J Clin Aesthet Dermatol. 2025;18(10):25–32.
by Glynis Ablon, MD, FAAD
Dr. Ablon is with the Ablon Skin Institute and Research Center in Manhattan Beach, California.
FUNDING: Research funding was provided by GlobalMed Technologies.
DISCLOSURES: The author reports no conflicts of interest relevant to the content of this article.
Abstract: Objective: Acne vulgaris is a major health and social concern for many adolescents and adults. The goal of this study was to further assess the efficacy and safety of a United States Food and Drug Administration cleared light-emitting diode (LED) therapy (Omnilux Clear, GlobalMed Technologies) for treating adolescents and adults with mild-to-moderate facial acne. The device is a wearable facial mask designed for home use that simultaneously emits light in red (633 nm) and blue (415 nm) wavelengths. Methods: The study enrolled male (n=15) and female (n=15) patients aged 14 to 45 years old. Patients were required to have an Investigators Global Assessment (IGA) score of 2 (mild) or 3 (moderate). Patients applied the treatment at home 4 times weekly, never more than once daily, and allowed 24 hours between treatments. The primary efficacy endpoints were the change from baseline in inflammatory and noninflammatory lesion counts, and the proportion of patients achieving a ≥1-grade reduction in IGA scores from baseline. Other assessments included quality of life and tolerability questionnaires. Results: After 7 weeks, there were significant reductions in inflammatory and noninflammatory lesion counts (for each, p<0.0001) and most patients (86%) achieved ≥1-grade reduction in IGA scores, meeting study success criteria. The few reported adverse events were mild and transient. Limitations: The primary limitation of this study was the open-label study design. Conclusion: These results provide strong support for this wearable LED device for the safe and effective home treatment of adolescents and adults with mild-to-moderate acne. Keywords: Acne vulgaris, light-emitting diode therapy, phototherapy, LED mask, home therapy
Introduction
Acne vulgaris is a major health and social concern for many individuals in the United States. The Global Burden of Disease Study reported that acne vulgaris had an estimated prevalence of 9.38% across all ages.1 Based on a search of health insurance databases, more than 5.1 million Americans sought medical treatment for acne in 2013.2 As it might be expected, 33.7% of these patients were adolescents; however, 53.8% were aged 18 to 44 years old and 9.6% were aged 45 to 64 years old. There is evidence that the global prevalence of acne is increasing among both adolescents and adults3 with a significantly greater number of women being affected.4 Acne is often stigmatizing5 and can have a significant effect on the quality of life and mental health of affected individuals, although the emotional impact may vary among age groups.6-8
The pathogenesis of acne is complex and involves 4 primary features: increased sebum gland activity; increased bacterial proliferation, specifically Cutibacterium acnes (C. acnes); abnormal hyperkeratinization and obstruction of sebaceous follicles; and the release of mediators of inflammation.9 Consequently, numerous therapies have been advanced for treating the various aspects of the disorder. Most treatments are pharmacological, with varying degrees of efficacy,10-12 and all with advantages and disadvantages.13
In addition, numerous nonpharmacological treatments, such as phototherapy, have been developed for treating acne. Phototherapy uses nonthermal light in visible wavelengths to achieve a therapeutic effect.14 Different wavelengths of light are used depending on the target chromophore. The availability of light-emitting diodes (LEDs) increased their clinical application for a variety of medical and cosmetic uses. Two wavelengths of light that have demonstrated therapeutic benefit for treating acne are blue (415 nm)15-18 and red (633 nm).19-21 Blue light generates nitric oxide and reactive oxygen species, which target bacterial porphyrins with bactericidal effects on C. acnes,22 thereby reducing inflammation.14 Red light penetrates deeper to target sebaceous glands14 and downregulates lipid production,23 producing anti‑inflammatory effects by altering cytokine release from macrophages,22 possibly destroying C. acnes bacteria.21 Several studies have demonstrated the safety and efficacy of alternating red and blue light20,21 or simultaneous administration for treating acne.24
A United States Food and Drug Administration (FDA)-cleared LED mask has been developed for the home treatment of individuals with acne-prone skin (Omnilux Clear, GlobalMed Technologies). The device combines blue (415 nm) and red (633 nm) light to safely deliver maximally beneficial results. The objective of this open-label clinical research study was to further demonstrate the efficacy and safety of this LED mask as a stand-alone home treatment for adults and adolescents with mild-to-moderate facial acne.
