 J Clin Aesthet Dermatol. 2022;15(11 Suppl 1):S3–S15
J Clin Aesthet Dermatol. 2022;15(11 Suppl 1):S3–S15
by Jo Ann LeQuang
Ms. LeQuang is Owner of LeQ Medical in Angleton, Texas; Director of Scientific Communications at NEMA Research, Inc., in Naples, Florida; and Founding Director of No Baby Blisters in Colorado Springs, Colorado.
FUNDING: Castle Biosciences provided funding for the development of this article.
DISCLOSURES: Ms. LeQuang reports no conflicts of interest relevant to the content of this article.
ABSTRACT: Risk-stratification of cancer, traditionally performed through staging, directs optimal disease management decisions with the result of improved patient outcomes. Many forms of cutaneous cancer have overall excellent survival rates, but conventional staging methods are imperfect in identifying high-risk patients. Gene expression profiling (GEP) is a clinically available, objective metric that can be used in conjunction with traditional clinicopathological staging to help clinicians stratify risk in patients with skin cancer, even in those who lack traditional risk markers. For patients with melanoma, the 31-GEP test provides personalized prognostic information that can guide risk-appropriate clinical management and surveillance decisions. The i31-GEP integrates 31-GEP results with clinicopathological features to provide a risk of recurrence (i31-GEP for ROR) and likelihood of having a positive sentinel lymph node biopsy (SLNB) (i31-GEP for SLNB) for patients with melanoma. For patients with cutaneous squamous cell carcinoma who have at least one risk factor, the 40-GEP test allows for better risk stratification by identifying the high-risk patients who are most likely to develop metastasis. These tests can be easily integrated into clinical practice to help guide treatment choices.
Keywords: 31-GEP, 40-GEP, i31-GEP, gene expression profiling, melanoma, cutaneous squamous cell carcinoma, risk of recurrence, risk stratification, prognosis, sentinel lymph node biopsy
Today’s skin cancer therapeutics offer the potential to better treat and manage patients with malignant melanoma and cutaneous squamous cell carcinoma (cSCC) and improve long-term survival outcomes. Prior to the introduction of immunotherapy, metastatic melanoma was typically a terminal condition, with an average life expectancy of 6 to 12 months and five-year overall survival (OS) rates in the single digits.1 However, with the approval of immune checkpoint inhibitors (ICIs), patients with metastatic melanoma are now experiencing higher survival rates,2,3 with three-, four-, and five-year survival rates ranging from 34 percent to over 50 percent in patients treated with a single ICI or a combination of ICIs.4–6 Among patients with cSCC, five-year disease-free survival rates are over 90 percent, with most patients experiencing tumor clearance following surgical excision and/or radiation.7,8
Early identification of patients at higher risk of developing metastasis is important so that clinicians can implement timely and effective treatments. Such patients are sometimes “hidden” among the seemingly low-risk patients because some aggressive cancers do not exhibit high-risk clinicopathological signs. Gene expression profile (GEP) testing has emerged as an important tool to assist clinicians in identifying patients with melanoma or cSCC who are at high risk for metastasis and poor outcomes. This article discusses the use of the 31-GEP for melanoma (DecisionDx®-Melanoma, Castle Biosciences, Inc., Friendswood, Texas), the i31-GEP algorithm integrating clinicopathologic features with 31-GEP results to predict sentinel lymph node biopsy (SLNB) positivity and risk of recurrence (ROR) for melanoma, and the 40-GEP for cSCC (DecisionDx®-SCC, Castle Biosciences, Inc.) in clinical practice to personalize risk-aligned treatment and achieve better patient outcomes.
31-GEP in Melanoma
Melanoma staging. Current American Joint Committee on Cancer (AJCC) melanoma staging9 uses three pathological tumor features: Breslow thickness, ulceration status, and SLN status. However, current guidelines do not adequately address all the potential nuances of melanoma prognosis, and clinicians also consider additional patient and tumor characteristics (e.g., mitotic rate) when developing management plans.10,11 For example, patients considered to be at low risk of dying from their disease according to AJCC staging and the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Cutaneous Melanoma (v 3.2022)10 can still experience tumor recurrence and die. Thus, clinical judgment plays a crucial role in guiding therapeutic options.
Early detection of metastatic melanoma is linked to significantly improved therapeutic response, and early treatment increases survival rates; this is because patients with low tumor burden (<10 lesions, with no lesion >3cm in size) respond better to therapy than those with high tumor burden (≥10 lesions or at least 1 lesion >3cm in size).12,13 Similarly in cSCC, asymptomatic disease recurrence is independently associated with better survival (hazard ratio 0.47, 95% confidence interval [CI] 0.29–0.78, p=0.003).14
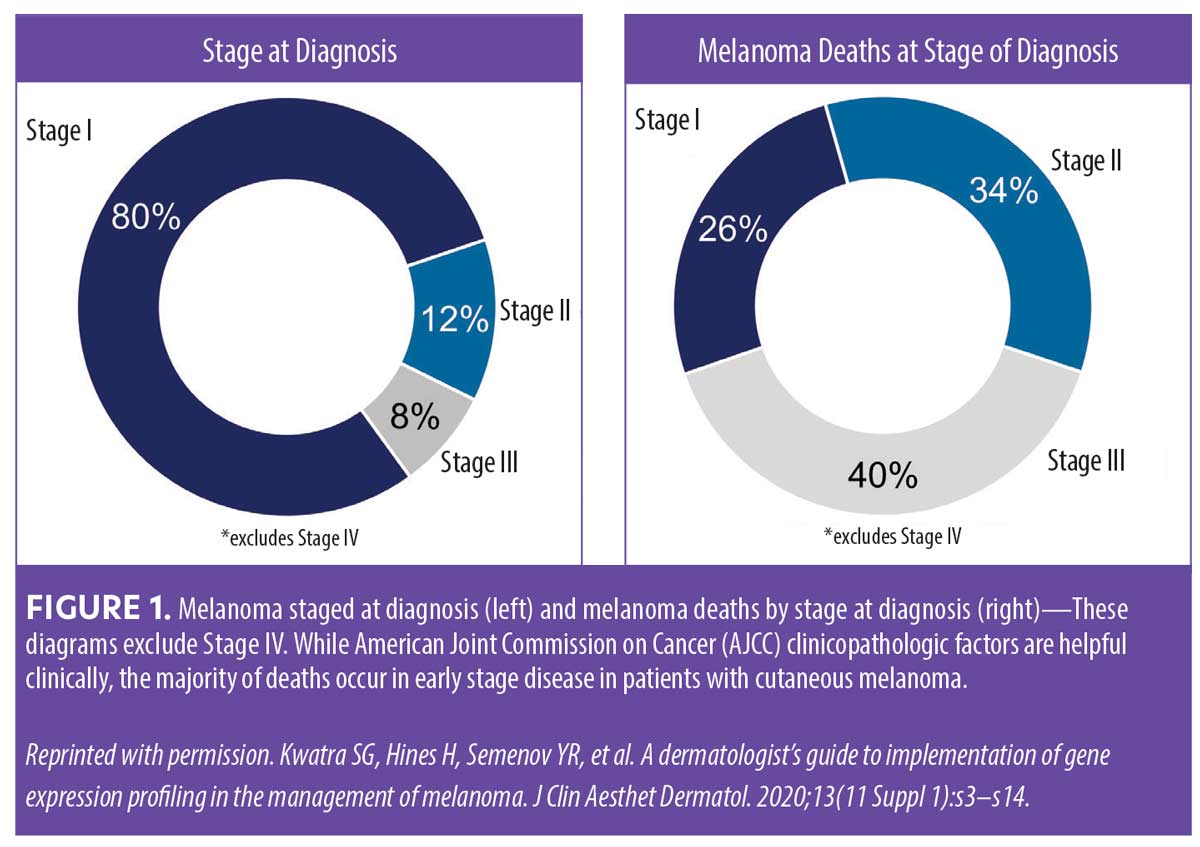
Eighty percent of melanomas are initially diagnosed at Stage I, but staging-only paradigms do miss patients with tumors classified as Stage I that have high-risk tumor biology.15 The majority of melanoma-related deaths occur in patients diagnosed with early-stage disease (localized Stage I–II at time of diagnosis) (Figure 1).15 The data shown in Figure 1 suggest that, among patients initially diagnosed with Stage I melanoma, there is a subpopulation of patients with high-risk melanoma that may benefit from more intensive disease management. Thus, when determining prognosis in patients with melanoma, it is crucial to differentiate those at high risk of recurrence/metastasis from those who are at low risk to identify metastases early and provide prompt and appropriate treatment for improved outcomes.16
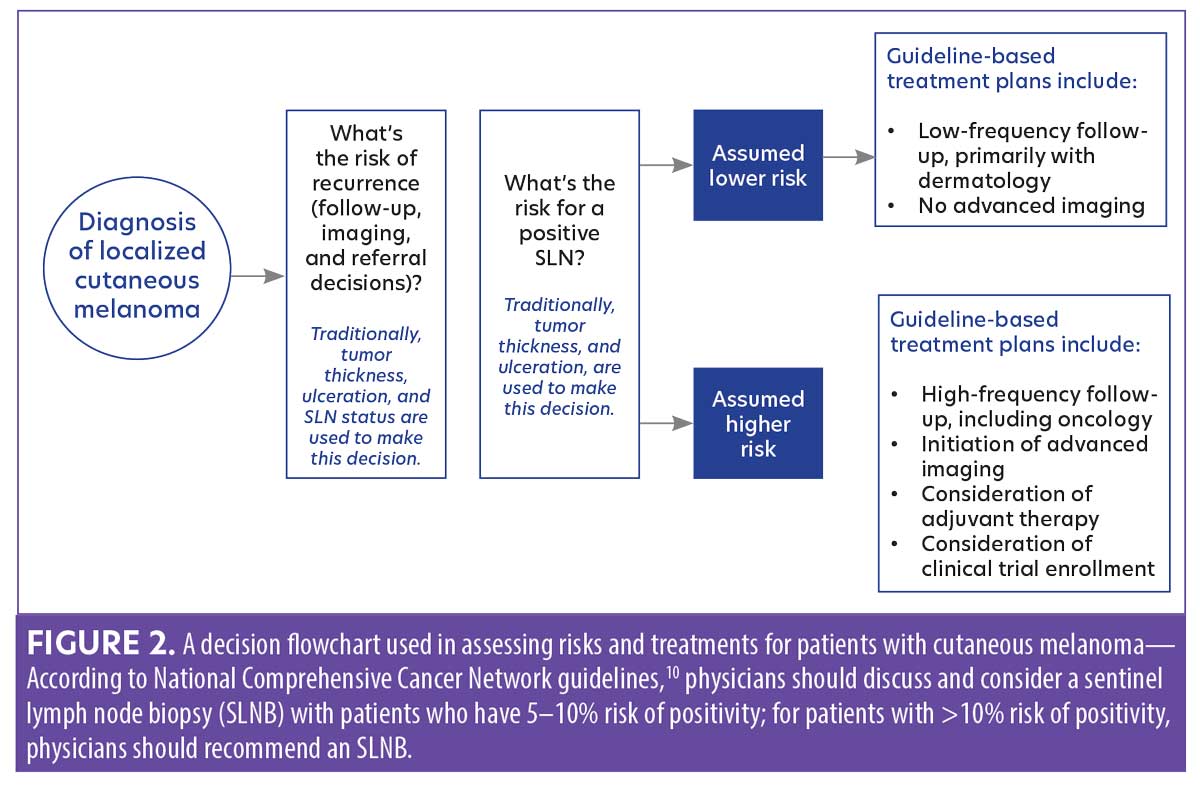
The role of 31-GEP testing in melanoma. Dermatologists have historically used a clinicopathological decision tree to assess risk in patients diagnosed with localized cutaneous melanoma (Figure 2). NCCN guidelines recommend using the individual patient’s ROR to determine decisions about disease management, and that an individual’s risk for a positive SLNB should drive recommendations for biopsy.10,17 31-GEP testing can help identify patients with early-stage melanoma who are at high risk of metastasis.
