J Clin Aesthet Dermatol. 2019;12(2):37–38
 by Melody Maarouf, MHS; Chantal Saberian, MD; Robert J. Segal, MD; and Vivian Y. Shi, MD
by Melody Maarouf, MHS; Chantal Saberian, MD; Robert J. Segal, MD; and Vivian Y. Shi, MD
Ms. Maarouf is with the University of Arizona College of Medicine in Tucson, Arizona. Dr. Saberian is with the Lebanese American University School of Medicine in Beirut, Lebanon. Drs. Segal and Shi are with the Division of Dermatology, Department of Medicine, University of Arizona in Tucson, Arizona.
FUNDING: No funding was received for this study.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
Abstract: The practice of adorning the body with permanent ink dates back to the late Neolithic period. Today, a large proportion of the younger generation has at least one tattoo. Despite the recent popularity of tattoos, there are prolific reports within the literature detailing the adverse cutaneous reactions that occur following the intradermal injection of tattoo inks. Such reactions can occur immediately or years later. In addition to these known reactions, consumer preference for “animal-friendly” products has shifted the ingredients used in tattoos and has ushered in the era of “vegan tattoos.” Because of its recent emergence and the lack of regulation of intradermal pigment by the United States Food and Drug Administration, we remain unsure of the potential reactions of these new ingredients. Currently, we can only predict complications by extrapolating from the known reactions of the topical administration of these same plant-based ingredients. In this article, we elucidate some potential reactions in an effort to warn the dermatologic community of the need to educate patients and encourage Federal reporting and regulation.
KEYWORDS: Tattoo, vegan tattoo, cutaneous complications, hypersensitivity reactions, Federal product regulation
Body inking dates back to the late Neolithic period. Nearly 5,200 years later, permanent and henna tattooing are popular cultural accessories, with 36 percent of people under the age of 40 years having at least one tattoo.1,2 With its increasing popularity, there is an understandable misperception that tattoos are safe, despite absent toxicological testing of tattoo ink colorants.1
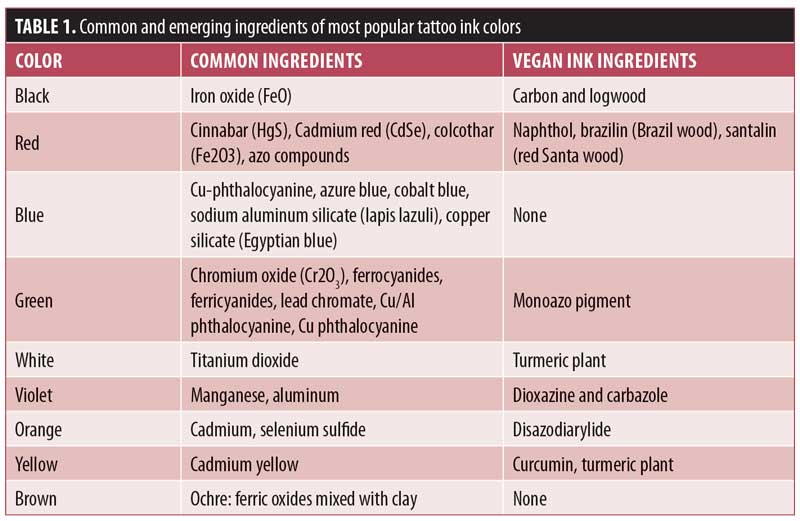
Permanent tattoos inks are composed of pigments and additives derived from animals, plants, and/or metals (Table 1).1 The base ingredient of temporary henna tattoo ink comes from Lawsonia inermis, a tropical shrub, with added essential oils, coffee, tea, indigo, azoic dye, or paraphenylenediamine (PPD) to decrease drying time and intensify the dark color.2
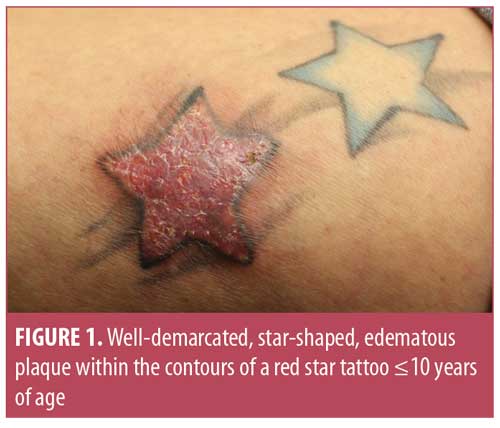
 The intradermal injection of foreign substances is associated with potential complications, including blood-borne viral infections (hepatitis B and C, human immunodeficiency virus); superficial or systemic bacterial infections (Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, and nontuberculous mycobacteria); phototoxicity; hypersensitivity; and Koebner’s response. Red pigment (cinnabar, mercury sulphate) is most often implicated (Figures 1 and 2) for cell-mediated, delayed hypersensitivity reactions (Table 1).2,3 The azo pigment decomposes into products such as amino-naphthol-AS and naphthol-AS, with increased production following exposure to ultraviolet B radiation. The concept of phototoxic potential might explain why red tattoos often lead to reactions.4 Eczematous and phototoxic reactions can occur either immediately or months to years after the tattoo was done, suggesting a lifelong