Methods
Study population. Participants in this study were healthy male and female patients aged 14 years or older with any Fitzpatrick skin type who were seeking treatment for mild-to-moderate facial acne vulgaris. Specifically, patients were required to have more than 10 but less than 50 inflammatory facial acne lesions (papules, pustules, and nodules); more than 10 but less than 100 noninflammatory facial acne lesions (open and closed comedones); and not more than one facial nodule at enrollment. Patients were also required to have an Investigator’s Global Assessment (IGA) score of 2 (mild) or 3 (moderate) at enrollment (Table 1). Female patients of childbearing potential or who were premenses agreed to practice effective contraception for the duration of the study, and all patients expressed their willingness to comply with study requirements, such as attending scheduled study visits, and using only sponsor-provided skincare products.
Reasons for exclusion from study participation included the presence of any facial dermatological conditions that could interfere with clinical evaluations, such as acne conglobata, acne fulminans, or significant rosacea; the presence of an underlying disease or facial dermatological condition requiring the use of a topical or systemic therapy that could interfere with lesion counts; a history of lupus erythematous, photosensitive eczema, albinism, photosensitive disorders, or light-induced seizures; current use of any photosensitizing medication or herbal supplements; a history of herpes simplex in the planned treatment area or immunosuppressive disease; a history of hypersensitivity to facial makeup, soap, washes, or sunscreens; current active infections, broken skin, extremely dry skin, or open lesions in the planned treatment area; patients who have not undergone the following washout periods for the following topical facial treatments: astringents and abrasives, nonallowed moisturizers, sunscreens, or antimicrobial soaps (one week); topical antibiotics or other topical anti-acne drugs, chemical peels, microdermabrasion, or photodynamic therapy (two weeks); topical anti-inflammatory agents, corticosteroids, retinoids, or laser therapy (four weeks); or the following washout periods for the systemic medications: oral corticosteroids, antibiotics, or other systemic acne treatments (four weeks) or retinoids (six months); refusal to abstain from sunbathing or use of tanning booths; use of an investigational drug or device within 30 days of enrollment, or participation in a concurrent research study; pregnancy, nursing, or planned pregnancy during the course of the study.
Investigational device. The device used in this study was an FDA-cleared, wearable LED device designed for home use. It is a flexible silicone mask that contains LEDs that produce a cool light in the red (633 nm) and blue (415 nm) wavelengths, which are effective for treating facial acne vulgaris. The device is worn on the face and is held in place by adjustable Velcro straps.
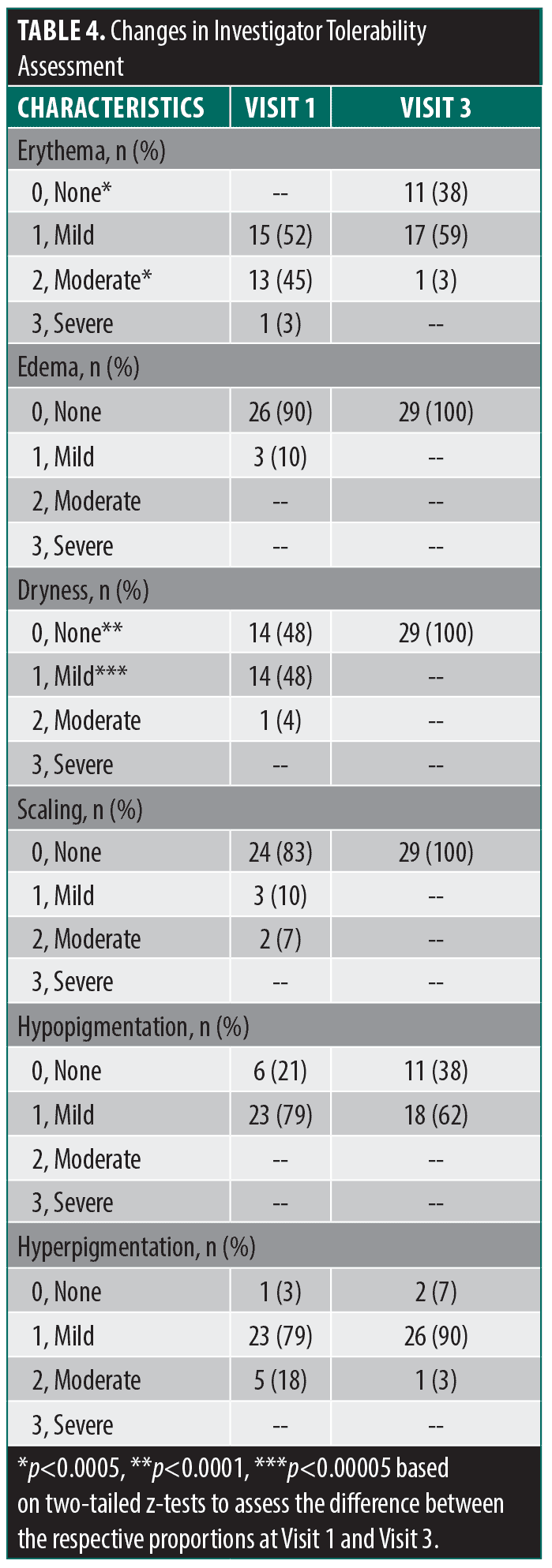
Study assessments. Investigator tolerability assessment.25 Using this assessment, the investigator determined how well patients tolerated the LED treatment with an emphasis on erythema, edema, dryness, scaling, hypopigmentation and hyperpigmentation on the global facial area using a 4-point scale from 0 (none) to 3 (severe). This assessment was administered at Visit 1 (Day 0), Visit 2 (Day 21), and Visit 3 (Day 49).
