J Clin Aesthet Dermatol. 2024;17(2):43–46.
J Clin Aesthet Dermatol. 2024;17(2):43–46.
by Zoe Diana Draelos, MD, FAAD; Pearl E. Grimes, MD, FAAD; Jacqueline Watchmaker, MD, FAAD; and
Diane B. Nelson, RN, MPH
Dr. Draelos is with Dermatology Consulting Services PLLC in High Point, North Carolina. Dr Grimes is with The Grimes Center of Medical and Aesthetic Dermatology and the Vitiligo and Pigmentation Institute of Southern California in Los Angeles, California. Dr. Watchmaker is with Southwest Skin Specialists in Scottsdale, Arizona. Ms. Nelson is with Skinbetter Science, a Dermatological Beauty brand of L’Oréal USA, Inc. in Phoenix, Arizona.
FUNDING: This study was funded by skinbetter science, LLC, a Dermatological Beauty brand of L’Oréal USA, Inc.
DISCLOSURES: Drs. Draelos, Grimes and Watchmaker were consultants for this study; Diane Nelson is an employee of skinbetter science, LLC.
ABSTRACT: Objective. A topical serum comprised of plant-based adaptogens was purposefully developed to support the ability of the skin to adapt and achieve balance. The study described herein evaluated changes in the expression of target genes related to skin homeostasis following topical exposure.
Methods. Utilizing an in vitro epidermal skin model, quantitative polymerase chain reaction (qPCR) analysis of gene expression was conducted following 48-hour exposure to 15μL of the study product (MYS serum) to the surface of each tissue (N=4). Biomarkers that play a key role in skin homeostasis were analyzed: Aryl hydrocarbon receptor (AhR), chloride channel accessory 2 (CLCA2), metallothionein 1A (MT1A), 1F (MT1F), and 1G (MT1G), and thioredoxin reductase 1 (TXNRD1). Statistically significant changes were calculated using unpaired t-test analysis (p<0.05) versus control (saline). A linear Fold Change (FC) value >2 was considered statistically significant.
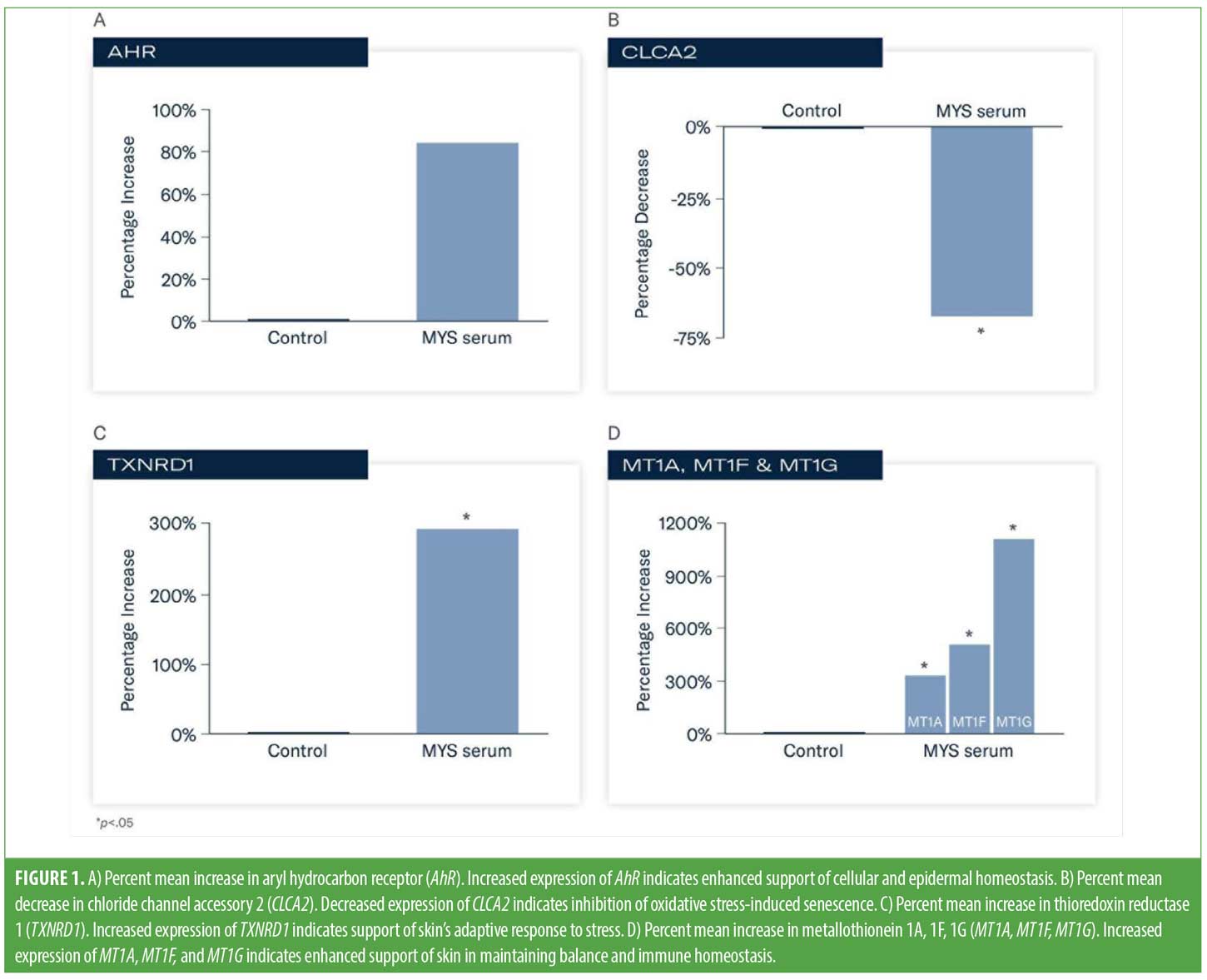
Results. An 85 percent (FC=1.85) increase in expression of AhR vs. control occurred following exposure to MYS serum indicating enhanced support of cellular and epidermal homeostasis, and the skin barrier’s response to stress. Statistically significant increases in expression occurred with TXNRD1 (293%; FC=3.93), MT1A (307%; FC=4.07), MT1F (529%; FC=6.29), and MT1G (163%; FC=12.63) vs. control, indicating support of skin’s adaptive response to stress and immune homeostasis. Significantly decreased levels of CLCA2 were demonstrated (69%; FC=-3.24) indicating inhibition of oxidative stress-induced senescence.
Conclusion. Utilizing an in vitro epidermal skin model, a serum comprised of plant-based adaptogens demonstrated changes in the expression of target genes that play important roles in skin’s ability to respond to stress and achieve homeostasis.
Keywords: adaptogens, homeostasis, in vitro gene analysis, anti-aging biomarkers