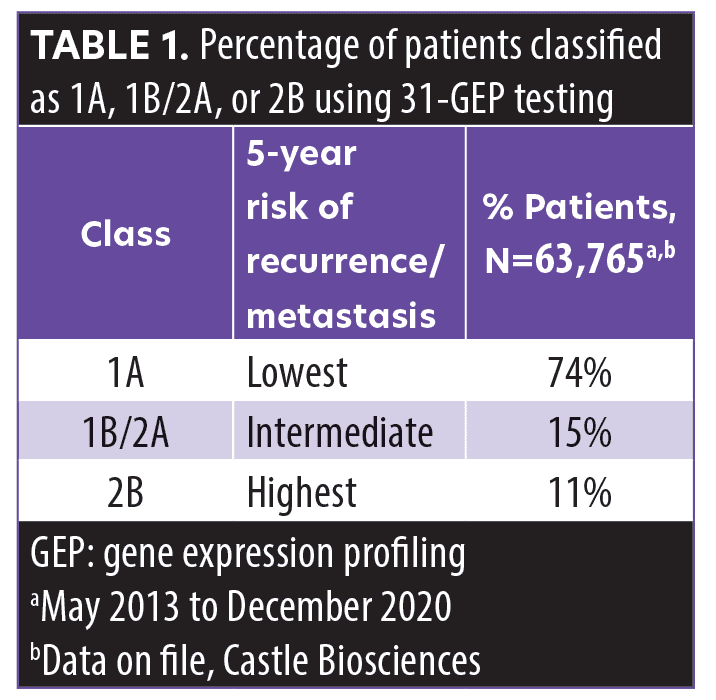
The 31-GEP test is an independent molecular risk stratification tool that uses a gene transcription assay to assess the expression of a validated panel of genes in primary cutaneous malignant melanoma tumors.18 The test evaluates 28 signature genes in pathways related to metastatic cutaneous melanoma and three control genes using reverse transcription polymerase chain reaction (RT-PCR) on primary tumor tissue from a confirmed diagnosis of Stage I, II, or III melanoma. A validated algorithm is then used to classify patients as Class 1A (low risk), Class 1B/2A (intermediate risk), or Class 2B (high risk) (note that Class 1B/2A are combined into an intermediate grouping).18–22 Table 1 displays the percentage of patients (based on clinical orders data from 2013 to 2020 [data on file, Castle Biosciences]) who were classified as Class 1A, Class 1B/2A, or Class 2B.
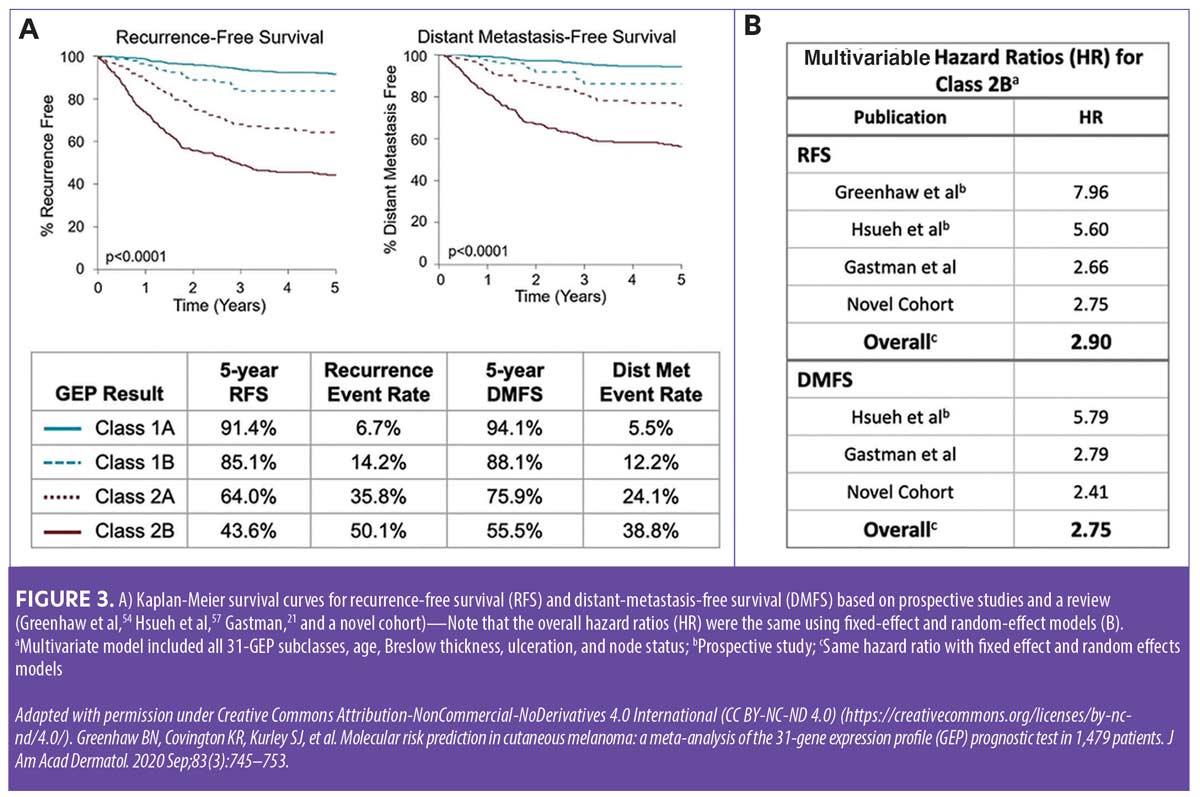
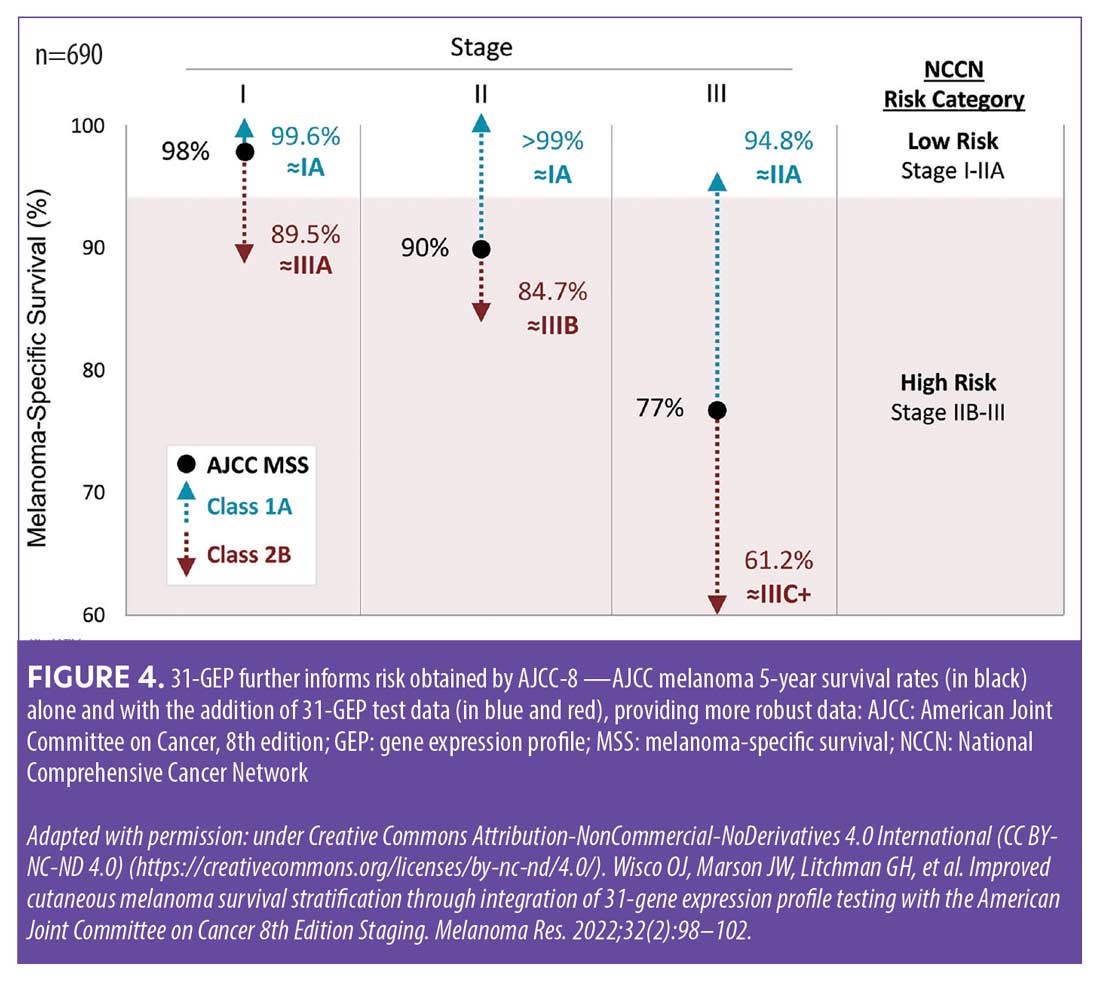
There are marked differences between a 31-GEP Class 1A result and a Class 2B result—patients who receive a Class 1A result have significantly higher five-year recurrence-free survival (RFS) rates compared to those with higher risk 31-GEP results (Figure 3).19–21 Figure 4 illustrates how the addition of 31-GEP results to AJCC five-year survival data provides a more robust picture of risk.22 Based solely on AJCC-8 (8th edition) staging data, patients with Stage I, II, and III melanoma have five-year melanoma-specific survival (MSS) rates of 98 percent, 90 percent, and 77 percent, respectively.23 However, when 31-GEP results are factored in, the five-year MSS rate for Stage I patients with a Class 1A 31-GEP test result increases to 99.6 percent, whereas the five-year MSS rate for Stage I patients with a Class 2B result drops to 89.5 percent. Of particular note, the subset of patients with Stage III tumors and a Class 1A 31-GEP result have lower rates of recurrence/metastasis than the subset of patients with Stage I tumors and a Class 2B 31-GEP result.19
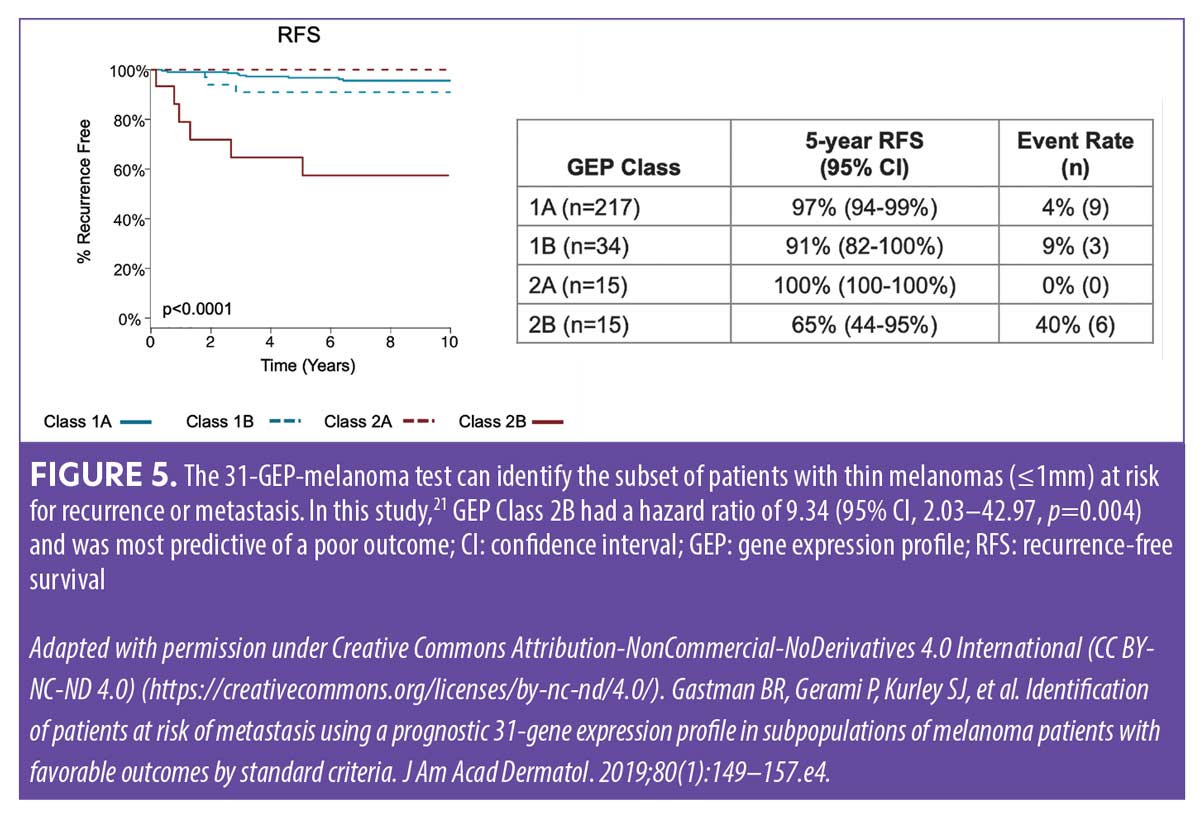
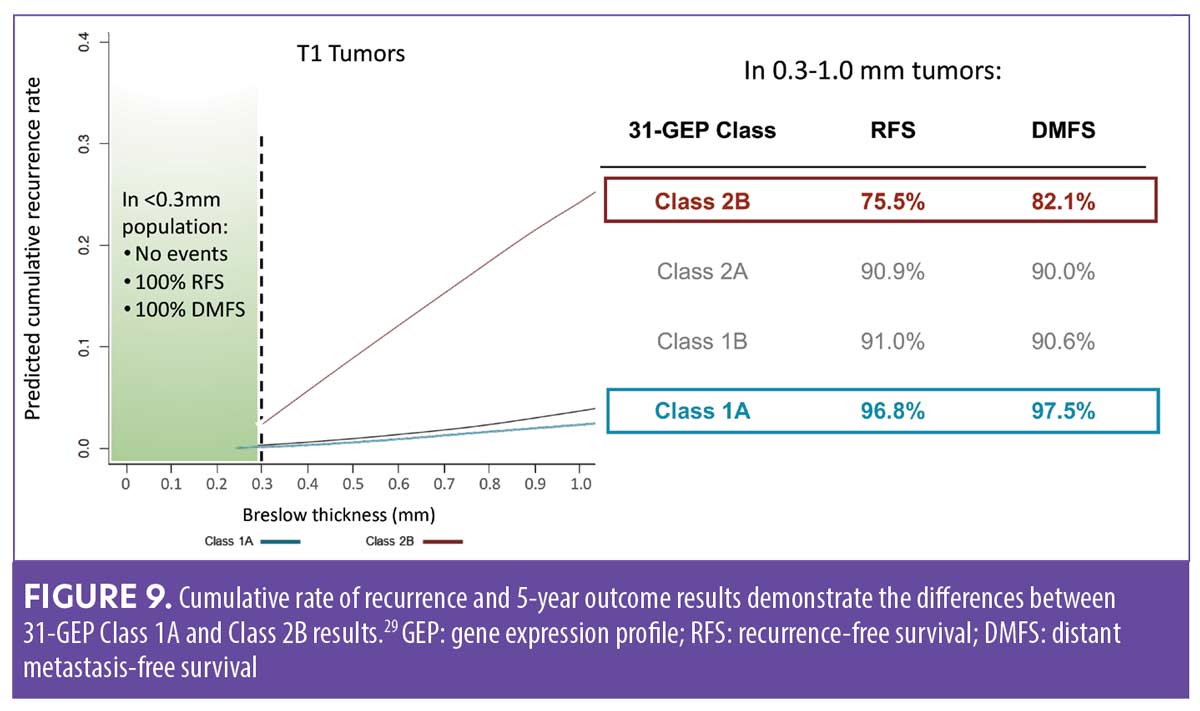
Thin melanomas (≤1mm) are routinely treated in dermatology practices without referral to surgical oncologists and have favorable survival rates of 99 percent at five years.9,23 However, adding 31-GEP testing can identify certain patients with thin melanomas—all of whom would be considered low risk by staging alone—as having a higher risk for recurrence/metastasis (Figure 5). These higher-risk patients with thin melanomas may benefit from further imaging and more intensive follow-up, which may improve outcomes.21
SLNB and 31-GEP. Current guidelines for recommending SLNB are based on Breslow thickness and ulceration status—SLNB is recommend for patients with T2 to T4 tumors who are considered to have a greater than 10-percent likelihood of having a positive SLN; those with T1a tumors (and no other high-risk features) are generally recommended to forego SLNB; and those with T1b tumors should consider an SLNB.17,24 The 31-GEP can help guide decisions about which patients should receive an SLNB. In a study of patients with melanoma,21 259 patients