 J Clin Aesthet Dermatol. 2022;15(11):18–21.
J Clin Aesthet Dermatol. 2022;15(11):18–21.
by Olga Marushchak, DO; Matthew Gagliotti, JD; Anjali S. Vekaria, MD; and Gary Goldenberg, MD
All authors are with the Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Marushchak is additionally with the Touro College of Osteopathic Medicine in New York, New York.
FUNDING: The Icahn School of Medicine at Mount Sinai and Bausch Health Americas, Inc. provided funding for this study
DISCLOSURES: Dr. Goldenberg is a consultant for Abbvie, ALMA, Almirall, Amgen, Arcutis, Eclipse, Novartis, Pfizer, Regeneron/Sanofi, Scibase, Valeant/Ortho Dermatologic, a speaker for Abbvie, Amgen, ALMA, Novartis, Pfizer, Regeneron/Sanofi, an investigator for Biofrontera, and an executive officer for Verrica.
ABSTRACT: Background: The current mainstay treatment of perimenstrual acne consists of systemic hormonal therapies, which can be problematic due to their side effects, stigma, or pill burden. Topical treatments are often used as well; however, data on their efficacy in treating this type of hormonal acne are limited.
Objective. We sought to evaluate the efficacy and tolerability of clindamycin phosphate and benzoyl peroxide 1.2%/3.75% combination gel in treating perimenstrual acne in adult women.
Methods. The single-group interventional pilot study was performed on 22 adult female subjects with perimenstrual acne. The subjects applied the investigational drug daily and were assessed every 14 days for a total of 99 days. Treatment success was evaluated by the investigators using the acne physician global assessment (PGA) scoring system. Drug tolerability assessment was based on the subject-reported adverse events, as well as physician-evaluated erythema, scaling, and dryness.
Results. The study demonstrated a significant improvement in PGA score and lesion count, as well as patient-reported outcomes. The medication was well-tolerated in all subjects.
Limitations. Limited sample size; lack of concurrent comparison group.
Conclusion. Clindamycin phosphate and benzoyl peroxide 1.2%/3.75% combination gel presents an important topical option for perimenstrual acne.
Keywords. Perimenstrual acne, hormonal acne, clindamycin phosphate and benzoyl peroxide combination gel, general dermatology, medical dermatology, drug response, clinical trial
Acne is an extremely common dermatologic problem, affecting many individuals globally. It is typically thought of as a disorder affecting adolescents. However, many individuals continue to be symptomatic well into adulthood or even initially develop acne well past their teenage years. Among those over the age of 20, acne is more commonly seen in females than in males.1–3
It is a common belief among the public, as well as health care professionals, that women often experience perimenstrual flares of acne. However, the exact prevalence of premenstrual acne remains uncertain.4 A study examining the effect of menstrual cycle on acne found that 44 percent of women with acne had premenstrual flares and symptoms were more commonly seen in women over the age of 33.5 Another study determined that 63 percent of women had an increased number of inflammatory acne lesions in the late luteal phase of their menstrual cycle.6
Hormones play a significant role in the pathophysiology of acne. Androgens, such as testosterone and dihydrotestosterone, bind to androgen receptors found in sebaceous glands and mediate sebum production. Increased androgen production may lead to an accumulation of sebum and keratin in hair follicles providing a growth medium for Cutibacterium acnes and resulting in open and closed comedones.7 Therefore, hormonal therapies, including oral contraceptive pills and spironolactone, are typically helpful in controlling acne symptoms in some females and have long been a mainstay of treatment.8 Yet for many women, these therapies are problematic due to side effects, stigma, or pill burden. Topical treatments are also commonly prescribed; however, at present, no topical therapy has been demonstrated to be effective in women with this type of hormonal acne. In this study, we investigated the effectiveness of clindamycin