 by Nikoo Cheraghi, MD; Armand Cognetta, Jr., MD; and David Goldberg, MD
by Nikoo Cheraghi, MD; Armand Cognetta, Jr., MD; and David Goldberg, MD
Dr. Cheraghi is with the University of Minnesota, Dermatology, Minneapolis, Minnesota. Dr. Cognetta is with Florida State University College of Medicine, Dermatology, Tallahassee, Florida. Dr. Goldberg is with Skin Laser & Surgery Specialists of New York and New Jersey, New York, New York; Icahn School of Medicine at Mount Sinai, New York, New York; and University of Medicine and Dentistry of New Jersey-Rutgers Medical School, Newark, New Jersey.
J Clin Aesthet Dermatol. 2017;10(8):12–15
Funding: No funding was provided for this study.
Disclosures: Dr. Cognetta was an early advisor on the medical advisory board for Topex (now known as Sensus Healthcare) during the sale of the company, and organized an advisory board composed of a dermatologist, radiation oncologist, and medical physicist. He was given a stock option for his advisory role during the company’s formative stages in 2012. Drs. Cheraghi and Goldberg have no conflicts of interest relevant to the content of this article.
Keywords: Radiotherapy, brachytherapy, keloid, electron beam, superficial radiation, orthovoltage radiation
Abstract: Background. Radiotherapy has been used historically to treat a wide variety of dermatologic conditions, including nonmelanoma skin cancers, lymphomas, and inflammatory skin conditions. Recently, radiotherapy has been used increasingly as a valuable tool in the postsurgical treatment of large or recalcitrant keloids.
Objective. The objective of this review was to explore the use of radiation therapy as an adjuvant to surgically excised keloids.
Design. A PubMed search of all published English literature regarding the applications of radiotherapy for the treatment of keloids was performed using a combination of keywords including radiation, radiotherapy, brachytherapy, electron beam, superficial radiation, orthovoltage radiation, and keloid. The results were analyzed and collated.
Results. A comprehensive review of radiotherapy for the adjuvant treatment of keloids was outlined.
Conclusion. Radiotherapy appears useful as an adjuvant therapy to surgically excised keloids. Dermatologists should be well versed in radiotherapy to provide optimal care for patients with recalcitrant keloids.
Introduction
Radiotherapy is used primarily in dermatology to treat nonmelanoma skin cancers, mycosis fungoides, lymphomas, and keloids. Keloids can be difficult to treat, and treatment with excision often leads to recurrence. Adjuvantly, keloids are treated with postexcisional radiation therapy with excellent results and minimal complications or recurrences. As such, radiation therapy should be kept in a dermatologist’s repertoire of treatment options for difficult-to-treat keloids. In this review, the use of various types of radiation therapy for keloids is highlighted.Radiotherapy is used primarily in dermatology to treat nonmelanoma skin cancers, mycosis fungoides, lymphomas, and keloids. Keloids can be difficult to treat, and treatment with excision often leads to recurrence. Adjuvantly, keloids are treated with postexcisional radiation therapy with excellent results and minimal complications or recurrences. As such, radiation therapy should be kept in a dermatologist’s repertoire of treatment options for difficult-to-treat keloids. In this review, the use of various types of radiation therapy for keloids is highlighted.