Optimal skin health relies on the ability of skin to efficiently respond to the constant bombardment of external insults. Skin innately strives to achieve and maintain homeostasis, but this ability naturally declines with age and with continued exposure to a range of exogenous stressors.1 The epidermis is a highly dynamic tissue that is constantly challenged by both internal and external factors that threaten equilibrium, necessitating constant and rapid adjustments to maintain balance.1–5 Various structural components and layers of the skin assume different and unique roles to provide both a physical barrier to protect against mechanical damage, ultraviolet (UV) exposure/damage, and environmental toxins, and an immune barrier to protect against possible pathogens.2,6,7,8 Skin aging leads to a loss of physiological integrity with increasing susceptibility of the skin to infection, increasing skin fragility, and impairing the ability of skin to function optimally.9,10 Decreasing immune function and increasing low-grade, chronic inflammation are also associated with aging skin.10,11
Skin cells utilize various, complex signaling pathways that sense environmental fluctuations and respond via intracellular communication to promote adaptation to the changing environment.2,6,7 Homeostasis depends on a multifaceted network of cell types and proteins, such as cytokines. Effective activation of the complement system, involving inflammatory factors, the extracellular matrix (ECM), intracellular adaptive stress response signaling pathways, and cellular tissue structure morphology all play a critical role in supporting homeostasis of the skin.7,12
Additionally, the need to efficiently replace aging senescent (SEN) cells and repair cellular damage is vital to maintain the integrity of the skin barrier.5 Cellular senescence can occur in response to DNA damage, telomere shortening and dysfunction, oxidative stress, and mitochondrial dysfunction reducing the cell’s ability to proliferate, promoting skin inflammation.5 Skin aging is associated with an accumulation of SEN dermal fibroblasts. Keratinocytes require active metabolism to fuel their growth, proliferation, and differentiation.5 Dermal fibroblasts synthesize and organize the ECM, communicating with the adjacent stem-cell niche as core factors in skin homeostasis associated with aging. Plant and botanical adaptogens represent a unique means for aiding skin adaptation to environmental changes in the pursuit of homeostasis, as they have the ability to support the ECM, reduce inflammation, and inhibit oxidative stress pathways.7,12
Adaptogens. The definition of adaptogens continues to evolve with our expanded understanding of their multiple benefits and uses. Currently, adaptogens are generally described as natural compounds or plant extracts that enhance resistance, adaptability, and survival of organisms under stress.7 Plant adaptogens must be able to act on multiple pathways and molecular targets, and to activate adaptive defense signaling pathways to be considered adaptogenic.7,12