with a negative SLNB had a five-year distant metastasis-free survival (DMFS) of 82 percent (range: 77–87%), with 21 percent (54/259) of the patients experiencing tumor metastasis. Among patients in this study with a negative SLNB, those with a Class 1A 31-GEP result (n=100) had a 92.9-percent five-year DMFS rate (95% CI=87.9–98.1%), with nine percent of the patients experiencing one or more metastatic events beyond the regional nodal basin (9 total events), while those with a Class 2B result (n=85) had a 64.2-percent five-year DMFS rate (95% CI=54.5–75.7%), with 36 percent of the patients experiencing one or more metastatic events beyond the regional nodal basin (31 total events).21
In a recent study, a neural network algorithm integrating the 31-GEP with clinical and pathological features (i31-GEP) was developed (n=1,398) and validated (n=1,674) to accurately assess the risk of metastasis to the SLN.25 Researchers reported the continuous 31-GEP risk score had the largest variable importance score and log-likelihood value compared to other i31-GEP covariates. The i31-GEP increased the number of patients (27.7% vs. 8.5%) predicted to have less than a five-percent likelihood of SLN positivity compared to using Breslow thickness and ulceration status alone. In particular, among patients with T1 tumors originally classified as having a 5- to 10-percent likelihood of SLN positivity by current guidelines, 63 percent were reclassified by the i31-GEP as having less than a five-percent likelihood or greater than 10-percent likelihood of SLN positivity, allowing for a more personalized approach to disease management.25
Approximately 12 percent of patients with melanoma undergoing SLNB will have a positive result, SLNB-associated complications can occur in 11.3 percent of patients, and SLNB can produce false-negative results.26–33 Thomas et al32 found that among 5,351 SLNB-negative patients with melanoma, 10.4 percent experienced recurrences, with local or in-transit recurrence and distant recurrence being the most common types. Patients with local or in-transit recurrence were more likely to have distant recurrence, which was predictive for worse survival.32
In contrast to patients with a false-negative SLNB result, a meta-analysis by Balch et al26 reported a significant association between negative SLNB, older age, and worse survival. In this study, investigators collected data from the AJCC Melanoma Staging Database of 7,756 patients with melanoma who presented without clinical evidence of regional lymph node or distant metastasis and who underwent SLNB. The investigators found that the incidence of sentinel node metastasis significantly decreased as patient age increased, yet higher mortality rates and primary melanoma features associated with more aggressive biology were associated with older age. Patients under the age of 20 years had the highest incidence of SLN metastasis but also had the most favorable survival outcomes.26 These data highlight the importance of identifying the subset of high-risk patients among those with negative SLNBs, as well as identifying the patients for whom an SLNB may be avoided without undue risk (e.g., elderly patients).