risk.1 PPD, a common and potent sensitizer, is present in 16 percent of henna preparations and can cause severe Type IV hypersensitivity skin reactions.2 More serious and long-term complications arising within permanent tattoos include pseudolymphomatous reactions, discoid lupus erythematosus, and malignant lesions. Additionally, tattoo ink migration to sentinel lymph nodes resembles metastatic melanoma on gross inspection and histopathology,1,5 which leads to unnecessary and invasive diagnostic testing. This might be an important consideration for individuals opting to get semipermanent tattoos that last one to three years. Semipermanent tattoo inks comprise smaller particles that get metabolized and deposited in draining lymph nodes. Additionally, magnetic resonance imaging (MRI) scans can react with iron oxide-based pigments to trigger low-grade burns, which should prompt the use of prophylactic measures with ice pack compressions or pressure dressings during imaging.6
The intradermal injection of foreign substances is associated with potential complications, including blood-borne viral infections (hepatitis B and C, human immunodeficiency virus); superficial or systemic bacterial infections (Streptococcus pyogenes, methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, and nontuberculous mycobacteria); phototoxicity; hypersensitivity; and Koebner’s response. Red pigment (cinnabar, mercury sulphate) is most often implicated (Figures 1 and 2) for cell-mediated, delayed hypersensitivity reactions (Table 1).2,3 The azo pigment decomposes into products such as amino-naphthol-AS and naphthol-AS, with increased production following exposure to ultraviolet B radiation. The concept of phototoxic potential might explain why red tattoos often lead to reactions.4 Eczematous and phototoxic reactions can occur either immediately or months to years after the tattoo was done, suggesting a lifelong risk.1 PPD, a common and potent sensitizer, is present in 16 percent of henna preparations and can cause severe Type IV hypersensitivity skin reactions.2 More serious and long-term complications arising within permanent tattoos include pseudolymphomatous reactions, discoid lupus erythematosus, and malignant lesions. Additionally, tattoo ink migration to sentinel lymph nodes resembles metastatic melanoma on gross inspection and histopathology,1,5 which leads to unnecessary and invasive diagnostic testing. This might be an important consideration for individuals opting to get semipermanent tattoos that last one to three years. Semipermanent tattoo inks comprise smaller particles that get metabolized and deposited in draining lymph nodes. Additionally, magnetic resonance imaging (MRI) scans can react with iron oxide-based pigments to trigger low-grade burns, which should prompt the use of prophylactic measures with ice pack compressions or pressure dressings during imaging.6
In addition to inauspicious reactions, awareness for consumer preferences has modified the industry’s options for ink ingredients. Now, vegan tattoos are available that employ a host of plant-based materials (Table 1). For instance, black ink, traditionally derived from the soot of charred animal bones, is instead made from a pigment extracted from logwood (Haematoxylon campechisnum). Pigment carrier solutions, traditionally made from animal fat (glycerin), are also exchanged for plant-based fats (glycerol).1 Though progression to more “natural” ingredients might reduce the incidence of certain known adverse reactions, what types of reactions can we expect from this new era of animal-friendly tattoos?