Adaptogenic compounds are often derived from plants or herbal sources with very rich phytochemical compositions.13 Most adaptogens are found within three families: Araliaceae (e.g., Panax ginseng), Fabaceae (e.g., Rhodiola) and Lamiaceae (mint family, including many garden and aromatic herbs). Well known adaptogenic agents include flavonoids, phenols, curcumin, terpenoids, and volatile oils. Beneficial properties of these compounds may include anti-inflammatory, antibacterial, antifungal, anti-aging/anti-photoaging, skin brightening, and anti-hair loss effects.7 Adaptogens include receptors for various stress hormones, reproductive hormones, and neurotransmitters, among others.12 Adaptogens can influence and increase the body’s resistance to negative stressors and facilitate protective benefits.4,7,13
To date, many of the 109 plant adaptogens which have already been identified as having anti-stress or adaptogenic activity are currently utilized in traditional medicinal systems.7,12 Adaptogens are increasingly being used in skincare for the management of certain dermatological conditions and in formulations designed for aging skin. Plant adaptogens have been shown to be beneficial in numerous dermatologic conditions, including psoriasis, atopic dermatitis, and acne.7 Recent evidence suggests that combinations of adaptogens may have synergistic benefits to increase resistance to stress, mitigate inflammation, and prevent premature aging.12 Continued research is required to improve our understanding of their unique mechanisms of action and effects in the skin.
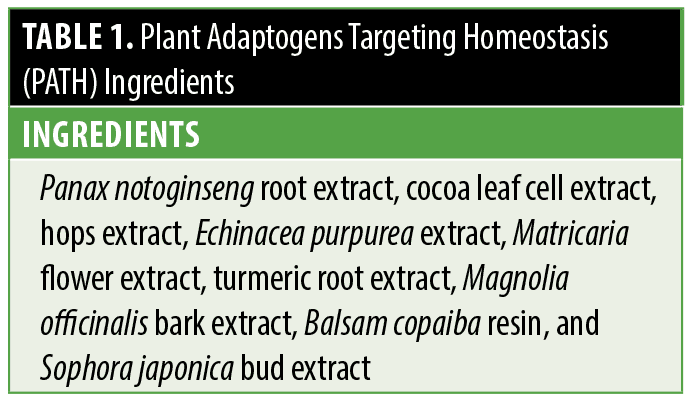
The incorporation of adaptogens into skincare presents a unique opportunity in optimizing global skin health and quality. Towards this end, rigorous screening and analysis of a diverse group of plant extracts was conducted to identify and categorize their specific activity and impact on pathways in the skin. Owing to their individual and combined benefits, a novel serum comprised of Plant Adaptogens Targeting Homeostasis (PATH) was purposefully developed to support skin’s natural ability to adapt and achieve balance, and visibly improve skin quality (Table 1). The adaptogenic compounds used in PATH have important multifunctional properties, such as inflammatory and tyrosinase inhibitory activity, support of aging skin, and antioxidant capacity. Additionally, many of these agents have been found to reinforce skin barrier function.14–20 Performing gene expression analysis with adaptogenic compounds can improve our understanding of their effects on specific gene functions and biological pathways in the skin. A separate study investigated the effects of a novel, adaptogenic compound (MYS serum) comprised of PATH technology on the visible signs of skin quality in participants with photoaged skin.21 Herein, we describe testing conducted utilizing an in vitro skin model to help identify the mechanistic activity of a novel serum. The purpose of this evaluation was to quantify changes in the expression of target genes related to skin homeostasis following topical exposure to MYS serum.