phosphate and benzoyl peroxide 1.2%/3.75% combination gel, the only FDA-approved fixed combination of 1.2% clindamycin phosphate and 3.75% benzoyl peroxide medication, for perimenstrual acne. This therapy is effective in the treatment of comedonal (non-inflammatory) and inflammatory acne when used once daily for twelve weeks.9 The combination of topical clindamycin with benzoyl peroxide has a synergistic effect in addition to decreasing the risk of inducing C. acnes resistance.10 Given its ease of use and efficacy and safety profile demonstrated in previous clinical studies in general population with acne vulgaris, it is an ideal topical agent to study in patients with perimenstrual acne.9,10
Methods
Study design. This open-label, single-group, interventional pilot study was approved by the institutional review board of Icahn School of Medicine at Mount Sinai and performed on female subjects with self-reported perimenstrual acne. The baseline visit (Day 1) was scheduled for one week prior to the first day of their menstruation. The subjects completed an acne-specific quality of life (acne QOL) questionnaire, a self-assessment, and a patient satisfaction survey (PSS) at the initial visit. Their skin was assessed for inflammatory and non-inflammatory acne vulgaris. The investigators performed a lesion count (counting papules, pustules, and comedones) and physician global assessment (PGA), and the patients were instructed to record their menstruation for the duration of the study. During the subsequent visit on Day 15, the subjects were reassessed and received clindamycin phosphate and benzoyl peroxide 1.2%/3.75% combination gel and instructions on its daily application to the face. The subjects continued to return to the outpatient clinic every 14 days to have their skin assessed until their final visit on Day 99, one week after their third menstruation while on the treatment and fourth menstruation since the enrollment.
Participants. The subject population consisted of 22 females over the age of 18 who had a self-reported complaint of perimenstrual acne that had occurred monthly for at least 6 months prior to the enrollment. The subjects were screened to include only those who had not used any topical prescription medications on the study area within 30 days prior to baseline visit and were willing to forego any other therapy to the treatment area for the duration of the study. The study excluded post-menopausal, pregnant, and lactating women.
Outcome measures. Primary outcome was determined by treatment success, a static endpoint defined as a score of 0 (clear) to 1 (almost clear) on a scale of 0 to 5 at the final study visit on Day 99 on the acne PGA scoring system. Secondary outcome measures included assessment of PGA and lesion count changes at each visit and drug tolerability based on the subject-reported adverse events (AE) as well as physician-evaluated erythema, scaling, and dryness on a point scale from 0 (none) to 3 (severe). Patient-reported outcomes included acne QOL score and subject self-assessment at all time points and subject satisfaction survey at baseline and Day 99.
Statistical analysis. Statistical analysis was performed using Student’s t-test to assess proportion of PGA of 0 or 1, changes in PGA score, lesion counts, and QOL total score from the baseline, subject self-assessment at all time points, and subject satisfaction survey at baseline and Day 99. A value of p<0.05 was considered significant. All data available were used for the analysis at each time point. Missing data were not imputed.
Results
Patient characteristics. Of the 22 patients screened, 21 were enrolled and 16 completed the study. All subjects were women between 23 and 40 years of age. At the baseline visit, average PGA was 3 (SE 0.18), average number of lesions was 16 (SE 2.19), average acne QOL was 49.8 (SE 6.36), average subject self-assessment score was 5.6 (SE 0.21), and average subject satisfaction survey score was 4.9 (SE 0.65). The reason for the discontinuation of all five patients was consent withdrawal by patients. The study treatment was not associated with any AEs or serious adverse events (SAEs) leading to discontinuation.