Because vegan tattoos only recently became available, the medical literature is sparse with reported reactions to plant-based inking. Therefore, we must glean an idea of what the future holds by identifying noted reactions following the topical use of these ingredients. The allergic contact dermatitis (ACD) literature is full of such reactions following cutaneous exposure to turmeric within massage oils, chlorhexidine antiseptic solutions, and food-coloring agents. Bircher et al7 reported ACD in a woman who repeatedly applied a temporary tattoo made from the extract of the jagua fruit (Genipa americana L.) that contained geniposide and genipin, compounds known to have antioxidative, anti-inflammatory, and antiviral activities. With such encouraging properties, consumers might gravitate towards these products with little risk suspicion.
Despite the growing popularity of tattoos, most reactions are not well-known to consumers or even state-licensed tattoo artists. Tattoo inks are considered cosmetic products under the United States (US) Food and Drug Administration’s (FDA) Federal Food, Drug, and Cosmetic Act. The FDA does not screen marketed pigments and will investigate only after formal complaints arise.8 There are no other standardized requirements for safety testing, and enforcement of regulations is left to state and local jurisdictions.9 Thus, an absence of recorded assessments of tattoo ink composition and toxicology poses problems in current and evolving tattoo practices. Unlike in the US, the European Commission routinely gathers information about potentially dangerous cosmetic products and sends alerts via its Rapid Alert System for Nonfood Products. Since 2009, reports have implicated more than 20 inks used in the US.9 Currently, the toxicology of tattoo inks is being investigated by the FDA’s Arkansas-based National Center for Toxicological Research.10
Reactions to tattoos can be chronic and difficult to treat, as well as necessitate long follow-up. As tattoo practices evolve, we should expect to see cutaneous complications caused by new ingredients. Thus, it is important that consumers, providers, and tattoo artists be aware of these potential complications and report adverse effects to the FDA to encourage the organized Federal regulation of tattoo inks and practices.
References
- Laux P, Tralau T, Tentschert J, et al. A medical-toxicological view of tattooing. Lancet. 2016;387(10016):395–402.
- Sweeney SM. Tattoos: a review of tattoo practices and potential treatment options for removal. Curr Opin Pediatr. 2006;18(4):391–395.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Ther (Heidelb). 2016;6(1):13–23.
- Gaudron S, Ferrier-Le Bouedec MC, Franck F, D’Incan M. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72(2):97–105.
- Ortiz AE, Alster TS. Rising concern over cosmetic tattoos. Dermatol Surg. 2012;38(3):424–429.
- Ross JR, Matava MJ. Tattoo-induced skin “burn” during magnetic resonance imaging in a professional football player: a case report. Sports Health. 2011;3(5):431–434.
- Bircher AJ, Sigg R, Scherer Hofmeier K, et al. Allergic contact dermatitis caused by a new temporary blue-black tattoo dye – sensitization to genipin from jagua (Genipa americana L.) fruit extract. Contact Dermatitis. 2017; 77(6):374–378.
- United States Food and Drug Administration. Tattoos & permanent makeup: fact sheet. Available at: https://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm108530.htm. Accessed January 30, 2018.
- Misiko H. Questions raised about tattoo inks, cancer. Available at: https://www.washingtonpost.com/national/health-science/questions-raised-about-tattoo-inks-cancer/2014/09/22/f1398ec8-2176-11e4-86ca-6f03cbd15c1a_story.html?utm_term=.6189dc6da5c5. Accessed January 30, 2018.
- Grabenhofer R. A tale of tattoos: toxicology. Available at: http://www.cosmeticsandtoiletries.com/research/chemistry/A-Tale-of-Tattoos-Toxicology-continued-322861291.html. Accessed January 30, 2018.
A New Era For Tattoos, with New Potential Complications
Categories:
J Clin Aesthet Dermatol. 2019;12(2):37–38
Ms. Maarouf is with the University of Arizona College of Medicine in Tucson, Arizona. Dr. Saberian is with the Lebanese American University School of Medicine in Beirut, Lebanon. Drs. Segal and Shi are with the Division of Dermatology, Department of Medicine, University of Arizona in Tucson, Arizona.