Methods
This study was conducted utilizing an in vitro full thickness, epidermal skin model (MatTek Corp., Massachusetts) comprised of human epidermal keratinocytes in a stratified corneal layer, an intact dermal-epidermal junction (DEJ), and viable fibroblasts obtained from neonatal tissue.
Prior to analysis, cell viability/cytotoxicity was assessed using a positive control (lactate dehydrogenase [LDH]) assay. Increased LDH activity is an indicator of damaged or dead cells. Cytotoxicity was assessed using media collected from the culture wells, using a LDH cytotoxicity detection kit. The detection kit is a colorimetric assay that allows measurement of the LDH activity released from damaged cells into the medium.
Quantitative polymerase chain reaction (qPCR) analysis of gene expression was conducted following 48-hour exposure and application of 15μL of MYS serum and 15μL of a control (saline) to the surface of each tissue (N=4 per group). Agents were applied to the surface of each tissue for 24 hours then washed off using sterile phosphate buffered saline (PBS). After a cotton swab was used to remove the excess PBS, test materials were reapplied to the surface of the tissues for another 24 hours (for a total exposure of 48 hours). The tissues were then rinsed again with sterile PBS and collected for gene expression analysis. Tissues were maintained at 37ºC with 5% CO₂ and ~95% humidity throughout the experiment.
Gene expression analysis was performed using validated assays for human gene expression. The genes selected were based on their regulation of homeostatic skin functions and included aryl hydrocarbon receptor (AhR), chloride channel accessory 2 (CLCA2), metallothionein 1A (MT1A), 1F (MT1F), and 1G (MT1G), and thioredoxin reductase 1 (TXNRD1). AhR is expressed in all skin cell types and plays an important role in homeostasis by regulating skin barrier function and controlling immune-mediated skin response to stress.22 CLCA2 plays a key role in supporting stressed, aging skin. Decreased levels indicate reduced accumulation of age-related effects.23 TXNRD1 has antioxidant properties and is essential for keratinocyte survival and protection against UV radiation.24 MT1A, MT1F, and MT1G are essential metal transporters (e.g., zinc) and provide a protective role in maintaining balance and homeostasis and reducing the skin’s stress response.25
Statistically significant changes were calculated using unpaired t-test analysis (p<0.05) vs. control group. A linear fold change (FC) value greater than two is considered statistically significant.
Results
Cytotoxicity analysis confirmed MYS serum was nontoxic to the skin tissue model (toxicity <20%; p<0.05).
Following exposure to MYS serum, an 85-percent (FC=1.85) increase in the expression of AhR versus control indicated enhanced support of cellular and epidermal homeostasis, and the skin barrier’s response to stress (Figure 1A). Statistically significant changes in expression occurred with TXNRD1, MT1A, MT1F, MT1G, and CLCA2 versus control (p<0.05). Decreased levels of CLCA2 (69%; FC=-3.24) occurred, indicating inhibition of oxidative stress-induced senescence (Figure 1B). TXNRD1 increased by 293 percent (FC=3.93) indicating support of skin’s adaptive response to stress (Figure 1C). Increased expression of MT1A (307%; FC=4.07), MT1F (529%; FC=6.29) and MT1G (163%; FC=12.63) was demonstrated, suggesting enhanced support of skin in maintaining balance and immune homeostasis (Figure 1D).