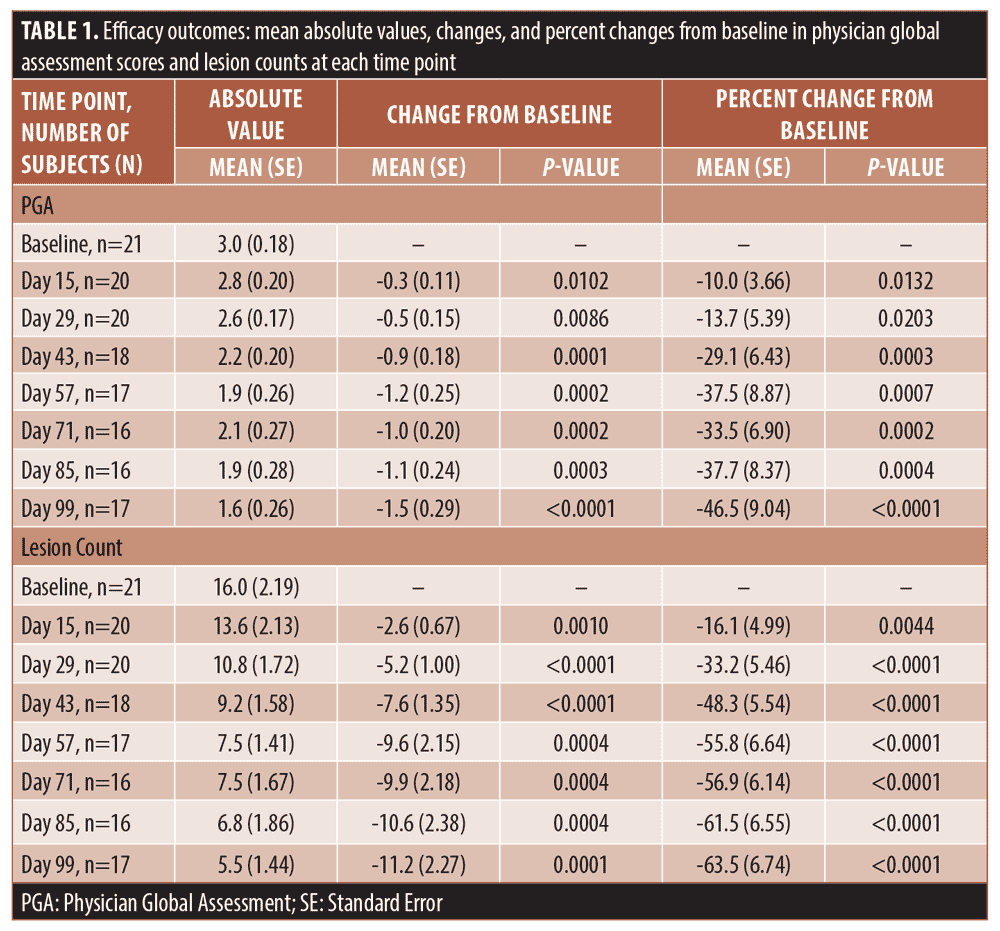
Efficacy outcomes. The primary efficacy endpoint analysis showed that 52.9 percent of the patients reached a PGA score of 0 (clear) or 1 (almost clear) at the final visit on Day 99, compared to 0 percent at the baseline.
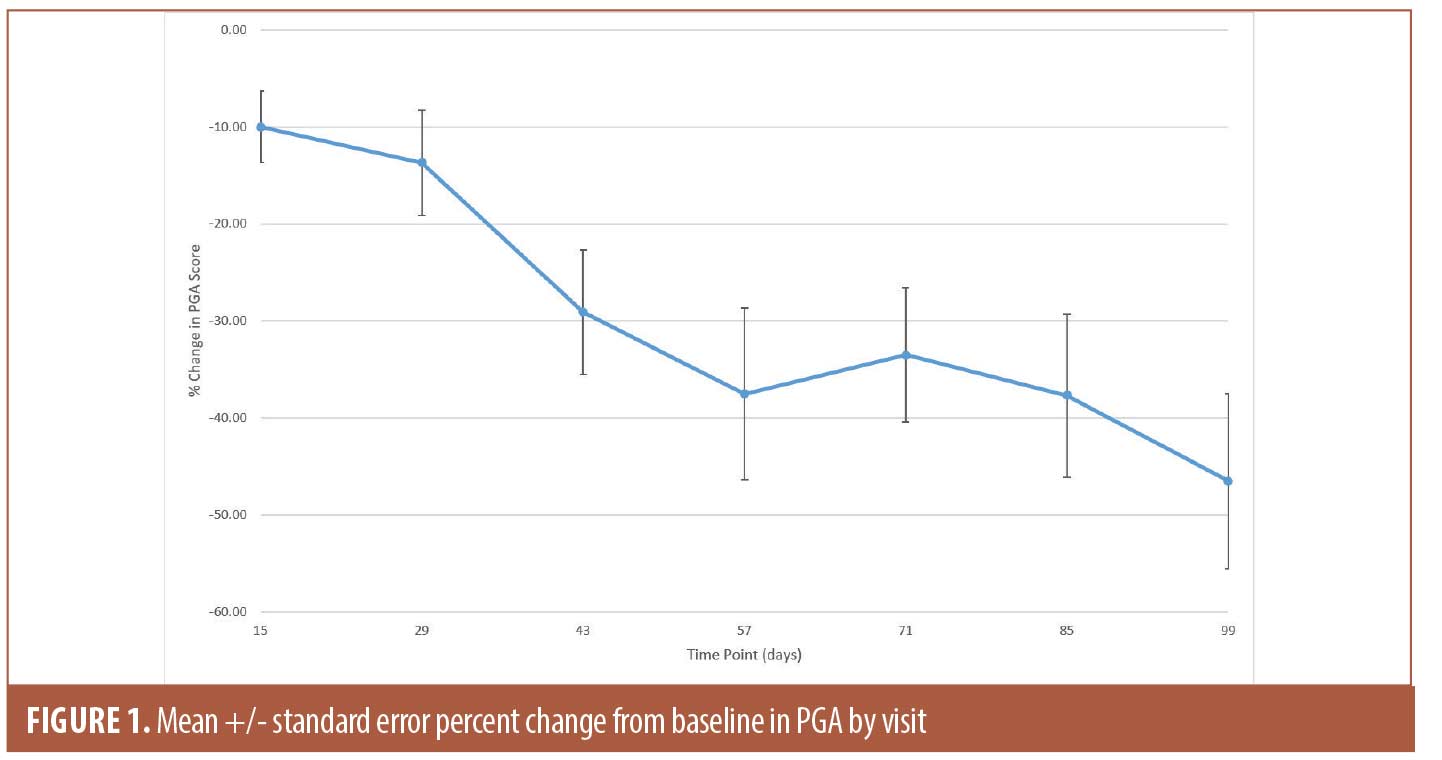
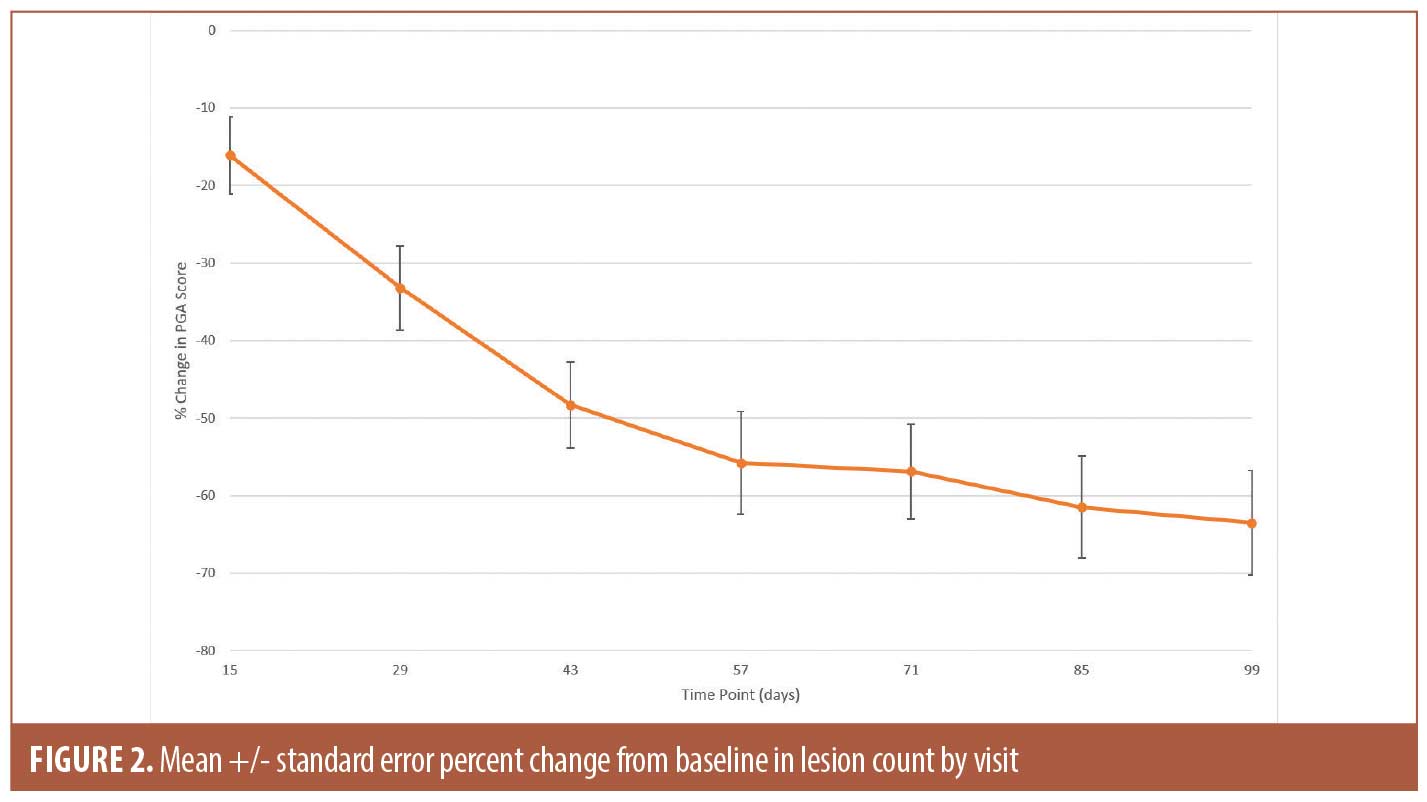
The secondary efficacy endpoints included measuring changes in PGA score and lesion counts at all time