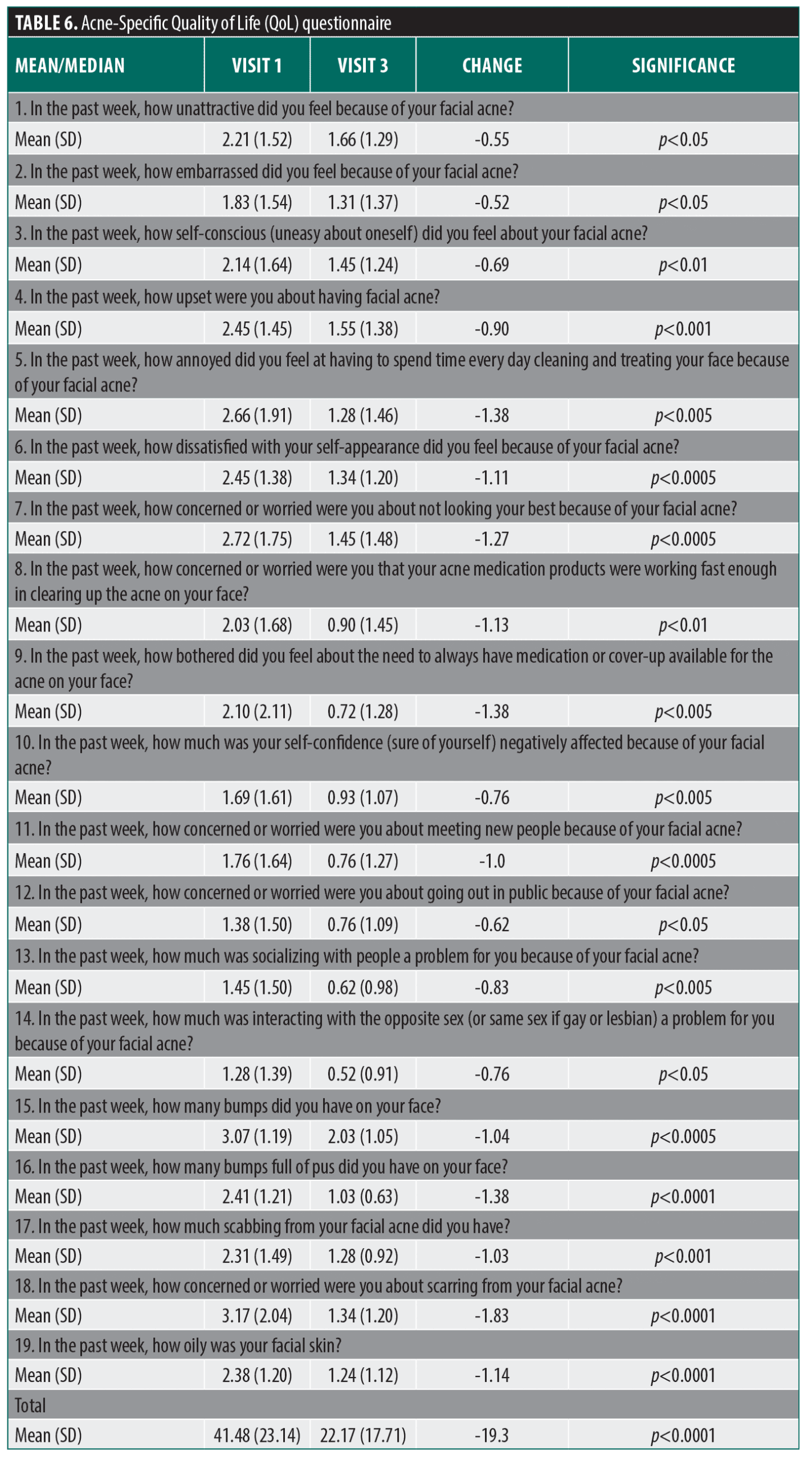
Acne-specific quality of life questionnaire (Acne QoL). This health-related quality of life questionnaire (QoL) was specifically developed for use in clinical trials to assess the impact of therapy on the quality of life among patients with facial acne.26 The self-administrated Acne QoL consists of 19 questions that can be answered without assistance or interpretation from study staff. The questionnaire was administered at Visit 1 and Visit 3.
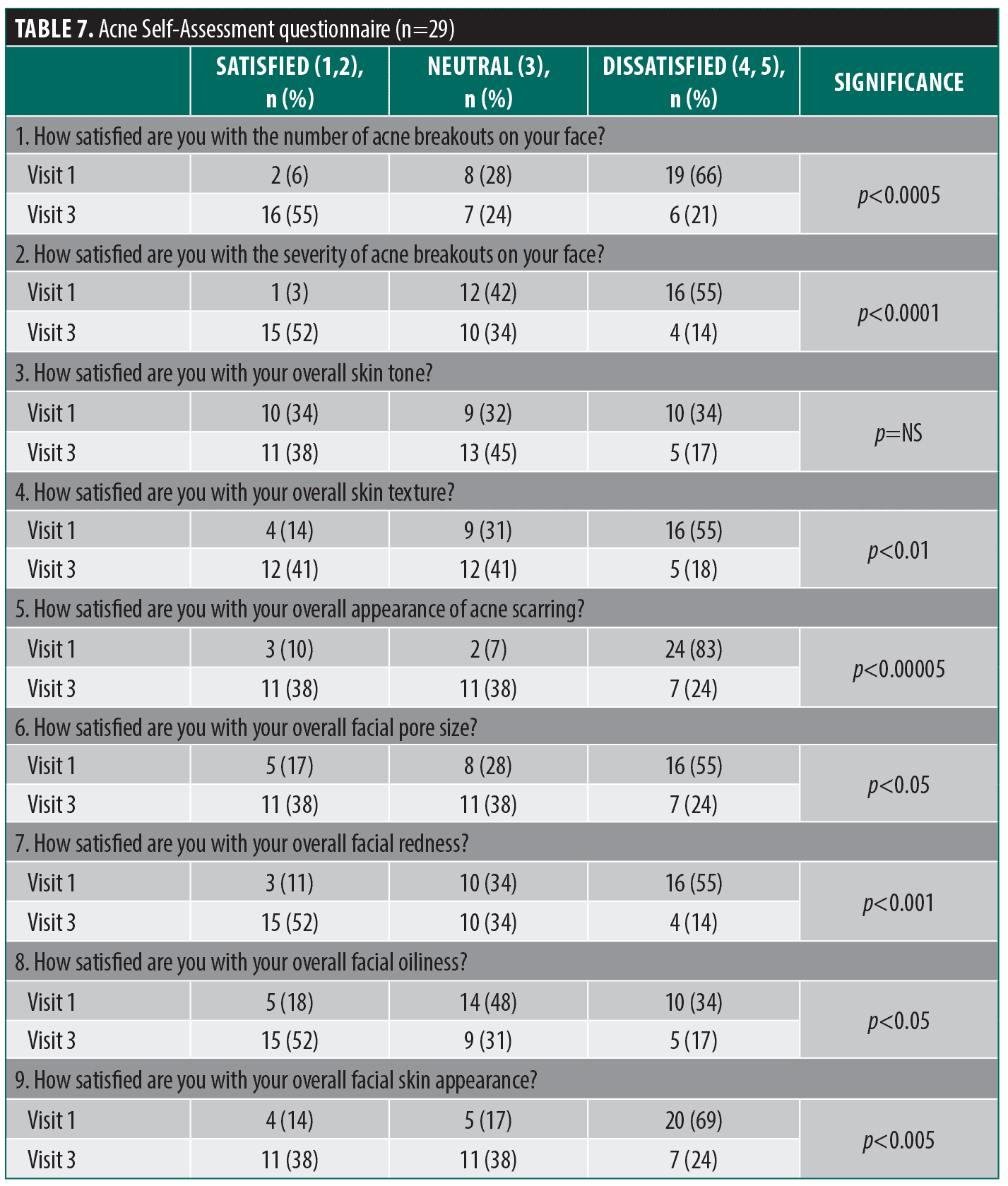
Acne self-assessment questionnaire. For this self-administered questionnaire, patients were asked a series of questions related to their facial acne and overall facial skin appearance. This questionnaire was administered at Visit 1 and Visit 3.
Patient tolerability self-assessment. This self-administered questionnaire assessed how well patients tolerated the LED treatment with respect to itching, burning and stinging. This questionnaire was administered at Visit 1, Visit 2, and Visit 3.
Study procedures. Visit 1 (Day 0). During the baseline visit, patient medical history and demographic information were obtained, a brief physical examination was performed, and a urine pregnancy test was done, if indicated. The investigator performed a baseline IGA, lesion counts for inflammatory and noninflammatory lesions, the Quantitative Global Acne Scarring Grading (QGAS), and tolerability assessment. Patients completed the tolerability self-assessment, the Acne QoL questionnaire, and the Acne Self-Assessment questionnaire. Digital imaging was performed. Patients received their first full-face LED treatment in the clinic.