Discussion
Aging and stress impair the ability of skin to sufficiently respond to internal and external stressors. The loss of physiological integrity increases the risk of infection, chronic low-grade inflammation, reduced immune function, and fragility. In addition, these changes impair optimal skin quality, leading to erythema, rough skin, skin dullness, enlarged pores, and uneven pigmentation.1 While agents typically used in topical skincare products, such as retinoids, alpha hydroxy acids, beta hydroxy acids, and antioxidants, can be highly effective in improving skin quality and appearance, they do not address or facilitate cellular or epidermal homeostasis. In contrast, plant adaptogens, by definition, are uniquely able to communicate with multiple pathways in the skin to activate the complement system, ECM, intracellular adaptive stress response signaling pathways, and inhibit oxidative stress pathways, among others, in order to facilitate homeostasis.7,12
To this end, initial investigations identified a group of plant adaptogens that demonstrated benefits in facilitating skin homeostasis. To improve our understanding of the mechanistic activity of this curated blend of adaptogens aimed at supporting the adaptive stress response in skin, we evaluated genes that play a key role in supporting homeostasis and the adaptive stress response in skin by analyzing their specific activity. Following topical exposure to MYS serum, enhanced expression of AhR, TXNRD1, MT1A, MT1F and MT1G were demonstrated, with decreased levels of CLCA2 expressed.
AhR is a ligand-dependent transcription factor, and its action is dependent upon which receptor the ligand is upregulating or downregulating, and the underlying health of the skin. Activation of AhR upregulates expression of barrier-related proteins, supporting terminal keratinocyte differentiation. Downregulation of AhR can lead to proliferation of proinflammatory cytokines.22 There was a substantial increase in the expression of AhR versus control, indicating enhanced support of cellular and epidermal homeostasis.
TXNRD1 regulates melanocyte homeostasis and pigmentation to protect against UVB-induced DNA damage and oxidative stress.26 When upregulated, TXNRD1 reduces oxidative stress in fibroblasts, facilitating homeostasis and cellular adaptation. In this analysis, there was a nearly 300-percent increase of TXNRD1 following topical exposure to MYS serum, indicating support of the skin’s adaptive response to stress. Similarly, there was significant increased expression of metallothionein (1A, 1F, 1G). MT1 is universally expressed in almost all organs to maintain homeostasis.25 MT1 has been shown to be an important stress protein that affects innate and adaptive immunity.25 These findings help support skin in maintaining balance and immune homeostasis. Lastly, CLCA2 is a p53-inducible SEN-associated gene that provokes cellular senescence, and downregulation of CLCA2 is associated with inhibition of oxidative stress-induced senescence.23 There was a significantly decreased level of CLCA2 demonstrating inhibition of oxidative stress-induced senescence.
The in vitro nature of this analysis is an inherent limitation of this study, as is the need to evaluate the effects of MYS serum in human subjects, which has been performed in a clinical study following this analysis.21
Conclusion
A topical serum comprised of plant-based adaptogens demonstrated in vitro changes in the expression of target genes that play important roles in skin’s ability to respond to stress and achieve homeostasis. These findings afford insight into the mechanistic activity of this novel serum in the skin.
Acknowledgments
The authors would like to thank Lynne Kolton Schneider, PhD, for her editorial assistance on this manuscript.
References
- Pomatto LCD, Davies KJA. The role of declining adaptive homeostasis in ageing. J Physiol. 2017;595(24):7275–7309.
- Eckhart L, Tschachler E, Gruber F. Autophagic control of skin aging. et al. Front Cell Dev Biol. 2019;7:143.
- Humphrey S, Brown SM, Cross SJ, et al. Defining skin quality: clinical relevance, terminology, and assessment. Dermatol Surg. 2021;47(7):974–981.
- Ray A, Gulati K, Anand R. Stress, adaptogens and their evaluation: an overview. J Pharma Reports. 2016;1:110.