Keloids
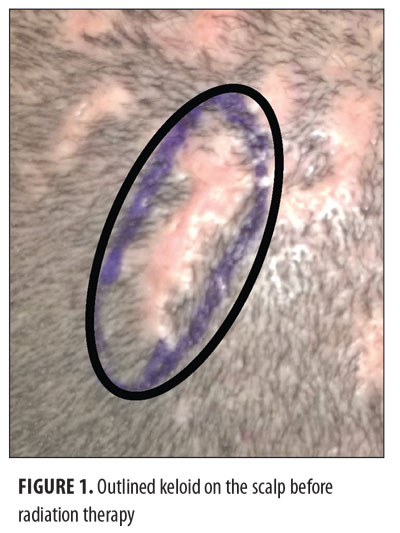
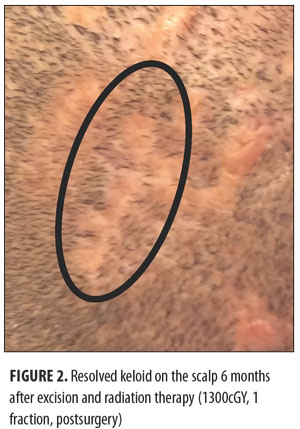
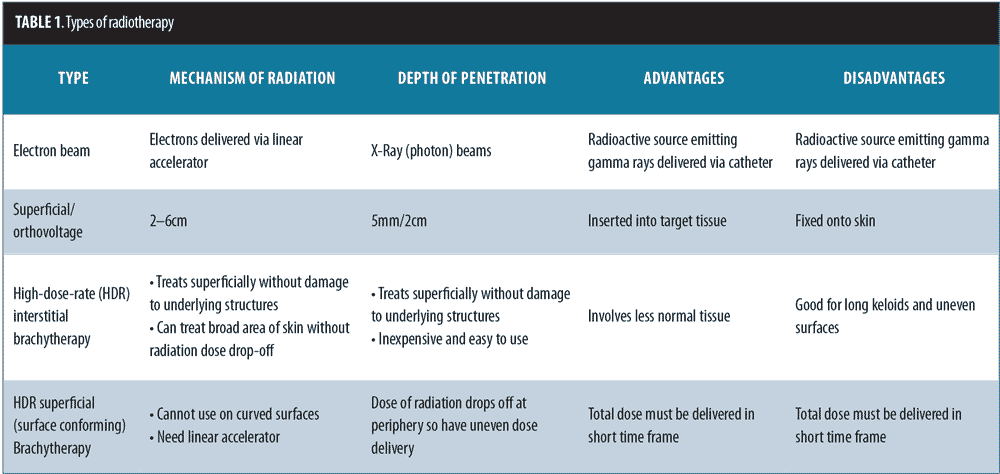
Keloids can be distressing for patients, as they can cause pain and itching and are also aesthetically displeasing. The current treatment options for keloids are limited. These include intralesional corticosteroids, silicone-based products, 5-fluorouracil, bleomycin, imiquimod, laser therapy, pressure treatments, surgical removal, cryotherapy, and radiotherapy.[1] The recurrence rate using surgical excision alone is 45 to 100 percent.[2] Surgical excision followed by radiotherapy is a helpful treatment option for large and more difficult-to-treat keloids that cannot otherwise be treated by or have failed more conservative measures.[1] The balance between proliferation and apoptosis is impaired in keloid fibroblasts, and because keloid fibroblasts are sensitive to x-ray irradiation, it is thought that x-ray radiation may prevent the recurrence of keloids by controlling fibroblast proliferation, arresting the cell cycle, and inducing premature cellular senescence.[3–5] The most commonly used radiotherapy modalities include electron beam irradiation, superficial and orthovoltage radiation therapy, and brachytherapy (Figures 1 and 2). Although there may be no significant difference in efficacy between the different radiotherapy modalities, each modality has its own advantages and drawbacks (Table 1).[6]
Electron Beam Radiotherapy
Electron beam radiotherapy (EBRT) uses a linear accelerator to deliver energy levels to a depth of 2 to 6cm and has the advantage of treating superficially without causing significant damage to deeper structures.[7,8] Postsurgical excision followed by EBRT has a low rate of recurrence depending on several factors, such as the treatment protocol that was used and keloid site.[9–13] Various treatment protocols have been described for the treatment of keloids with EBRT. The dose of radiotherapy used is dependent on the location of the keloid: keloids located in high-stretch tension areas including the chest, scapular region, and suprapubic region may need higher doses in contrast to low-tension areas such as the neck or earlobes.[9] For example, Ogawa et al suggested that the earlobes can be treated effectively with EBRT using a dose of 10Gy fractioned over two days, while the chest, scapular region, and suprapubic region should be treated with 20Gy fractioned over four days.[10] Other authors have suggested a higher dose of 15Gy for earlobe keloids and keloids on the cartilaginous part of the auricle with low recurrence rates.[12,14] A large study examining 834 keloids treated with excision followed by a total dose of 18Gy in two fractions had a relapse rate of 9.59 percent. This study showed that there were increased relapse rates in men, persons under 28 years in age, persons in whom radiation occurred more than 24 hours after surgery, keloids located in high-stretch tension sites, keloid size longer than 5cm, and if grafting was performed. Moreover, no radiation-induced malignancies were observed after a median follow-up of 40 months, and there was a low adverse effect profile of 9.38 percent, primarily composed of pigmentary changes.[15] These findings suggest that postsurgical radiation is more effective within 24 hours of excision.