Using the i31-GEP to guide SLNB decisions has an overall positive net benefit in patients with T1a, T1b, T2a, and T2b tumors. The i31-GEP results have clinical utility to guide surgical discussions and recommendations in a risk-appropriate manner within current risk-based guidelines.
Imaging and 31-GEP. In a retrospective review of 353 consecutive patients with malignant melanoma seen at a single institution from January 2006 to January 2016, investigators evaluated the impact of imaging intensity on the rates of asymptomatic surveillance-detected recurrence (ASDR) versus symptomatic recurrence (SR) and subsequent treatment outcomes.14 All patients were Stage IIB to III and had access to immunotherapy and targeted therapy. Patients were divided into two groups: one group was imaged regardless of whether they had symptoms, and the other group had symptom-guided imaging. The researchers found that shorter imaging intervals identified more ASDR and that ASDR had better prognostic factors (such as normal lactate dehydrogenase and ≤3 metastatic sites) than SR, as well as better survival outcomes on ICIs.14 These results not only highlight the importance of early identification and prompt treatment of metastases, but also the importance of identifying high-risk patients considered by conventional staging to be low-risk who may benefit from more intensive imaging surveillance.1 Integrating the 31-GEP test into clinical practice can help identify these patients.
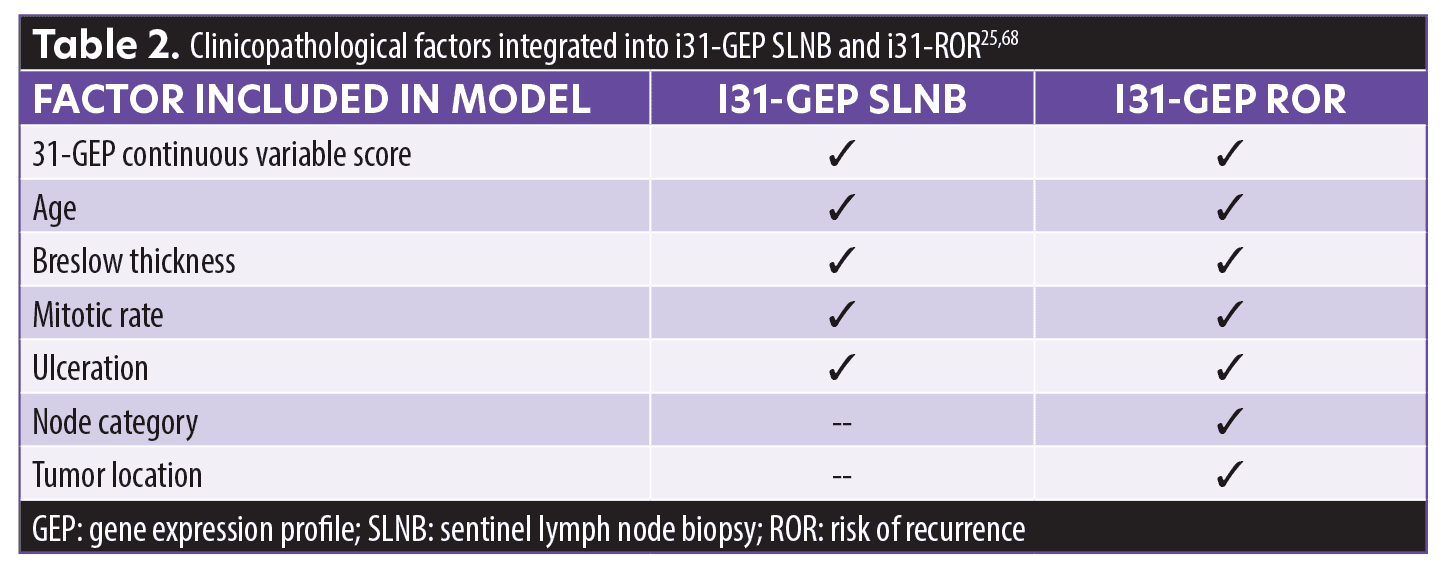
Artificial intelligence and 31-GEP. In 2021, two i31-GEP algorithms, a neural network-based model for more precise prediction of the likelihood of SLN positivity (i31-GEP SLNB) and a Cox model for an individualized risk of recurrence (i31-GEP ROR), were launched to improve the precision of melanoma treatment plans for better patient outcomes. These validated models25,34 integrate 31-GEP results with specific clinicopathological factors (Table 2) to provide patient-specific risk of SLN positivity, five-year RFS, DMFS, and MSS (RFS and DMFS are not currently provided by the AJCC-8 staging system) (Figures 6 and 7).10,13,23

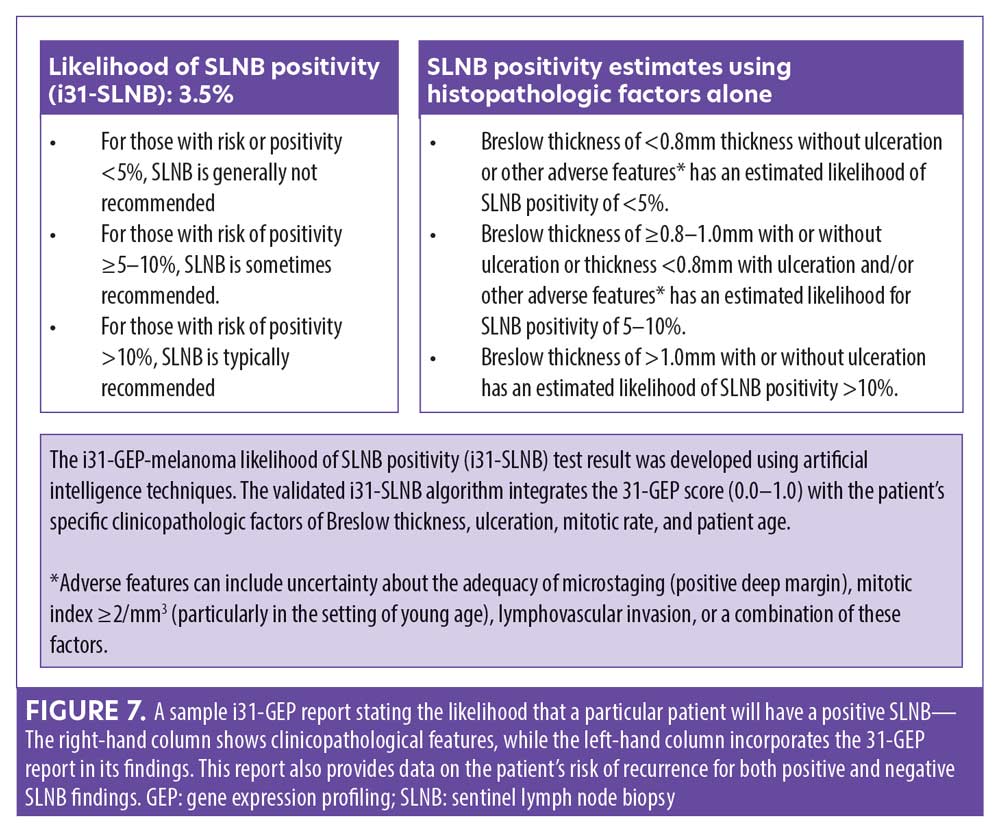
Integrating 31-GEP into clinical practice. Workflow considerations are an important aspect of providing thorough patient care. An expert panel convened to offer guidance on how the test could be used effectively (Figure 8).35 31-GEP testing is validated for all melanomas 0.3mm in diameter or larger. The 0.3-mm cutoff value was derived from a study of cumulative rates of recurrence and five-year outcomes of thin tumors (Figure 9).36 However, clinical judgment should always be exercised when determining if 31-GEP testing for an individual patient is appropriate.
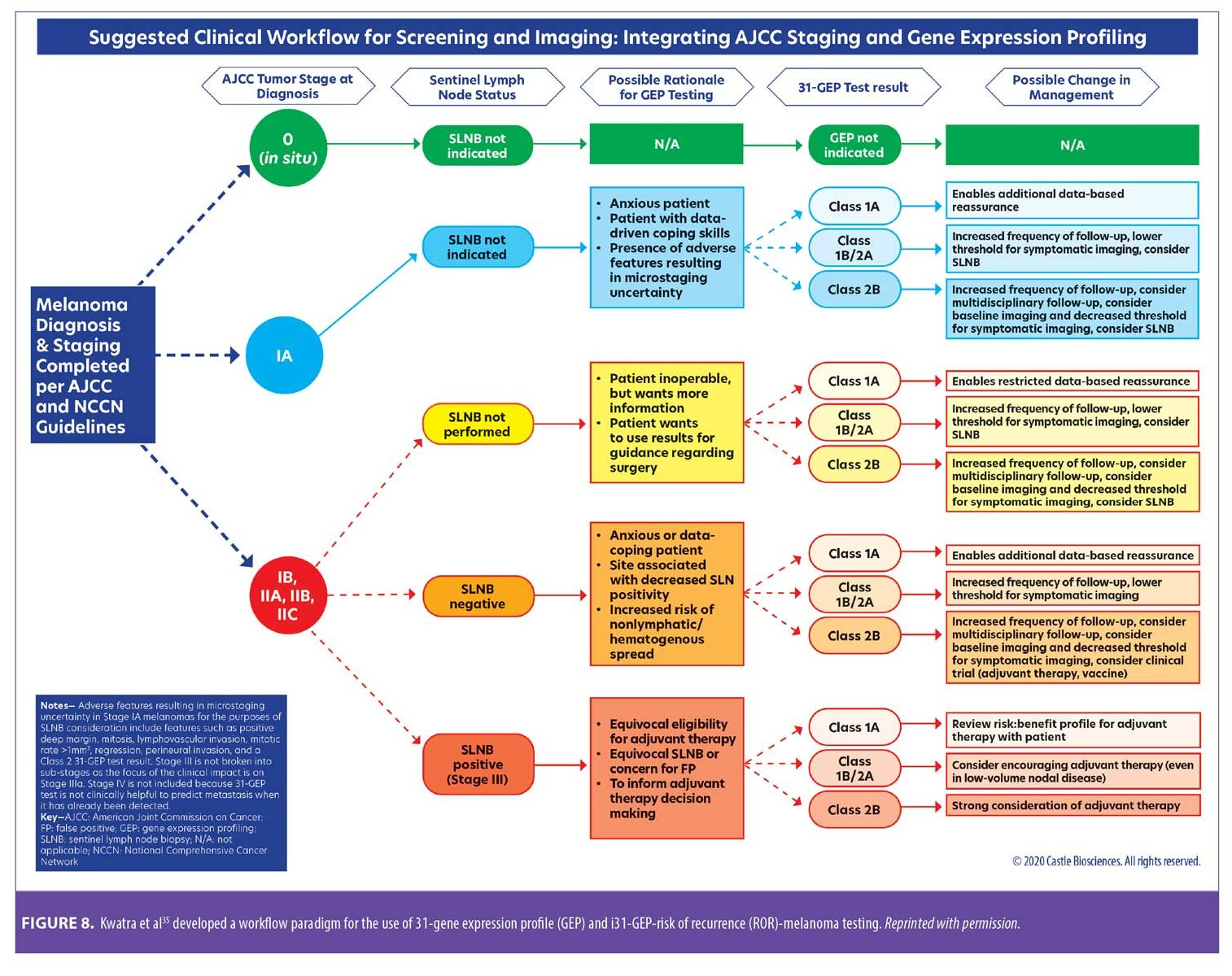
The decision to undergo an SLNB must always be considered in the context each patient’s clinical and pathological features (e.g., patient age, frailty, comorbidities). For patients with tumor thickness of 0.3 to 1.0mm, 31-GEP testing provides important information that can help guide the decision as to whether the patient should have an SLNB.