- Wang J, Cui B, Chen Z, et al. The regulation of skin homeostasis, repair and the pathogenesis of skin diseases by spatiotemporal activation of epidermal mTOR signaling. Front Cell Devel Biol. 2022;10:950973.
- Chen Y, Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm Allergy. 2014;13:177–190.
- Liu X-X, Chen C-Y, Li L, et al. Bibliometric study of adaptogens in dermatology: pharmacophylogeny, phytochemistry, and pharmacological mechanisms. Drug Design, Devel Ther. 2023;17:341–361.
- Zorina A, Zorin V, Kudlay D, et al. Molecular mechanisms of changes in homeostasis of the dermal extracellular matrix: both involutional and mediated by ultraviolet radiation. Int J Mol Sci. 2022;23:6655.
- Lopez-Otin C, Blasco A, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217.
- Pilkington SM, Bulfone-Paus S, Griffiths CEM, et al. Inflammaging and the skin. J Investig Dermatol. 2021;141:1087–1095.
- Xia S, Zhang X, Zheng S, et al. An update on inflamm-aging: mechanisms, prevention, and treatment. J Immunol Res. 2016;8426874:1–12.
- Panossian AG, Efferth T, Shikov AN, et al. Evolution of the adaptogenic concept from traditional use to medical systems: pharmacology of stress- and aging-related diseases. Med Res Rev. 2021;41:630–703.
- Todorova V, Ivanov K, Delattre C, et al. Plant adaptogens – history and future perspectives. Nutrients. 2021;13:2861.et al; Nutrients 2021;13(8):2861.
- Barbalho SM, de Sousa Gonzaga HF, de Souza GA, et al. Dermatological effects of Curcuma species: a systematic review. Clin Exp Dermatol. 2021;46(5):825–833.
- Park MY, Han SJ, Moon D, et al. Effects of red ginseng on the elastic properties of human skin. J Ginseng Res. 2019;44:738–746.
- Pham QL, Jang H-J, Kim K-B. Anti-wrinkle effect of fermented black ginseng on human fibroblasts. Int J Mol Med. 2017;39(3):681–686.
- Ren Q and Lin J. el the biomarkers of oxidative stress: a systematic review and meta-analysis. Phytother Res. 2023.
- Saboori S, Falahi E, Rad EY, et al. Effects of ginseng on C-reactive protein level: a systematic review and meta-analysis of clinical trials. Complement Ther Med. 2019;45:98–103.
- Vaughn AR, Branum A, Sivamani RK. Effects of turmeric (Curcuma longa) on skin health: a systematic review of the clinical evidence. Phytother Res. 2016;30(9):1243–1264.
- Yang Y, Ren C, Zhang Y, et al. Ginseng: an nonnegligible natural remedy for healthy aging. Aging Dis. 2017;8(6):708–720.
- Draelos ZD, Grimes PE, Watchmaker J, Nelson DB. A Multi-center Trial Evaluating a Serum Comprised of Plant-based Adaptogens Targeting Skin Quality. J Clin Aesthet Dermatol. 2024;17(2):15–19.
- Fernández-Gallego N, Sanchez-Madrid F, Cibrian D. Role of AHR ligands in skin homeostasis and cutaneous inflammation. Cells. 2021;10:3176.
- Tanikawa C, Nakagawa H, Furukawa Y, et al. CLCA2 as a p53-inducible senescence mediator. Neoplasia Press. 2012;14(2):141–149.
- de la Vega MR, Krajisnik A, Zhang DD, et al. Targeting NRF2 for improved skin barrier function and photoprotection: focus on the achiote-derived apocarotenoid bixin. Nutrients. 2017;9:1371.
- Dai H, Wang L, Li L, et al. Metallothionein 1: a new spotlight on inflammatory diseases. Front. Immunol. 2021;12:739918.
- Carpenter EL, Wyant MB, Indra A, et al. Thioredoxin reductase 1 modulates pigmentation and photobiology of murine melanocytes in vivo. J Invest Dermatol. 2022;142(8):1903–1911.