Superficial and Orthovoltage Radiation Therapy
Superficial and orthovoltage radiotherapy have a depth of penetration of 5mm and 2cm, respectively. Superficial radiation therapy (SRT) has been used for treatment of keloids, as it entirely targets the skin, avoiding deeper tissues (Dmax=0). One study used 60kV (for chest keloids) or 100kV (for earlobe keloids) irradiation and a dose of 10Gy in a single fraction after excision of keloids; this study noted that the majority of keloids could be controlled by resection with immediate radiotherapy with a probability of relapse of nine percent at one year and 16 percent at five years.[16] Another study including 194 keloids treated with excision followed by postoperative superficial radiation therapy (55kV or 100kV depending on site and total of 16Gy or 40Gy dose) showed 91-percent reduction in itching and 96-percent reduction in pain. With total radiation doses greater than 21Gy, there was a greater risk of pigmentary changes.[17] Moreover, SRT (dose of 12Gy divided into 3 fractions) was shown to be superior in a randomized trial when compared to multiple sessions of cryotherapy followed by intralesional triamcinolone. In fact, the cryotherapy/intralesional triamcinolone group experienced more side effects including ulceration, necrosis, and telangiectasia as well as a more prolonged course of treatment, higher recurrence rate, and less satisfaction.[18]
In a large study by Speranza et al, 234 keloids were treated 24-hours postexcision with orthovoltage radiation therapy using a dose of 15Gy divided into three daily fractions. With this regimen, 60 percent of patients reported a satisfaction level of 8 or higher on a 10-point scale. Twenty-seven percent of patients developed telangiectasias as a late adverse event; this was the greatest predictor of dissatisfaction.[19]
Brachytherapy
Brachytherapy has also been very effective for management of keloids postexcision. It involves placing a radioactive source (commonly iridium-192 or cobalt) into or onto the target tissue.[20] High-dose-rate (HDR) brachytherapy can be performed in an outpatient setting and delivers more than 12Gy per hour.[21] HDR interstitial brachytherapy, during which a catheter is inserted into the surgical site with delivery of radiation through the catheter, was shown to have excellent results with a 3.4-percent recurrence rate at seven years using a dose of 12Gy divided into four fractions and delivered within 48 hours.[22] Another study of 67 keloids treated with postexcision HDR interstitial brachytherapy using iridium-192 and a dose of 12Gy in two fractions showed a 3.1-percent recurrence rate.[23] Although HDR interstitial brachytherapy has an advantage over external radiotherapy (including superficial/ orthovoltage radiation therapy and electron beam) of involving less normal tissue, the administration of this form of radiation therapy may be cumbersome for the physician and patient, as many treatment sessions must be administered within a short period of time. An even more cumbersome form of brachytherapy is low-dose-rate brachytherapy during which a patient is confined in a lead-coated chamber for up to 64 hours while the brachytherapy is being administered. Although this has been used effectively for the treatment of keloids, it is now rarely used due to its inconvenience.[24] An easier form of brachytherapy is HDR superficial or surface-conforming brachytherapy during which the catheter is fixed externally onto the skin. This form of brachytherapy is helpful for long keloids. Kuribayashi et al[25] studied 36 keloids with a median length of 8.5cm treated with excision followed by HDR superficial brachytherapy using an iridium-192 source. The applicator was fixed to the skin with a spacer, and the keloids on high-tension sites were treated with 20Gy in four daily fractions, while keloids in other areas were treated with 15Gy in three daily fractions; there was a 9.7-percent recurrence at 12 months. HDR superficial brachytherapy can be advantageous over electron beam radiotherapy for keloids with uneven surfaces, as the applicator can be molded to the surface of the skin so that there are no variations in the distance between the skin surface and radiation source.[25]