40-GEP in cSCC
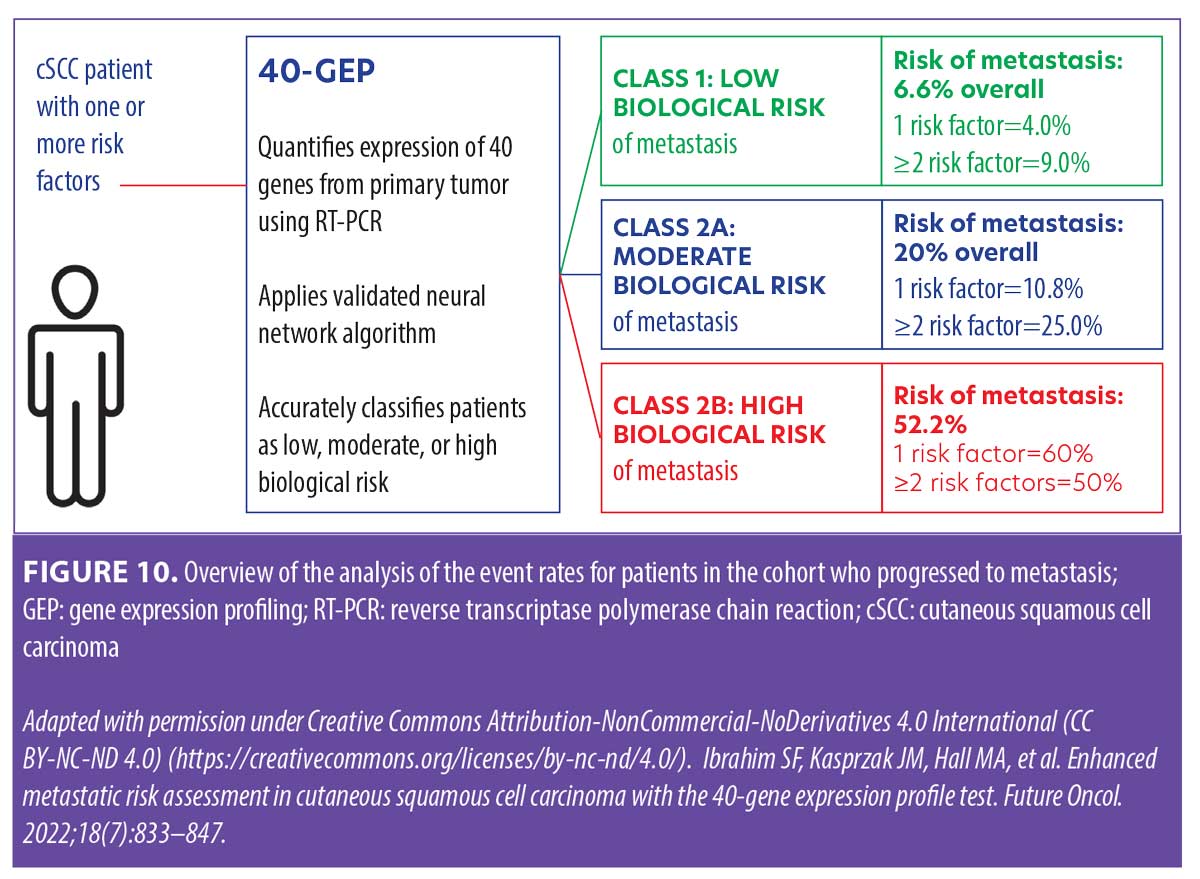
A patient with cSCC who has one or more risk factors qualifies for 40-GEP testing. The 40-GEP test quantifies expression of 40 genes from the primary tumor using RT-PCR and applies a neural-network algorithm37 to accurately classify patients as low (Class 1), moderate (Class 2A), or high (Class 2B) biological risk of metastasis within three years of diagnosis (Figure 10). This information can help guide risk-appropriate treatment decisions made by the clinician and patient.
Two staging systems are currently utilized in patients with cSCC—the AJCC-8 system9 and the Brigham and Women’s Hospital (BWH) Tumor Staging System38—the latter outperforms over the former.39 Overall, the traditional clinicopathologic risk factors have a low positive predictive value in cSCC.37,40 The validated 40-GEP test improves in the risk stratification of patients with cSCC who have at least one of the risk factors listed in Table 3.41 The 40-GEP test is not intended to be used for locally recurrent tumor tissue.
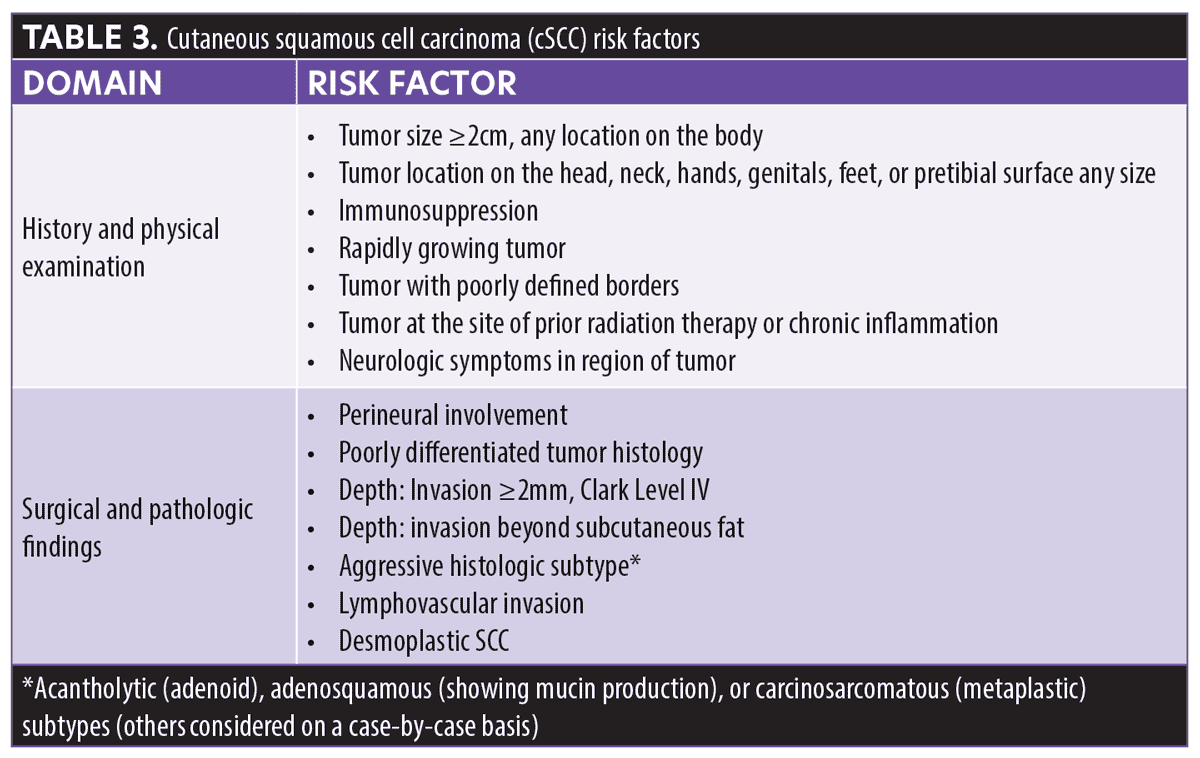
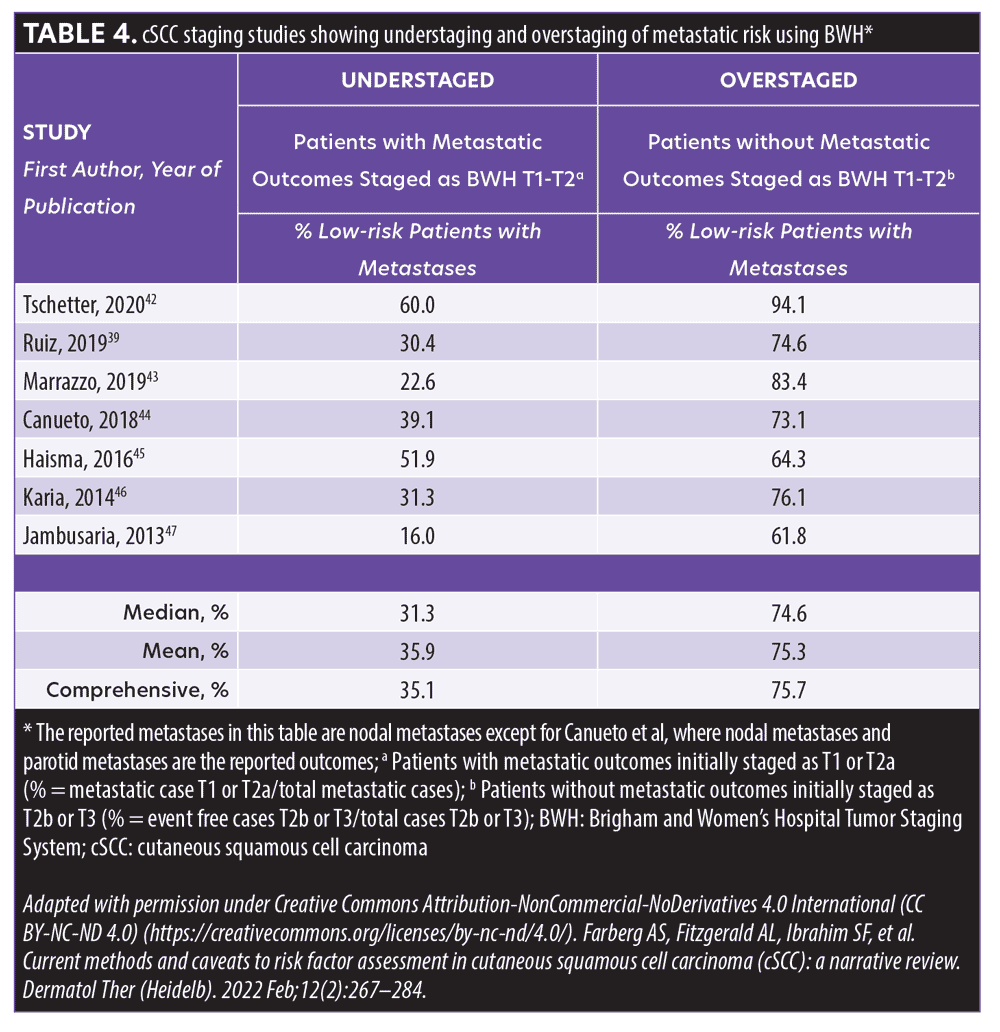
In the AJCC-8 staging system, those with tumor diameters greater than 2cm but no larger than 4cm are classified as T2; patients with a tumor diameter of 4cm or greater or a tumor of any size with any one high-risk factor (e.g., deep invasion [beyond the subcutaneous fat or >6mm], minor bone erosion, perineural invasion [PNI] ≥0.1mm) are classified as T3. While AJCC-8 differentiates tumors staged as T3 to be at greater risk for poor outcomes compared to tumors staged as T2, the indistinguishable metastasis rate within the study performed by Ruiz et al39 suggests that the factors used to create these particular stages are inadequate. The BWH algorithm enumerates four risk factors: 1) a tumor diameter of 2cm or greater, 2) tumor invasion beyond subcutaneous fat, 3) perineural invasion 0.1mm or greater, and 4) poor differentiation. The BWH scale identifies T1 as no risk factors, T2a as one risk factor, T2b as 2 to 3 risk factors, and T3 as all four risk factors and/or bone invasion. Although patients identified as T2b or T3 are considered high risk, there is a clear differential for mortality among the T2a, T2b, and T3 groups. Under-staging and over-staging of risk for metastatic outcomes does occur using this system (Table 4).39,42–48 These limitations emphasize the utility of the 40-GEP in complementing the information provided by the conventional staging systems with independent prognostic data, which can help clinicians more accurately identify those patients with cSCC who are at greater risk of metastasis based on their tumor biology.
 The limitations of conventional staging systems may result in clinicians imposing their own differentiation between low- and high-risk in patients with cSCC. The NCCN guidelines have broader categories for defining high risk, while the BWH and AJCC-8 models have narrower categories. The NCCN adds risk factors, such as rapid tumor growth, neurologic symptoms, and selected histologic subtypes, among others.49 Other risk factors may be omitted in conventional staging systems, such as compromised immunity or lymphovascular invasion, but clinicians should still take these risk factors into account. Each clinician may weigh these supplemental factors differently. Even straightforward tumor characteristics, such as depth, can be subject to different interpretations among clinicians due to multiple types of measurement. The challenge in cSCC risk stratification is that overly sensitive criteria may expose low-risk patients to expensive, time-consuming, and potentially distressing procedures (e.g., nodal radiation, imaging, SLNB, accelerated follow-up schedules), while missing those high-risk patients who will experience potentially life-threatening metastases.
The limitations of conventional staging systems may result in clinicians imposing their own differentiation between low- and high-risk in patients with cSCC. The NCCN guidelines have broader categories for defining high risk, while the BWH and AJCC-8 models have narrower categories. The NCCN adds risk factors, such as rapid tumor growth, neurologic symptoms, and selected histologic subtypes, among others.49 Other risk factors may be omitted in conventional staging systems, such as compromised immunity or lymphovascular invasion, but clinicians should still take these risk factors into account. Each clinician may weigh these supplemental factors differently. Even straightforward tumor characteristics, such as depth, can be subject to different interpretations among clinicians due to multiple types of measurement. The challenge in cSCC risk stratification is that overly sensitive criteria may expose low-risk patients to expensive, time-consuming, and potentially distressing procedures (e.g., nodal radiation, imaging, SLNB, accelerated follow-up schedules), while missing those high-risk patients who will experience potentially life-threatening metastases.
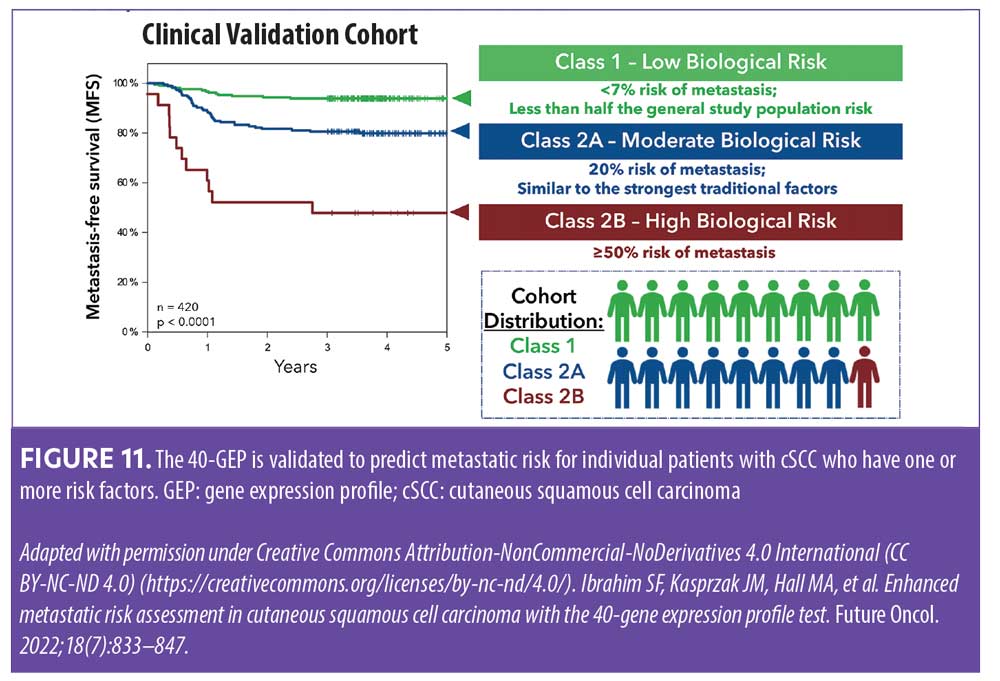
Kaplan-Meier survival curves show that using the 40-GEP test to identify metastatic risk in patients with cSCC offers distinct stratification based on the test’s classes (Figure 11).40 Furthermore, a multivariate model demonstrated 40-GEP tests results were independent factors for determining metastatic risk having similar and higher hazard ratios for Class 2A and Class 2B (2.3, p=0.013; 6.9, p<0.001, respectively) when compared to common high-risk factors.40 These data validate the 40-GEP as a useful tool that provides additive and independent prognostic data for individual patients, augmenting risk classification of cSCC.
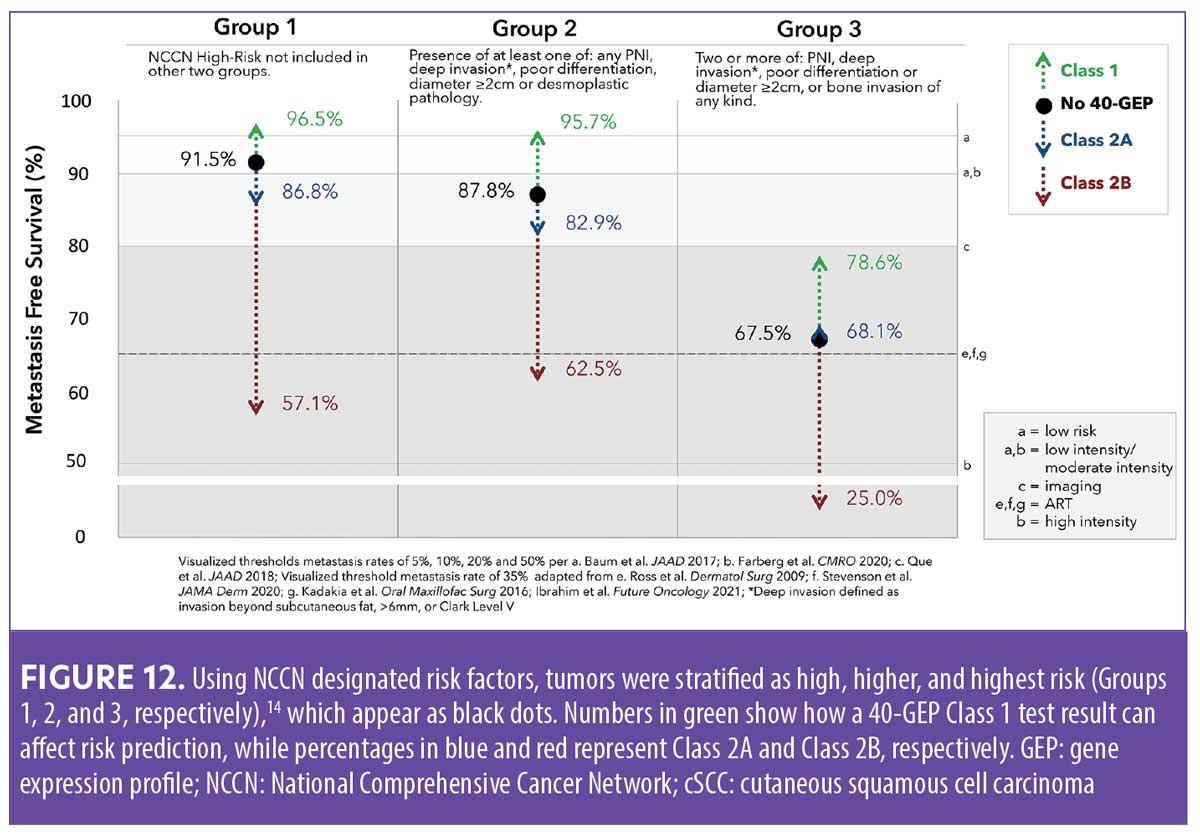
In the first real-world study evaluating the first year of clinical orders of the 40-GEP test (n=2,468),50 nearly all patients (99.8%) had one or more risk factors that would be classified by NCCN as high risk or very high risk, 75.3 percent of the patients had two or more risk factors, and the median number of risk factors per patient was three. Summary metrics from one year of clinical orders of the 40-GEP test demonstrated that clinicians are ordering the test for the intended use population. Survey findings revealed that when incorporating 40-GEP testing into their decision-making process for high-risk cSCC patients, clinicians do so in a risk-aligned manner. Results from this initial 40-GEP real-world study also determined 68.8 percent of patients were Class 1, 28.3 percent were Class 2A, and 2.9 percent were Class 2B.50 Combining staging data from conventional grading systems with 40-GEP results can provide a more accurate and personalized prognostic picture for individual patients with high-risk cSCC (Figure 12).
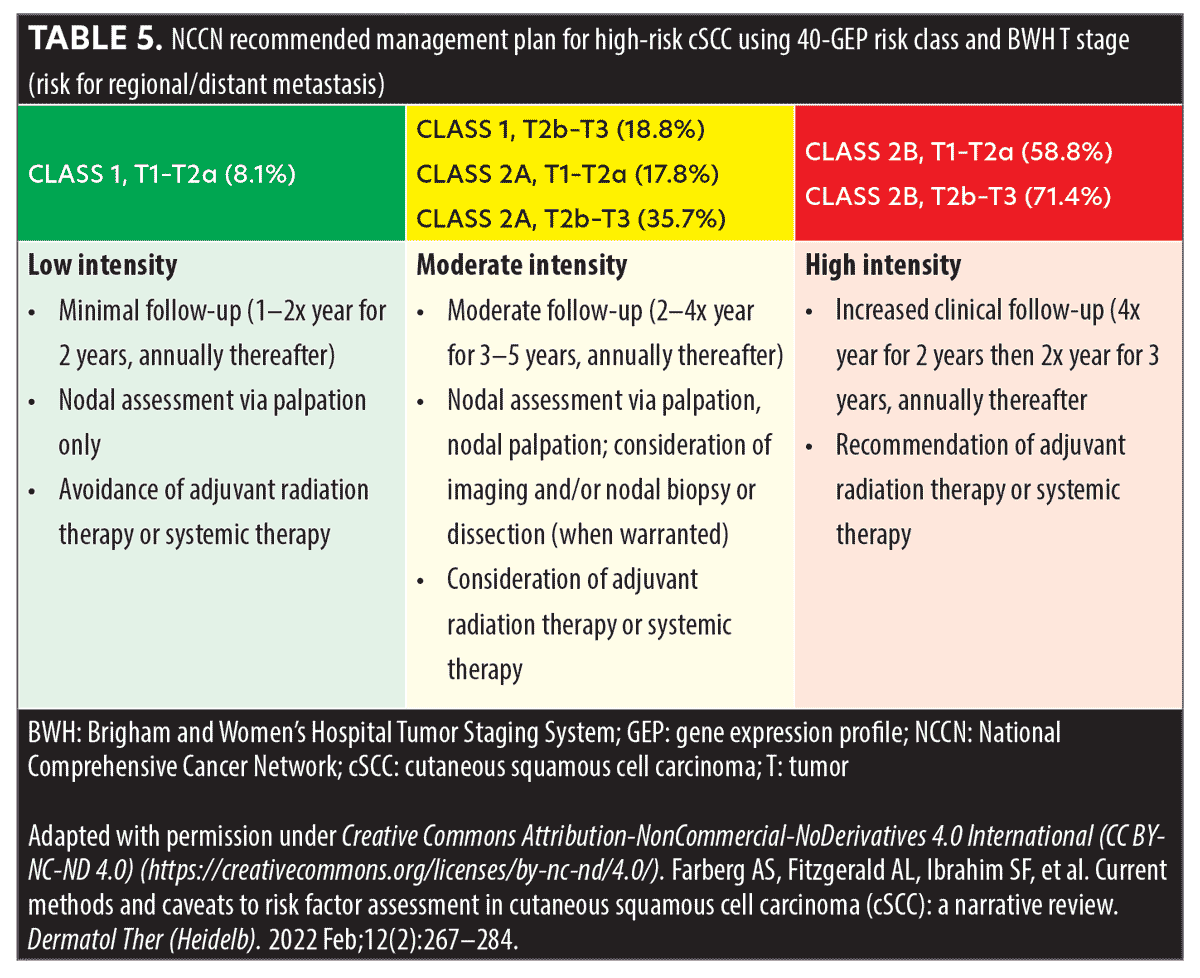
Integrating 40-GEP into clinical practice. A 40-GEP test result can be integrated into the normal flow of clinical practice when evaluating patients based on conventional prognostic risk factors and staging.51 Treatment plans would include surgery, when feasible, for all patients. Patients at a lower risk of metastasis (those with <10% risk) could be recommended for a clinical nodal exam, but patients at elevated risk should consider or be recommended for nodal imaging and/or nodal biopsy. Consideration for radiation, chemotherapy, and/or immunotherapy should be applied to patients at a moderate risk of metastasis (those with >10% risk); however, all patients who are at a higher risk of metastasis should be recommended for radiation, chemotherapy, and/or immunotherapy.18,51 Suggested follow-up schedules are 1 to 2 times a year for those at low risk, 2 to 4 times a year for those at a moderate risk, and four times or more a year for those at high risk (Table 5).51
Practice pearls in GEP Testing integration
It is often helpful to print out the i31-GEP or 40-GEP results to give to the patient. However, it is to the patients’ greatest benefit to clearly circle the relevant information for them and cross out or otherwise indicate the portions of the report that are not relevant to their case.
GEP testing allows more tailored, individualized patient treatment and can substantially change patient management, which may also cause changes to the clinical workflow but can improve outcomes.
There is substantial evidence published in peer-reviewed journals supporting the utility of GEP testing in clinical practice.19–22,24,25,34,37,40,50–67
To date, 31-GEP has been used in over 84,000 patients and the 40-GEP test has been used for over 8,000 high-risk SCC patients.
31-GEP is covered by Medicare and many private insurers; a patient assistance program is also available.
40-GEP became commercially available in September of 2020 for SCC patients with one or more high-risk factors.
Conclusion
There are no optimal staging systems for cutaneous cancers, such as malignant melanoma or cSCC, although the conventional systems based on clinicopathological features can be informative and helpful. The addition of GEP testing has changed how these patients are managed. Genomic information from the primary tumor can help identify patients with high-risk tumor biology who may benefit from more intense treatment and follow-up surveillance, due to their increased likelihood of recurrence, metastasis, or other poor outcomes. In contrast, patients with low-risk tumor biology may be spared from unnecessarily intense clinical management. These tests are straightforward to order and interpret, and Class result printouts can be given to patients when discussing their treatment options so they are more aware of the decision-making process regarding their care. The use of 31- and 40-GEP testing in clinical practice is a valuable tool in the dermatology armamentarium.
Acknowledgments
This article is a review of the presentation, “The Use of Gene Expression Profile Testing to Personalize Risk in Patients with Skin Cancer,” by Dr. Brent Moody (Skin Cancer and Surgery Center, PLC, Nashville, Tennessee), which was presented during Maui+Derm 2022, January 24–28, 2022, in Maui, Hawaii.
References
- Weiss SA, Wolchok JD, Sznol M. Immunotherapy of melanoma: facts and hopes. Clin Cancer Res. 2019;25(17):
5191–5201. - NCI staff. Deaths from metastatic melanoma drop substantially in the United States. 21 Apr 2020. National Institute of Health. National Cancer Institute. https://www.cancer.gov/news-events/cancer-currents-blog/2020/metastatic-melanoma-deaths-drop. Accessed 13 Jun 2022.
- Islami F, Ward EM, Sung H, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. JNCI J Natl Cancer Inst. 2021;113(12):1658–1669.
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356.
- Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492.
- Hamid O, Robert C, Daud A, et al. 5-year survival outcomes in patients (pts) with advanced melanoma treated with pembrolizumab (pembro) in KEYNOTE-001. J Clin Oncol. 2018;36:9516.
- Eigentler TK, Leiter U, Häfner HM, et al. Survival of patients with cutaneous squamous cell carcinoma: results of a prospective cohort study. J Invest Dermatol. 2017;137(11):2309–2315.
- American Academy of Dermatology website. Skin cancer types: squamous cell carcinoma treatment. https://www.aad.org/public/diseases/skin-cancer/types/common/scc/treatment. Accessed 10 Aug 2022.
- Amin MB, Edge SB, Greene FL, et al (eds). American Joint Commission on Cancer Staging Manual (8th ed). Switzerland: Springer; 2017.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: cutaneous melanoma. Version 3.2020: 2020. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. Accessed 10 May 2022.
- Klapperich ME, Bowen GM, Grossman D. Current controversies in early-stage melanoma: questions on management and surveillance. J Am Acad Dermatol. 2019 Jan;80(1):15–25.
- Bataille V. Early detection of melanoma improves survival. Practitioner. 2009;253(1722):29–32.
- Poklepovic AS, Carvajal RD. Prognostic value of low tumor burden in patients with melanoma. Oncology (Williston Park). 2018;32(9):e90–e96.8.
- Ibrahim AM, Le May M, Bossé D, et al. Imaging intensity and survival outcomes in high-risk resected melanoma treated by systemic therapy at recurrence. Ann Surg Oncol. 2020;27(10):3683–3691.
- National Cancer Institute site. Surveillance, epidemiology, and end results program (SEER). cancer stat facts: melanoma of the skin. National Institute of Health. https://seer.cancer.gov/statfacts/html/melan.html. Accessed 18 Aug 2020.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474.
- Swetter SM, Thompson JA, Albertini MR, et al. NCCN Guidelines® insights: melanoma: cutaneous, version 2.2021. J Nat Comprehen Cancer Network. 2021;19(4):364–376.
- Baum CL, Wright AC, Martinez JC, et al. A new evidence-based risk stratification system for cutaneous squamous cell carcinoma into low, intermediate, and high risk groups with implications for management. J Am Acad Dermatol. 2018;78(1):141–147.
- Greenhaw BN, Covington KR, Kurley SJ, et al. Molecular risk prediction in cutaneous melanoma: a meta-analysis of the 31-gene expression profile prognostic test in 1,479 patients. J Am Acad Dermatol. 2020 Sep;83(3):745–753.
- Hsueh EC, DeBloom JR, Lee JH, et al. Long-term outcomes in a multicenter, prospective cohort evaluating the prognostic 31-gene expression profile for cutaneous melanoma. JCO Precis Oncol. 2021;5.
- Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80(1):149–157.e4.
- Prado G, Cook RW, Covington KR, et al. Improvement of risk assessment in cutaneous melanoma (CM) by a prognostic 31-gene expression profile (31-GEP) test over AJCC-based staging alone (poster). Presented at 2018 Fall Clinical Dermatology Conference. Las Vegas, NV: 18–21 Oct 2018.
- Keung EZ, Gershenwald JE. American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care (8th ed). Expert Rev Anticancer Ther. 2018;18(8):775–778.
- Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72(5):780–785.
- Whitman ED, Koshenkov VP, Gastman BR, et al. Integrating 31-gene expression profiling with clinicopathologic features to optimize cutaneous melanoma sentinel lymph node metastasis prediction. JCO Precision Oncol. 2021;5:1466–1479.
- Balch CM, Thompson JF, Gershenwald JE, et al. Age as a predictor of sentinel node metastasis among patients with localized melanoma: an inverse correlation of melanoma mortality and incidence of sentinel node metastasis among young and old patients. Ann Surg Oncol. 2014;21(4):1075–1081.
- Bamboat ZM, Konstantinidis IT, Kuk D, et al. Observation after a positive sentinel lymph node biopsy in patients with melanoma. Ann Surg Oncol. 2014;21(9):3117–3123.
- Joyce KM, McInerney NM, Piggott RP, et al. Analysis of sentinel node positivity in primary cutaneous melanoma: an 8-year single institution experience. Ir J Med Sci. 2017;86(4):847–853.
- Moody JA, Ali RF, Carbone AC, et al. Complications of sentinel lymph node biopsy for melanoma: a systematic review of the literature. Eur J Surg Oncol. 2017;43(2):270–277.
- Ellis MC, Weerasinghe R, Corless CL, Vetto JT. Sentinel lymph node staging of cutaneous melanoma: predictors and outcomes. Am J Surg. 2010;199(5):
663–668. - Grossman D, Okwundu N, Bartlett EK, et al. Prognostic gene expression profiling in cutaneous melanoma: identifying the knowledge gaps and assessing the clinical benefit. JAMA Dermatol. 2020;156(9):1004–1011.
- Thomas DC, Han G, Leong SP, et al. Recurrence of melanoma after a negative sentinel node biopsy: predictors and impact of recurrence site on survival. Ann Surg Oncol. 2019;26(7):2254–2262.
- Scoggins CR, Martin RC, Ross MI, et al. Factors associated with false-negative sentinel lymph node biopsy in melanoma patients. Ann Surg Oncol. 2010;17(3):
709-717. - Jarell A, Gastman BR, Dillon LD, et al. Optimizing treatment approaches for patients with cutaneous melanoma by integrating clinical and pathologic features with the 31-gene expression profile test. J Am Acad Dermatol. 2022 Jul 7:S0190-9622(22)02253-8. Epub ahead of print.
- Kwatra SG, Hines H, Semenov YR, et al. A dermatologist’s guide to implementation of gene expression profiling in the management of melanoma. J Clin Aesthet Dermatol. 2020;13(11 Suppl 1):s3–s14.
- Marks E, Caruso H, Kurley S, et al. Establishing an evidence-based decision point for clinical use of the 31-gene expression profile test in cutaneous melanoma. SKIN J Cutaneous Med. 2019;3(4):239–249.
- Wysong A, Newman JG, Covington KR, et al. Validation of a 40-gene expression profile test to predict metastatic risk in localized high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2021;84(2):361–369.
- Venables ZC, Tokez S, Hollestein LM, et al. Validation of four cutaneous squamous cell carcinoma staging systems using nationwide data. Br J Dermatol. 2022;186:835–842.
- Ruiz ES, Karia PS, Besaw R, Schmults CD. Performance of the American Joint Committee on Cancer staging manual (8th ed) vs the Brigham and Women’s Hospital tumor classification system for cutaneous squamous cell carcinoma. JAMA Dermatol. 2019;155(7):819–825.
- Ibrahim SF, Kasprzak JM, Hall MA, et al. Enhanced metastatic risk assessment in cutaneous squamous cell carcinoma with the 40-gene expression profile test. Future Oncol. 2022;18(7):833–847.
- Fox M, Brown M, Golda N, et al. Nodal staging of high-risk cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2019;81(2):548–557.
- Tschetter AJ, Campoli MR, Zitelli JA, Brodland DG. Long-term clinical outcomes of patients with invasive cutaneous squamous cell carcinoma treated with Mohs micrographic surgery: a 5-year, multicenter, prospective cohort study. J Am Acad Dermatol. 2020;82(1):139–148.
- Marrazzo G, Zitelli JA, Brodland D. Clinical outcomes in high-risk squamous cell carcinoma patients treated with Mohs micrographic surgery alone. J Am Acad Dermatol. 2019;80(3):633–638.
- Cañueto J, Burguillo J, Moyano-Bueno D, et al. Comparing the eighth and the seventh editions of the American Joint Committee on Cancer staging system and the Brigham and Women’s Hospital alternative staging system for cutaneous squamous cell carcinoma: implications for clinical practice. J Am Acad Dermatol. 2019;80(1):106–113.
- Haisma MS, Plaat BEC, Bijl HP, et al. Multivariate analysis of potential risk factors for lymph node metastasis in patients with cutaneous squamous cell carcinoma of the head and neck. J Am Acad Dermatol. 2016;75(4):722–730.
- Karia PS, Jambusaria-Pahlajani A, Harrington DP, et al. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):
327–334. - Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402–410.
- Farberg AS, Fitzgerald AL, Ibrahim SF, et al. Current methods and caveats to risk factor assessment in cutaneous squamous cell carcinoma (cSCC): a narrative review. Dermatol Ther (Heidelb). 2022 Feb;12(2):267–284.
- Schmults CD, Blitzblau R, Aasi SZ, et al. NCCN Guidelines® Insights: squamous cell skin cancer, version 1.2022. J Nat Compreh Cancer Network. 2021;19(12):1382–1394.
- Hooper PB, Farberg AS, Fitzgerald AL, et al. Real-world evidence shows clinicians appropriately use the prognostic 40-gene expression profile (40-GEP) test for high-risk cutaneous squamous cell carcinoma (cSCC) patients. Cancer Invest. 2022 Sep 15:1–12.
- Farberg AS, Hall MA, Douglas L, et al. Integrating gene expression profiling into NCCN high-risk cutaneous squamous cell carcinoma management recommendations: impact on patient management. Curr Med Res Opin. 2020;36(8):1301–1307.
- Greenhaw BN, Zitelli JA, Brodland DG. Estimation of prognosis in invasive cutaneous melanoma: an independent study of the accuracy of a gene expression profile test. Dermatol Surg. 2018;0:1–7.
- Gerami P, Cook RW, Wilkinson J, et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin Cancer Res. 2015;21(1):175–183.
- Zager JS, Gastman BR, Leachman S, et al. Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer. 2018;18(1):130.
- Hsueh EC, DeBloom J, Lee J, et al. Interim analysis of survival in a prospective, multi-center registry cohort of cutaneous melanoma tested with a prognostic 31-gene expression profile test. J Hematol Oncol 2017;10(152): Epub 29 August 2017.
- Gastman BR, Zager JS, Messina JL, et al. Performance of a 31-gene expression profile test in cutaneous melanomas of the head and neck. Head Neck. 2019;41(4):871–879.
- Cook RW, Middlebrook B, Wilkinson J, et al. Analytic validity of DecisionDx-Melanoma, a gene expression profile test for determining metastatic risk in melanoma patients. Diagn Pathol. 2018;13(1):13.
- Keller J, Schwartz TL, Lizalek JM, et al. Prospective validation of the prognostic 31-gene expression profiling test in primary cutaneous melanoma. Cancer Med. 2019;8(5):2205–2212.
- Podlipnik S, Carrera C, Boada A, et al. Early outcome of a 31-gene expression profile test in 86 AJCC stage IB–II melanoma patients: a prospective multicentre cohort study. J Eur Acad Dermatol Venerol. 2019;33(5):857–862.
- Dillon LD, Gadzia JE, Davidson RS, et al. Prospective, multicenter clinical impact evaluation of a 31-gene expression profile test for management of melanoma patients. SKIN J Cutan Med. 2018;2:111–121.
- Mirsky R, Prado G, Svoboda R, et al. Management decisions made by physician assistants and nurse practitioners in cutaneous malignant melanoma patients: impact of a 31-gene expression profile test. J Drugs Dermatol. 2018;17(11):1220–1223.
- Schuitevoerder D, Heath M, Cook RW, et al. Impact of gene expression profiling on decision-making in clinically node negative melanoma patients after surgical staging. J Drugs Dermatol. 2018;17(2):196–199.
- Berman B, Ceilley R, Cockerell C, et al. Appropriate use criteria for the integration of diagnostic and prognostic gene expression profile assays into the management of cutaneous malignant melanoma: an expert panel consensus-based modified delphi process assessment. SKIN: J Cutan Med. 2019;3(5): 291–306.
- Dubin DP, Dinehart SM, Farberg AS. Level of evidence review for a gene expression profile test for cutaneous melanoma. Am J Clin Dermatol. 2019;20(6):763–770.
- Litchman GH, Prado G, Teplit RW, Rigel D. A systematic review and meta-analysis of gene expression profiling for primary cutaneous melanoma prognosis. SKIN: J Cutan Med. 2020;4(3):221–237.
- Marchetti MA, Coit DG, Dusza SW, et al. Performance of gene expression profile tests for prognosis in patients with localized cutaneous melanoma: a systematic review and meta-analysis. JAMA Dermatol. 2020;156(9):953–962.
- Farberg AS, Glazer AM, White R, et al. Impact of a 31-gene expression profiling test for cutaneous melanoma on dermatologists’ clinical management decisions. J Drugs Dermatol. 2017;16(5):428–431.