FUNDING: No funding was received for this study.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
Abstract: The practice of adorning the body with permanent ink dates back to the late Neolithic period. Today, a large proportion of the younger generation has at least one tattoo. Despite the recent popularity of tattoos, there are prolific reports within the literature detailing the adverse cutaneous reactions that occur following the intradermal injection of tattoo inks. Such reactions can occur immediately or years later. In addition to these known reactions, consumer preference for “animal-friendly” products has shifted the ingredients used in tattoos and has ushered in the era of “vegan tattoos.” Because of its recent emergence and the lack of regulation of intradermal pigment by the United States Food and Drug Administration, we remain unsure of the potential reactions of these new ingredients. Currently, we can only predict complications by extrapolating from the known reactions of the topical administration of these same plant-based ingredients. In this article, we elucidate some potential reactions in an effort to warn the dermatologic community of the need to educate patients and encourage Federal reporting and regulation.
KEYWORDS: Tattoo, vegan tattoo, cutaneous complications, hypersensitivity reactions, Federal product regulation
Body inking dates back to the late Neolithic period. Nearly 5,200 years later, permanent and henna tattooing are popular cultural accessories, with 36 percent of people under the age of 40 years having at least one tattoo.1,2 With its increasing popularity, there is an understandable misperception that tattoos are safe, despite absent toxicological testing of tattoo ink colorants.1
Permanent tattoos inks are composed of pigments and additives derived from animals, plants, and/or metals (Table 1).1 The base ingredient of temporary henna tattoo ink comes from Lawsonia inermis, a tropical shrub, with added essential oils, coffee, tea, indigo, azoic dye, or paraphenylenediamine (PPD) to decrease drying time and intensify the dark color.2
In addition to inauspicious reactions, awareness for consumer preferences has modified the industry’s options for ink ingredients. Now, vegan tattoos are available that employ a host of plant-based materials (Table 1). For instance, black ink, traditionally derived from the soot of charred animal bones, is instead made from a pigment extracted from logwood (Haematoxylon campechisnum). Pigment carrier solutions, traditionally made from animal fat (glycerin), are also exchanged for plant-based fats (glycerol).1 Though progression to more “natural” ingredients might reduce the incidence of certain known adverse reactions, what types of reactions can we expect from this new era of animal-friendly tattoos?
Because vegan tattoos only recently became available, the medical literature is sparse with reported reactions to plant-based inking. Therefore, we must glean an idea of what the future holds by identifying noted reactions following the topical use of these ingredients. The allergic contact dermatitis (ACD) literature is full of such reactions following cutaneous exposure to turmeric within massage oils, chlorhexidine antiseptic solutions, and food-coloring agents. Bircher et al7 reported ACD in a woman who repeatedly applied a temporary tattoo made from the extract of the jagua fruit (Genipa americana L.) that contained geniposide and genipin, compounds known to have antioxidative, anti-inflammatory, and antiviral activities. With such encouraging properties, consumers might gravitate towards these products with little risk suspicion.
Despite the growing popularity of tattoos, most reactions are not well-known to consumers or even state-licensed tattoo artists. Tattoo inks are considered cosmetic products under the United States (US) Food and Drug Administration’s (FDA) Federal Food, Drug, and Cosmetic Act. The FDA does not screen marketed pigments and will investigate only after formal complaints arise.8 There are no other standardized requirements for safety testing, and enforcement of regulations is left to state and local jurisdictions.9 Thus, an absence of recorded assessments of tattoo ink composition and toxicology poses problems in current and evolving tattoo practices. Unlike in the US, the European Commission routinely gathers information about potentially dangerous cosmetic products and sends alerts via its Rapid Alert System for Nonfood Products. Since 2009, reports have implicated more than 20 inks used in the US.9 Currently, the toxicology of tattoo inks is being investigated by the FDA’s Arkansas-based National Center for Toxicological Research.10
Reactions to tattoos can be chronic and difficult to treat, as well as necessitate long follow-up. As tattoo practices evolve, we should expect to see cutaneous complications caused by new ingredients. Thus, it is important that consumers, providers, and tattoo artists be aware of these potential complications and report adverse effects to the FDA to encourage the organized Federal regulation of tattoo inks and practices.
References
Share:
Recent Articles:
Categories:
Recent Articles:
Tags: