This ongoing column explores emerging treatment options, drug development trends, and pathophysiologic concepts in the field of dermatology.
J Clin Aesthet Dermatol. 2019;12(2):39–43
 by James Q. Del Rosso, DO
by James Q. Del Rosso, DO
Dr. Del Rosso is Research Director of JDR Dermatology Research in Las Vegas, Nevada; is with Thomas Dermatology in Las Vegas, Nevada; and is Adjunct Clinical Professor (Dermatology) with Touro University Nevada in Henderson, Nevada.
FUNDING: There was no funding related to the development, writing, or publication of this article.
DISCLOSURES: Dr. Del Rosso is a consultant, speaker, and/or researcher for several companies who market products used in the management of atopic dermatitis or have compounds under development. These include Almirall, Dermira, Galderma, Genentech, LaRoche Posay, Leo Pharma, Loreal, Ortho Dermatologics, Pfizer, Promius, Regeneron, Sanofi-Genzyme, Skinfix, Sonoma, Sun Pharma, and Taro.
Abstract: Atopic dermatitis (AD) is a chronic disorder that requires thorough patient education and a therapeutic management strategy designed to control flares, decrease recurrences, and reduce pruritus. In cases that cannot be controlled by proper skin care and barrier repair, topical therapy, and avoidance of triggers, systemic therapy is often required to control flares and maintain remission. It is important for clinicians to avoid becoming overly dependent on the intermittent use of systemic corticosteroid therapy to control flares, without incorporating other treatment options that might more optimally control AD over time. This article provides an overview of systemic therapies, including conventional oral therapy options and injectable biologic agents, that modulate the immune dysregulation in AD. Major emphasis is placed on the monoclonal antibodies currently available (e.g., dupilumab) for the treatment of AD, as well as those in latter stages of development, with a focus on agents targeting IL-4 and/or IL-13.
KEYWORDS: Atopic dermatitis, calcineurin inhibitors, phosphodiesterase-4 inhibitors, immunosuppressants, interleukin-4, interleukin-13
Many patients with atopic dermatitis (AD) are able to control their disease primarily with topical agents, including corticosteroids, calcineurin inhibitors, phosphodiesterase-4 (PDE4) inhibitors, moisturizers/barrier repair agents, wet wraps, and the avoidance of triggers.1,2 However, it is important to better define the word “control,” as AD is a chronic disorder characterized by marked flares of eczema and pruritus, variable periods of persistent eczema of lesser severity with itching, and complete remission, all of which vary in intensity, frequency, and duration among each individual affected by AD. Marked flares can often be mitigated with topical agents of adequate potency and duration, and, in selected cases, in conjunction with short courses of systemic corticosteroid (CS) therapy. The most difficult therapeutic challenges in AD are effective control of eczematous dermatitis (eczema) and pruritus, both of which are persistent but of a lesser overall severity, and the maintenance of remission after control of disease flares.1–5
Many patients with AD, including the parents/guardians of children with AD, deserve a discussion of what options exist beyond topical management alone and intermittent systemic CS therapy. This discussion often needs to be initiated by the clinician, as patients with AD or other chronic disorders depend on their clinician to direct them toward what is likely to be the most effective treatment for them at any given point in time. There are only so many oral CS courses or intramuscular CS injections a clinician can prescribe to help control AD flares without tipping the benefit versus risk balance toward too much risk. This same principle also applies to repeated use of topical CS therapy, which can progress to use so frequent that the risk for adverse effects is increased significantly. Skin barrier repair agents and steroid-sparing topical agents (e.g., pimecrolimus, tacrolimus, crisaborole) provide marked benefit in some cases of AD, especially on certain anatomic sites or when the affected body surface area (BSA) is not too extensive.1–3 However, most patients with AD would benefit from systemic therapies that are designed to achieve optimal suppression of AD, including eczematous dermatitis and/or pruritus. Daily diffuse application of a well-formulated moisturizer for skin barrier maintenance and the application of prescription topical therapies to persistent AD lesions remain part of the standard therapeutic regimen, especially for localized refractory and lichenified sites.1–6 Finding the optimal balance of therapeutic choices varies among individual patients and requires careful consideration of the overall clinical situation and specific patient-related factors, such as age, severity of AD signs and symptoms, and patient and clinician comfort levels with the treatments selected. Ultimately, the clinician should identify what is most likely to achieve an optimal level of control and express their treatment recommendations to the patient with realistic confidence and a proper benefit versus risk discussion.