points (Table 1). The mean percent change in PGA score showed gradual improvement throughout the study and reached 46.5 percent (SE 9.04; p<0.0001) at Day 99 (Figure 1). The improvement in the lesion count reached 63.5 percent (SE 6.74; p<0.0001) at Day 99 (Figure 2). All changes from baseline in lesion counts were statistically significant.
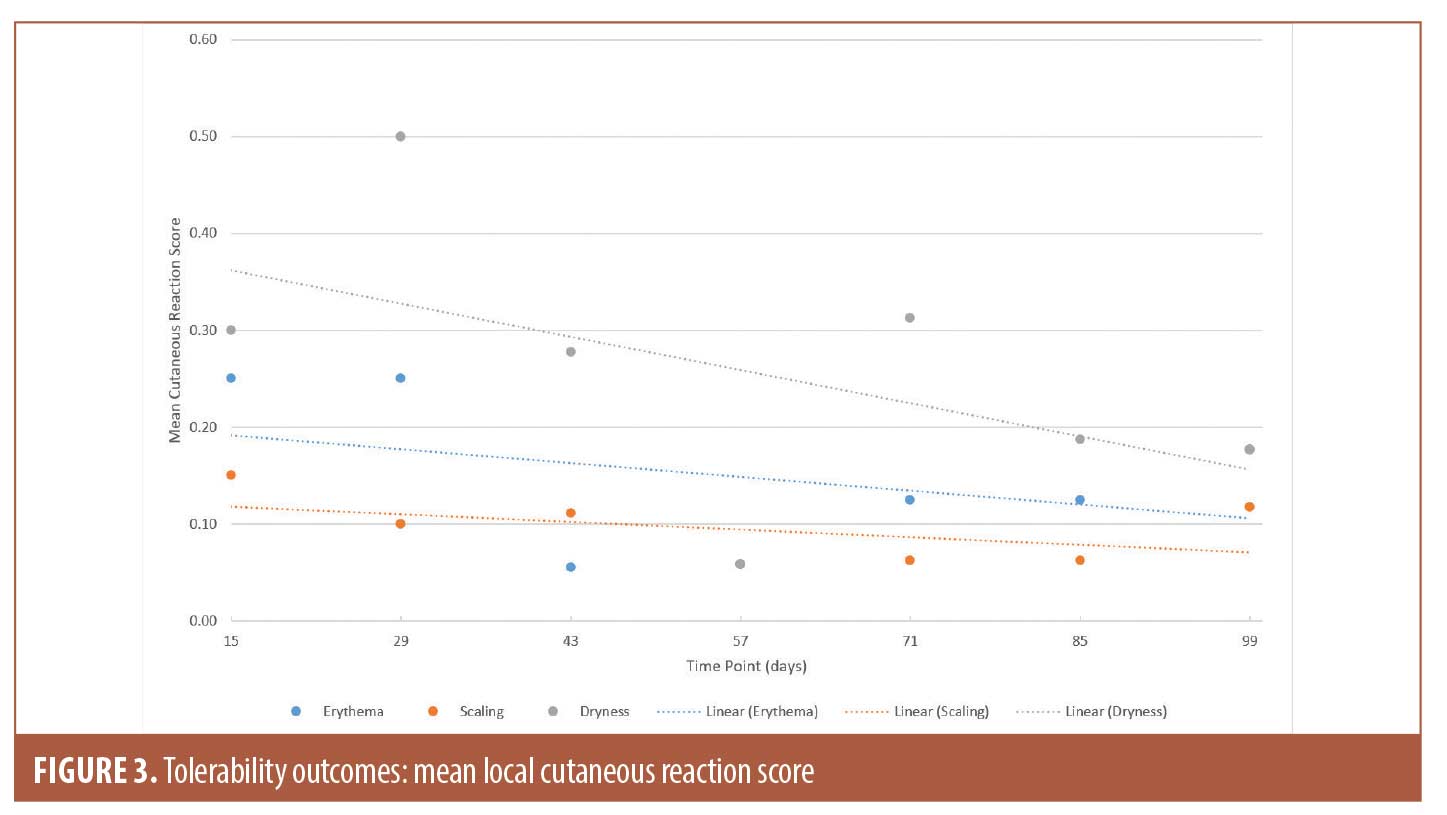
Tolerability outcomes. Physician-evaluated local cutaneous reactions indicated that the highest percentage of patients with erythema (18.2%) and dryness (40.9%) were identified on Day 29, while scaling was most prevalent on Day 15 (13.6%). Erythema, scaling, and dryness gradually improved during subsequent visits and were detected in 13.6 percent, 9.1 percent, and 13.6 percent of the subjects, respectively, on Day 99. For the duration of the study, majority of the reported cutaneous reactions were mild. To account for changes in the number of subjects experiencing irritation, as well as severity of the symptoms, the mean scores at all time points are presented in Figure 3. However, it should be noted that missing data were not imputed. Data for all three categories were missing for two subjects on Days 15 and 29, four subjects on Day 43, five subjects on Days 57 and 99, and six subjects on Days 71 and 85 due to subjects missing the visits.
