, Dover JS. Use of Hyaluronidase in patients with bee allergy. Dermatol Surg. 2014;40(10):1145–1147.
- Lee HK, Choi EJ, Lee PB, Nahm FS. Anaphylactic Shock Caused by the Epidurally-Administered Hyalurinidase. Korean J Pain. 2011;24(4): 221–225.
- Barbaud A, Weinborn, Garvey LH et al. Intradermal Tests With Drugs: An Approach to Standardisation. Front Med. 2020;7:156.
- Rajalakshmi AR, Kumar MA. Hyaluronidase hypersensitivity: A rare complication of peribulbar block. Indian J Ophthalmol. 2016;64(2):160–162.
- Vartanian J. Injected hyaluronidase reduces restylane- mediated cutaneous augmentation. Arch Facial Plast Surg. 2005;7(4):231–237.
- Shumate GT, Chopra R, Jones D, Messina DJ. Hee CK. In Vivo Degradation of Crosslinked Hyaluronic Acid Fillers by Exogenous Hyaluronidases. Dermatologic Surgery. 2018;44(8): 1075–1083.
- The Duration of Hyaluronidase and Optimal Timing of Hyaluronic Acid (HA) filler Reinjection after Hyaluronidase Injection. J Cosmet Laser Ther. 2017;20(1).
- Delorenzi C. New High Dose Pulsed Hyaluronidase Protocol for Hyaluronic Acid Filler Vascular Evenets. Aesthetic Surgical Journal. 2017;37(7):814–825.
- Newton DJ, MacLeod FK and Belch JJF. Mechanism influencing the vasoactive effects of lidocaine in the human skin. Anaesthesia. 2007;62 (2):146–150.
- Muckenschnabel I, Bernhardt G, Srpuss T and Buschauer A. Pharmackinetics and tissue distribution of bovine testicular hyaluronidase and vinblastine in mice: an attempt to optimize the mode of adjuvant hyaluronidase administration in cancer chemotherapy. Cancer Letters. 1998;71–84.
- Murray G, Convery C, Davies E and Walker L. Guideline for the management of hyaluronic acid induced vascular occlusion. J Clin Aesthet Dermatol. 2021;14(5):E61–E69.
- Bohen B, Bashey S and Wysong A. The use of hyaluronidase in cosmetic dermatology: A literature review. J Clin Investigat Dermatol. 2015;3(2):7.