Complications
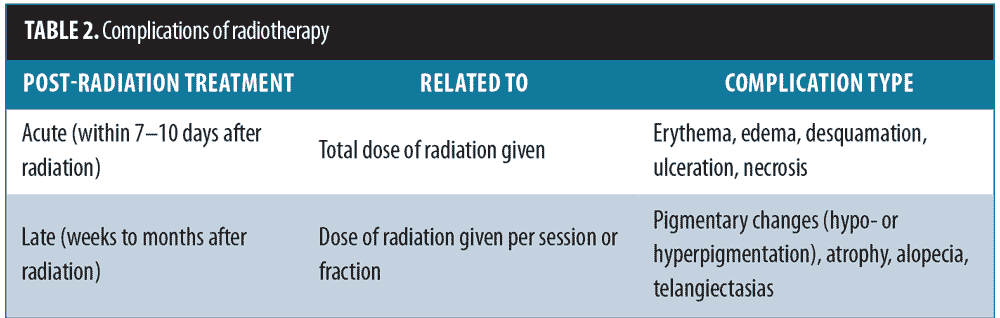
The acute skin side effects of temporary erythema are seen in almost all patients during the first 7 to 10 days after treatment and are related to the total dose of radiation that is given. The late side effects are observed weeks later and include pigmentary changes that are generally temporary and mild. The late side effects are related to the dose given per session or fraction (Table 2).[9,10,19,26] In order to reduce the chances of these side effects, it is important to use an emollient and a steroid ointment after radiation, reduce the single dose of irradiation per fraction while keeping the total dose unchanged, or lengthen the irradiation interval.[10] As patients with darker skin types are more prone to developing keloids, it is important to note that pigmentary changes were found most commonly in Types 5 and 6 African American individuals.[23] Recurrence of keloids treated with postsurgical radiation was found to be significantly increased in subjects who developed the keloid after infection and those with a family history of keloids.[6]
As keloids are a benign skin condition, there is a question of whether radiotherapy is an appropriate modality for treatment. A review of the literature by Ogawa et al in 2009[26] yielded a total of five cases of carcinogenesis associated with radiation therapy for keloids: fibrosarcoma, basal cell carcinoma, thyroid carcinoma, and breast carcinoma. In each of these cases, it is unclear whether an appropriate dose of radiation was used or if there was adequate protection of surrounding tissues. As such, the authors concluded that radiation is useful if sensitive tissues, such as the mammary gland or thyroid gland, are effectively protected.[26] Moreover, a consensus statement from the European Society for Therapeutic Radiology and Oncology noted that keloids are an acceptable indication for radiotherapy and placed no age restrictions provided that alternative therapies were ineffective.[27]
Conclusion
Various radiotherapy modalities including electron beam radiotherapy, superficial and orthovoltage radiotherapy, and brachytherapy are being used after excision for the treatment of keloids. Frequently, postsurgical radiation may be a preferable option, as there is a low risk of recurrence and complications. Dermatologists should be aware of the option of radiation therapy for difficult-to-treat keloids so that a true informed consent can be reached with the patient.
References
- Gold MH, Berman B, Clementoni MT, et al. Updated international clinical recommendations on scar management: part 1–evaluating the evidence. Dermatol Surg. 2014;40:817–824.
- Mustoe TA, Cooter RD, Gold MH, et al. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–571.
- Luo S, Benathan M, Raffoul W, et al. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg. 2001;107:87–96.
- Lee KS, Jung JB, Ro YJ, et al. Effects of x-irradiation on survival and extracellular matrix gene expression of cultured keloid fibroblasts. J Dermatol Sci. 1994;8:33–37.
- Ji J, Tian Y, Zhu YQ, et al. Ionizing irradiation inhibits keloid fibroblast cell proliferation and induces premature cellular senescence. J Dermatol. 2015;42:56–63.
- Klumpar DI, Murray JC, Anscher M. Keloids treated with excision followed by radiation therapy. J Am Acad Dermatol. 1994;31:225–231.
- Cognetta AB Jr, Mendenhall WM. Radiation Therapy for Skin Cancer. 1st ed. New York, NY: Springer; 2013.