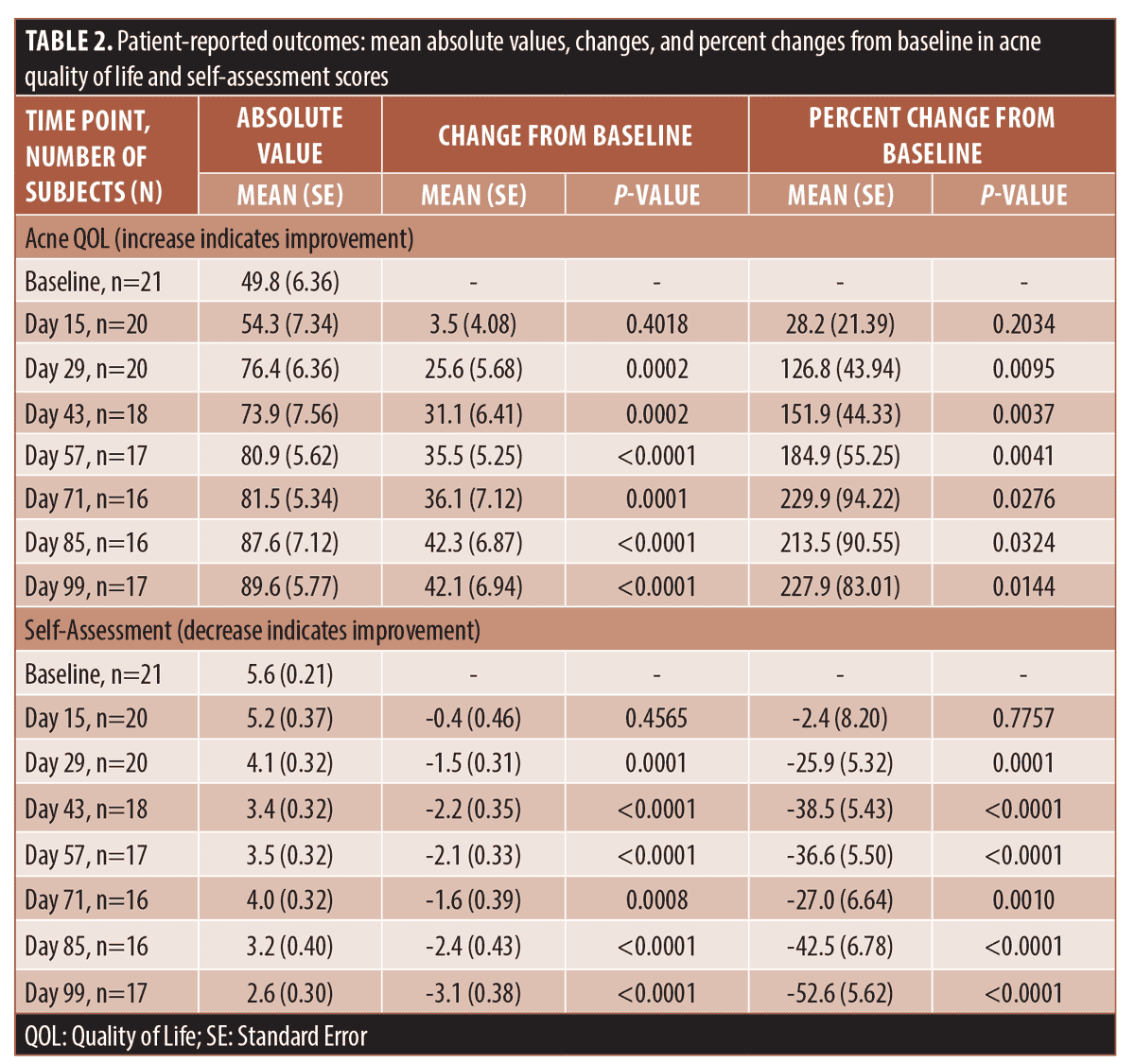
Patient-reported outcomes. The acne QOL progressed from the mean score of 49.8 (SE 6.36) at the baseline to 89.6 (SE 5.77; p<0.0001) and mean percent change of 227.9% (SE 83.01; p=0.0144) at Day 99, which indicated significant improvement. All changes from baseline in self-assessment score were statistically significant starting Day 29, when the percent change reached 25.9 percent (SE 5.32; p=0.0001), and continued improving till Day 99 with percent change of 52.6 percent (SE 5.62; p<0.0001). Table 2 presents the mean absolute values, changes and percent changes at each time point for the acne QOL and self-assessment survey responses. The subject satisfaction survey improved by the average of 162.9% (SE 71.24; p=0.0372) to reach 8.0 (SE 0.59) on Day 99, compared to 4.9 (SE 0.65) at the baseline.