The time has come for clinicians treating AD to consider moving from a rescue approach for flares to treating AD as a chronic, inflammatory, cutaneous and systemic disorder by using therapies that more selectively suppress the underlying disease pathophysiology, effectively treat eczema and pruritus, mitigate flares, and sustain long-term control of the disease. While topical therapies to manage epidermal barrier dysfunction and inflammation of AD should remain an important component of the total management approach for patients with AD, clinicians would be prudent to also consider therapies with better short-term and long-term safety profiles than the conventional oral agents that are currently available. In this article, an overview of the current conventional oral systemic therapeutic options for atopic dermatitis are presented, followed by an overview of the new systemic therapeutic options for AD, namely monoclonal antibody agents, including the currently available agent, duplimab, and other agents in latter stages of development, with a focus on compounds targeting IL-4 and/or IL-13. Other monoclonal antibodies that have been studied and/or are currently under evaluation for treatment of AD, such mepolizumab (anti-IL-5), nemolizumab (anti-IL-31), and omalizumab (anti-IgE), as well as other drug classes, will be discussed in future installments of “What’s New in the Medicine Chest.”
Conventional Systemic Therapeutic Options for Atopic Dermatitis—Oral Agents
When patients with moderate-to-severe AD and their clinicians are considering systemic therapy for AD, a variety of treatment options are available.3,5–12 Prior to 2018, available systemic therapies for AD were primarily oral agents, such as cyclosporin, methotrexate, azathioprine, and mycophenolate mofetil, all of which appear to modulate the underlying pathophysiologic pathways that contribute to AD.3,6–8 Each of these agents has variable amounts of data available regarding its use in children and adults for treatment of AD.3,6–12 However, none of these oral agents are approved by the United States Food and Drug Administration (FDA) for the treatment of AD, and all exhibit immunosuppressant properties.3,8 Oral antihistamines have also been used as part of the treatment regimen for AD, primarily as an adjunctive therapy to help reduce pruritus and/or decrease interference with sleep (i.e., sedating antihistamines).12 It is important to note that chronic or frequent use of systemic CS is best avoided in children and adults due to the risk of several significant AEs.6,10–12
Cyclosporin. Among the conventional systemic oral agents used in the management of AD, cyclosporin appears to exhibit the fastest onset of efficacy, but its use is limited by its safety profile, which includes risks of nausea, cephalgia, hypertension, nephrotoxicity, sequelae of chronic immunosuppression, gingival hyperplasia, and drug interactions.6,8,10 Cyclosporin is primarily recommended for treatment-resistant and/or uncontrolled AD, after which patients are usually transitioned to a safer, long-term approach; continuous use of cyclosporin beyond 12 to 24 months generally is not advisable.6,8,10
Methotrexate. Methotrexate therapy, another conventional systemic oral treatment for AD, can exhibit efficacy in as little as 4 to 8 weeks, but, like cyclosporin, warrants careful monitoring due to potential adverse events (AEs); these include nausea, bone marrow suppression (including pancytopenia), hepatotoxicity, pulmonary fibrosis, potential sequelae of immunosuppression, drug interactions, and the need to avoid alcohol intake.6,8,10 As with cyclosporin, long-term use of methotrexate should likely be avoided.
Azathioprine. Azathioprine is another conventional systemic oral treatment option for AD, but it is not usually considered an initial systemic option due to its slower onset of efficacy and potential toxicities. Potential AEs include bone marrow suppression, increased malignancy risk, other sequelae of immunosuppression, severe nausea/vomiting, abdominal pain, hepatotoxicity, drug hypersensitivity syndrome, and risk for drug-drug interactions (e.g., allopurinol).6,8,10
Mycophenolate mofetil. Finally, although data for use of mycophenolate mofetil as a treatment option for AD are more limited than cyclosporin data, mycophenolate mofetil appears to be the safest oral agent, when compared with cyclosporin, methotrexate, and azathioprine; it has an efficacy onset range of 4 to 12 weeks, making it a logical choice when transitioning patients to longer-term oral maintenance therapy after initial use of cyclosporin for treatment-refractory or severe AD. Potential AEs include gastrointestinal side effects, fatigue, hematologic changes, and potential sequelae of immunosuppression.6,8,10
Biologics for Treatment of Atopic Dermatitis
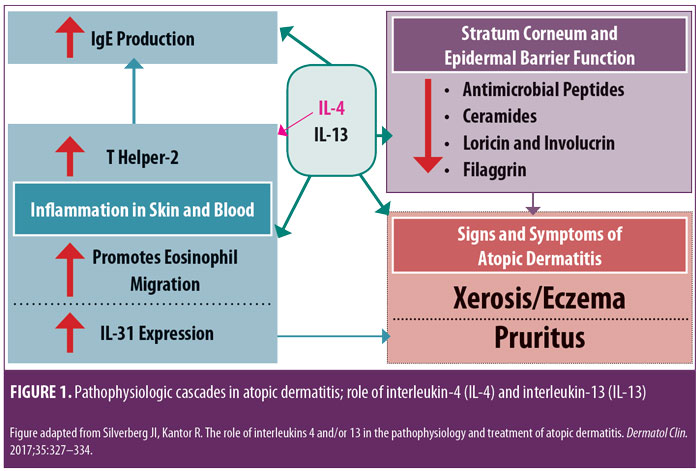
Research is in progress evaluating a variety of injectable and/or oral agents, including PDE4 inhibitors, Janus kinase (JAK) inhibitors, cannabinoid receptor agonists, kappa-opioid receptor agonists, and agents that target thymic stromal lymphopoietin (TSLP).14–17 A systematic review and meta-analysis of published studies evaluating the efficacy of biologics in AD treatment (published in April 2018) reported good evidence, to date, regarding agents that inhibit IL-4 and/or IL-13; a relative lack of evidence supporting efficacy in AD was noted thus far in studies with biologics modulating other targets, such as omalizumab (anti-IgE), infliximab ((anti-tumor necrosis factor), ustekinumab (anti-IL-12/23), and rituximab (anti-B-cell).19 IL-4 and IL-13 are reported to play prominent roles in AD with inflammation in skin and/or blood, epidermal barrier impairment, pruritis, and susceptibility to infection (Figure 1).18 Monoclonal antibodies that inhibit the effects of various ILs (i.e., IL-4, IL 13, IL-5, IL-17, IL-22, IL-31, IL-33) are showing therapeutic promise for the treatment of AD.
Monoclonal Antibody Interleukin-4 and Interleukin-13 Inhibitor
Dupilumab. Dupilumab is an injectable human IgG4 monoclonal antibody that inhibits IL-4 and IL-13 cytokine responses, including the expression and/or release of proinflammatory cytokines, chemokines, and IgE; binding of dupilumab occurs with both Types I and II IL-4 alpha receptors, found on hematopoietic cells and keratinocytes, respectively.13,20,21 In March 2017, duplimab was FDA-approved for the treatment of moderate-to-severe AD in adult patients (aged ?18 years) in whom the disease has not been adequately controlled with prescription topical therapies or in cases where such therapies are not advisable. In October 2018, duplimab was also approved as an add-on maintenance treatment in adolescent and adult patients (aged ?12 years of age) for moderate-to-severe asthma with an eosinophilic phenotype or oral–corticosteroid-dependent asthma.13 13 The dosing regimens for AD and asthma might differ between patients; however, the common regimen includes a 600mg loading dose (2×300mg2/mL injections), followed by a single 300mg injection every two weeks; with regard to asthma, dupilumab is not indicated or recommended for relief of acute bronchospasm or status asthmaticus.13
Clinical response. In the pivotal randomized, controlled trials (RCTs) evaluating dupilumab for AD, which included a Phase II, dose-ranging study, two 16-week monotherapy RCTs versus placebo, and a 52-week RCT that allowed for combination use with a topical CS, 1,472 subjects received dupilumab, with 739 treated for more than 52 weeks.13,20–22 Efficacy was substantiated by improvements in several assessment parameters versus placebo, both clinically and statistically, including positive changes in Investigator Global Assessment (IGA), marked reductions in Eczema Area Severity Index (EASI) scores, and significant decreases in pruritus, with clinical improvements sustained in the 52-week study without any loss of efficacy.13,20,21 Many patients reported a definite improvement in eczema and pruritus within the first few injections of dupilumab; however, onset of efficacy occurred later in some individuals (within 2 to 3 months after starting therapy). In patients currently undergoing other systemic therapies for severe AD (e.g., cyclosporin, methotrexate) who are starting dupilumab, researchers recommended that therapy be bridged without abrupt discontinuation of the patients’ previous therapy in order to avoid rebound exacerbation of AD while waiting for the clinical effects of dupilumab to manifest. Clinicians should then determine, on a case-by-case basis, the optimal approach to take when tapering patients off previous systemic therapy. 13,20–22
Safety. During the RCTs, no significant changes occurred in laboratory test results of the study subjects; thus, laboratory monitoring was not required by the FDA to be included in the approved product labeling for dupilumab.13 The most common AEs observed in the RCTs were injection site reactions and conjunctivitis
(10–16% in active arms vs. 2–9% in placebo arms); separately, hypersensitivity reactions (e.g., urticaria, serum sickness-type reactions) were observed in less than one percent of the active-treatment study subjects.13,20–22 Most cases of conjunctivitis did not require stopping dupilumab, and were treated with topical ophthalmic lubricants and anti-inflammatory agents, and appeared to resolve or markedly improve despite continued use of the drug; however, some cases were severe enough to require discontinuation of dupilumab therapy.13,20–23 New onset or worsening ocular symptoms warrant referral to an ophthalmologist for evaluation.13,23 Ocular abnormalities inherent to AD that are unrelated to dupilumab use, including conjunctivitis and blepharitis, are not uncommon; the cause of the conjunctivitis that occurs related to use of dupilumab is not fully understood.24
Dupilumab and concomitant systemic therapy. A complete review of publications on dupilumab are beyond the scope of this article; however, a few articles provide information on the effective and safe use of dupilumab in a subpopulation of patients previously treated with cyclosporin. In a 16-week RCT study of adults with AD (N=390), responses to dupilumab therapy in conjunction with a medium-potency topical CS were assessed in subjects with inadequate response to or intolerance of oral cyclosporin or those in whom it was clinically inadvisable to use cyclosporin.25 Researchers reported that, following individual clinical assessment, topical CS therapy was safely tapered and/or stopped in many patients. Results of the study indicate that dupilumab with concomitant topical CS therapy (when needed) might signi?cantly improve signs and symptoms of AD and patient quality of life, with no new safety signals noted by the investigators.25
Infection risk. Eight RCTs that assessed outcomes with dupilumab versus placebo in patients with AD were analyzed by meta-analysis, with an emphasis on the incidence of AEs.26 Regarding infection rate risks, dupilumab had a lower risk of skin infection (risk ratio: 0.54), compared with placebo, with similar to negligible risks noted for nasopharyngitis, urinary tract infection, upper respiratory tract infection, and herpes virus infection. These observations further support the concept that dupilumab is immunomodulatory through the mitigation of IL-4 and IL-13 signaling, without a significant increased risk of infection, which can occur with immunosuppressive agents. It is important to note that by counteracting certain immune dysfunctions that lead to epidermal barrier impairment and cascades of Th2-driven humoral and cutaneous inflammation, dupilumab might help to normalize certain immunologic processes that are dysregulated in AD. Continued research and pharmacovigilance will help elucidate the efficacy and safety factors associated with dupilumab in greater detail.
Monoclonal Antibody Interleukin-13 Inhibitors
Lebrikizumab. Lebrikizumab is an injectable monoclonal antibody that exhibits high-affinity binding to soluble IL-13, thus preventing pro-inflammatory signaling by inhibiting heterodimerization of the IL-13 alpha/IL-4 alpha complex.27 In a preliminary Phase II, dose/frequency-ranging 12-week RCT, 209 adults with moderate-to-severe AD were treated with one of three dosing regimens of active drug versus placebo. Following a two-week “run in” with medium-potency topical CS therapy (triamcinolone acetonide 0.1% applied twice daily with lower potency hydrocortisone 2.5% allowed for facial AD), patients were randomized to receive lebrikizumab 125mg every four weeks, a single dose of lebrikizumab (125mg or 250mg), or placebo. Primary efficacy endpoint was the percent of subjects achieving a 50-percent reduction in EASI at Week 12.27 Investigators reported that patients in the lebrikizumab 125mg every four weeks achieved markedly superior results compared with those in the single-dose lebrikizumab group and those in the control group. Superiority to placebo was also observed in other parameters (e.g., SCORAD-50, reduction in BSA). An increasing trajectory of favorable response based on the EASI-50 results was noted at the end of the study (12 weeks) in the group receiving lebrikizumab 125mg every four weeks. Overall, the safety profile was favorable in all study arms.27 Data from this early study in AD suggest that lebrikizumab for AD shows promise as a treatment for AD. Additional research is needed on whether further increases in the dose per injection or treatment frequency (i.e., interval between doses) and use of a loading dose improve lebrikisumab’s efficacy, without affecting safety, for initial and maintenance therapy for AD.
Tralokinumab. Tralokinumab, an IgG4 human monoclonal antibody that specifically neutralizes IL-13, was evaluated in a Phase IIb, dose-ranging, 12-week RCT of adult subjects (N=202) with moderate-to-severe AD.28,29 Patients were randomized to receive a 45mg (n=50), 150mg (n=51), or 300mg (n=51) subcutaneous injection of tralokinumab or placebo (n=50) every two weeks after a two-week “run in” with a mid-strength topical CS.29 Several efficacy parameters were assessed, with the coprimary endpoints being the change from baseline in total EASI score at Week 12 and the percent of IGA responders at Week 12 versus baseline (IGA score of clear/almost clear + at least a 2-grade reduction). Overall, AEs were generally similar among all study arms. Interestingly, six of the 204 subjects (2.9%) exhibited treatment-emergent conjunctivitis during the study (placebo, n= 2 [3.9%], tralokinumab 45mg, n =1 [2.0%], and tralokinumab 150mg, n=3 [5.9%]). Another important observation was that the serum level of dipeptidyl peptidase 4 might serve as a predictive biomarker for patients who could benefit from tralokinumab therapy.29 As with lebrikizumab, initial results with this agent for AD are encouraging and hopefully will be further supported by additional RCTs.
Summary Points
- AD is a chronic disorder that, from the outset, requires a management strategy designed to control flares, decrease recurrences, and reduce pruritus.
- Cases of AD that are not adequately controlled with conventional measures and topical therapy can usually be effectively treated with incorporation of systemic therapy. It is important to assess the benefits versus the risks of various options in each case.
- It is also important to avoid becoming dependent on the intermittent use of intramuscular and/or oral corticosteroid therapy to control flares. Incorporation of other treatment options that can more optimally control AD over time are recommended.
- With the use of oral immunosuppressive agents such as cyclosporin, methotrexate, mycophenolate mofetil, and azathioprine, baseline and periodic laboratory and clinical monitoring are very important. Each of these agents carries its own significant “side effects baggage” to keep track of with relevant testing.
- Dupilumab is a newer option shown to be effective in markedly decreasing signs and symptoms of AD. In the opinion of the author, based on the available data and experiences thus far, dupilumab therapy offers a more favorable overall safety profile in comparison with the available oral systemic agents.
- Lebrikizumab and tralokinumab, both inhibitors of IL-13, are currently under development and show promise based on preliminary studies in adult patients with moderate-to-severe AD.
References
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2—management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132.
- Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4—prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71(6):1218–1233.
- Del Rosso JQ, Harper J, Kircik L, et al. Consensus recommendations on adjunctive topical management of atopic dermatitis. J Drugs Dermatol. 2018;17(10):1070–1076.
- Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol. 2017;139(6):1723–1734.
- Thomson J, Wernham AGH, Williams HC. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a critical appraisal. Br J Dermatol. 2018;178(4):897–902.
- Prezzano JC, Beck LA. Long-term treatment of atopic dermatitis. Dermatol Clin. 2017;35(3):335–349.
- Admani S, Eichenfield LF. Atopic dermatitis. In: Lebwohl MG, Berth-Jones J, Heymann WR, Coulson I, Eds. Treatment of Skin Disease: Comprehensive Therapeutic Strategies. 4th edition. Philadelphia, PA: Elsevier-Saunders; 2014: 52–60.
- Akhavan A, Rudikoff D. Systemic agents for the treatment of atopic dermatitis. In: Rudikoff D, Cohen SR, Scheinfeld N (eds). Atopic Dermatitis and Eczematous Disorders. Boca Raton, FL: CRC Press/Taylor & Francis Group; 2014:187–199.
- Dhadwal G, Albrecht L, Gniadecki R, et al. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. section IV: treatment options for the management of atopic dermatitis. J Cutan Med Surg. 2018;22(1 Suppl):21S–29S.
- Mayba J, Gooderham M. Oral agents for atopic dermatitis: current and in development. In: Yamauchi PS (ed). Biologic and Systemic Agents in Dermatology. Cham, Switzerland: Springer International Publishing; 2018:319–330.
- Wolverton SE. Systemic corticosteroids. In: Wolverton SE (ed). Comprehensive Dermatologic Drug Therapy, 3rd edition. Philadelphia, PA: Elsevier-Saunders; 2013:143–168.
- Thomas K, Bath-Hextall F, Ravenscroft J, et al. Atopic eczema. In: Williams H. Bigby M, Diepgen T, et al (eds). Evidence-Based Dermatology, 2nd edition. Malden, MA: Blackwell Publishing; 2008: 128–163.
- Regeneron Pharmaceuticals and Sanofi-Genzyme. Dupixent (dupilumab) Injection, Full Prescribing Information. October 2018.
- Kusari A, Han AM, Schairer D, et al. Atopic dermatitis: new developments. Dermatol Clin. 2019;37(1):11–20.
- Patel N, Strowd LC. The future of atopic dermatitis treatment. Adv Exp Med Biol. 2017;1027:185–210.
- Edwards T, Patel NU, Blake A, et al. Insights into future therapeutics for atopic dermatitis. Expert Opin Pharmacother. 2018;19(3):265–278.
- Napolitano M, Marasca C, Fabbrocini G, et al. Adult atopic dermatitis: new and emerging therapies. Expert Rev Clin Pharmacol. 2018;11(9):867–878.
- Silverberg JI, Kantor R. The role of interleukins 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol Clinic. 2017;35(3):327–334.
- Snast I, Reiter O, Hodak E, et al. Are biologics efficacious in atopic dermatitis: a systematic review and meta-analysis. Am J Clin Dermatol. 2018;19(2):145–165.
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348.
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3S1):S28–S36.
- Hajar T, Hill E, Simpson E. Biologics for treatment of atopic dermatitis. In: Yamauchi PS (ed). Biologic and Systemic Agents in Dermatology. Cham, Switzerland: Springer International Publishing; 2018: 309–317.
- Treister AD, Kraff-Cooper C, Lio PA. Risk factors for dupilumab-associated conjunctivitis in patients with atopic dermatitis. JAMA Dermatol. 2018;154(10):1208–1211.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77(2):280–286.
- de Bruin-Weller M, Thaci D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol. 2018;178(5):
1083–1101. - Ou Z, Chen C, Chen A, et al. Adverse events of Dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018;54:303–310.
- Simpson E, Flohr C, Eichenfield LE, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with severe moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol. 2018;78(5):863–871.
- May RD, Monk PD, Cohen ES, et al. Preclinical development of CAT-354, an IL-13 neutralizing antibody, for the treatment of severe uncontrolled asthma. Br J Pharmacol. 2012;166(1):177–193.
- Wollenberg A, Howell MD, Guttman-Yassky E, et al. A Phase 2b dose-ranging efficacy and safety study of tralokinumab in adult patients with moderate to severe atopic dermatitis (AD). Poster presentation. Orlando, FL: American Academy of Dermatology Meeting. 3–7 Mar 2017.