- Miller RA, Spittle MF. Electron beam therapy for difficult cutaneous basal and squamous cell carcinoma. Br J Dermatol. 1982;106:429–435.
- Ogawa R, Mitsuhashi K, Hyakusoku H, Miyashita T. Postoperative electron-beam irradiation therapy for keloids and hypertrophic scars: retrospective study of 147 cases followed for more than 18 months. Plast Reconstr Surg. 2003;111:547–553.
- Ogawa R, Miyashita T, Hyakusoku H, et al. Postoperative radiation protocol for keloids and hypertrophic scars: statistical analysis of 370 sites followed for over 18 months. Ann Plast Surg. 2007;59:688–691.
- Song C, Wu HG, Chang H, et al. Adjuvant single-fraction radiotherapy is safe and effective for intractable keloids. J Radiat Res. 2014;55:912–916.
- Ogawa R, Akaishi S, Dohi T, et al. Analysis of the surgical treatments of 63 keloids on the cartilaginous part of the auricle: effectiveness of the core excision method. Plast Reconstr Surg. 2015;135:868–875.
- Wang LZ, Ding JP, Yang MY, Chen B. Forty-five cases of chest keloids treated with subcutaneous super-tension-reduction suture combined with postoperative electron-beam irradiation. Dermatol Surg. 2014;40:1378–1384.
- Kim K, Son D, Kim J. Radiation therapy following total keloidectomy: a retrospective study over 11 years. Arch Plast Surg. 2015;42:588–595.
- Shen J, Lian X, Sun Y, et al. Hypofractionated electron-beam radiation therapy for keloids: retrospective study of 568 cases with 834 lesions. J Radiat Res. 2015;56:811–817.
- Ragoowansi R, Cornes PG, Moss AL, Glees JP. Treatment of keloids by surgical excision and immediate postoperative single-fraction radiotherapy. Plast Reconstr Surg. 2003;111:1853–1859.
- Sakamoto T, Oya N, Shibuya K, et al. Dose-response relationship and dose optimization in radiotherapy of postoperative keloids. Radiother Oncol. 2009;91:271–276.
- Emad M, Omidvari S, Dastgheib L, et al. Surgical excision and immediate postoperative radiotherapy versus cryotherapy and intralesional steroids in the management of keloids: a prospective clinical trial. Med Princ Pract. 2010;19:402–405.
- Speranza G, Sultanem K, Muanza T. Descriptive study of patients receiving excision and radiotherapy for keloids. Int J Radiat Oncol Biol Phys. 2008;71:1465–1469.
- Alam M, Nanda S, Mittal BB, et al. The use of brachytherapy in the treatment of nonmelanoma skin cancer: a review. J Am Acad Dermatol. 2011;65:377–388.
- Williamson JF, Brenner DJ. Physics and biology of brachytherapy. In: Halperin EC, Perez CA, Brady LW, eds. Perez and Brady’s Principles and Practice of Radiation Oncology. 5th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008:423–475.
- Guix B, Henríquez I, Andrés A, et al. Treatment of keloids by high-dose-rate brachytherapy: a seven-year study. Int J Radiat Oncol Biol Phys. 2001;50:167–172.
- van Leeuwen MC, Stokmans SC, Bulstra AE, et al. High-dose-rate brachytherapy for the treatment of recalcitrant keloids: a unique, effective treatment protocol. Plast Reconstr Surg. 2014;134:527–534.
- Arnault JP, Peiffert D, Latarche C, et al. Keloids treated with postoperative Iridium 192* brachytherapy: a retrospective study. J Eur Acad Dermatol Venereol. 2009;23:807–813.
- Kuribayashi S, Miyashita T, Ozawa Y, et al. Post-keloidectomy irradiation using high-dose-rate superficial brachytherapy. J Radiat Res. 2011;52:365–368.
- Ogawa R, Yoshitatsu S, Yoshida K, Miyashita T. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124:1196–1201.
- Leer JW, van Houtte P, Seegenschmiedt H. Radiotherapy of non-malignant disorders: where do we stand? Radiother Oncol. 2007;83:175–177.