Discussion
Perimenstrual acne is a common disorder with a significant impact on quality of life in a large number of adult women; however, current treatment options are limited to oral hormonal agents, such as oral contraceptive pills and androgen receptor blockers. While topical agents are clinically used, current literature lacks evidence-based evaluation of their efficacy and safety.
The present study was the first to evaluate the efficacy and tolerability of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% combination gel in patients with perimenstrual acne. The main tools for assessing treatment efficacy were PGA and lesion count. According to the FDA guidelines, PGA of 0 or 1 (clear or almost clear, respectively) defines treatment success. However, it is also important to consider changes in lesion count, as this endpoint provides a complimentary quantitative measure of effect.11 By Day 99, the combination therapy reduced PGA to 0 or 1 in 52.9 percent of the subjects. Treatment with the combination gel also resulted in improvement in lesion counts, which was statistically significant at all time points and reached 63.5 percent on Day 99.
Overall, the medication was well tolerated with no AEs or SAEs reported throughout the study. The physician-evaluated local cutaneous reactions of erythema and dryness were consistent with the Phase 3 clinical trial comparing the efficacy and safety of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% combination gel once daily for 12 weeks with vehicle gel in patients with moderate to severe acne vulgaris.9 Most cutaneous reactions were mild and improved by Day 99. Interestingly, the study subjects reported no AEs throughout the whole study. The fact that the patients did not report cutaneous reactions as AEs highlights the importance of recognizing differences in perception of the balance between efficacy and tolerability between the physician and patient. Survey studies suggest that patients’ compliance may be influenced by the perceived efficacy and tolerability.12 In our study, the discrepancy between the physician and patient reported cutaneous reactions may be explained by significant improvement of the patients’ self-assessments early in the study.
Limitations. Study limitations included the limited sample size and lack of a concurrent comparison group to determine comparative effectiveness of the investigational drug, as the results could not be compared to other treatment options or vehicle by itself.
Conclusion
The combination of efficacy and tolerability of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% combination gel in patients with perimenstrual acne suggests that this medication could provide an important, clinically effective topical treatment option and address the drawbacks of the oral medications currently recommended for this condition. Clinicians should be aware of this potential alternative therapy to offer an individualized therapeutic approach to their patients.
References
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults. J Am Acad Dermatol. 2008 Jan;58(1):56–59.
- James WD. Clinical practice. Acne. N Engl J Med. 2005 Apr 7;352(14):1463–1472.
- Shen Y, Wang T, Zhou C, et al. Prevalence of acne vulgaris in Chinese adolescents and adults: a community-based study of 17,345 subjects in six cities. Acta Derm Venereol. 2012;92(1):40–44.
- Geller L, Rosen J, Frankel A, et al. Perimenstrual flare of adult acne. J Clin Aesthet Dermatol. 2014;7(8):30–34.
- Stoll S, Shalita AR, Webster GF, et al. The effect of the menstrual cycle on acne. J Am Acad Dermatol. 2001;45:957–960.
- Lucky, AW. Quantatative Documentation of a premenstrual flare of facial acne in adult women. Arch Dermatol. 2004;140:423–424.
- Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004;22(5):419–428.
- Ebede TL, Arch EL, Berson D. Hormonal treatment of acne in women. J Clin Aesthet Dermatol. 2009 Dec;2(12):16–22.
- Onexton gel [package insert]. Quebec, Canada: Valeant Pharmaceuticals International, Inc. http://www.valeant.com/Portals/25/Pdf/PI/Onexton-PI.pdf. Accessed on June 23rd, 2021.
- Langner A, Chu A, Goulden V, et al. A randomized, single-blind comparison of topical clindamycin + benzoyl peroxide and adapalene in the treatment of mild to moderate facial acne vulgaris. Br J Dermatol. 2008;158(1):122–129.
- U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry. Acne vulgaris: establishing effectiveness of drugs intended for treatment.
- Zouboulis CC, Fischer TC, Wohlrab J, et al. Study of the efficacy, tolerability, and safety of 2 fixed-dose combination gels in the management of acne vulgaris. Cutis. 2009;84(4):223–229.