The study coordinator dispensed the LED device to each patient and provided instructions for use. Patients were to apply the treatment for 10 minutes four times weekly, never more than once daily, and allow 24 hours between treatments. Patients were provided with written instructions for the home use of the LED device and use of the personal diary. Patients received a hydrating facial cleanser, a moisturizing lotion, and a moisturizing facial lotion with SPF 30 for home use (CeraVe Hydrating Facial Cleanser, CeraVe Moisturizing Lotion, and CeraVe Facial Moisturizing Lotion with SPF 30; L’Oréal). Patients were instructed to cleanse their face with the hydrating facial cleanser each morning followed by an application of the facial moisturizing lotion with SPF 30. Every evening, patients were to cleanse their face with the hydrating facial cleanser followed by a facial application of the moisturizing lotion.
Visit 2 (Day 21 ± 3 days). Patients were queried about any changes in their health and any potential adverse events (AEs). The investigator repeated the IGA, lesion counts, the QGAS, and tolerability assessment. Patients completed the tolerability self-assessment, and digital imaging was repeated. Diaries were reviewed for compliance, instructions for at-home use of the LED device were reviewed, and patients received an additional supply of hydrating facial cleanser, moisturizing lotion, and moisturizing facial lotion with SPF 30.
Visit 3 (Day 49 ± 3 days). Patients were again queried about any changes in their health and potential AEs. The investigator repeated the IGA, lesion counts, the QGAS, and tolerability assessment. Patients completed the tolerability self-assessment, the acne QoL questionnaire, the self-assessment questionnaire, and the patient satisfaction questionnaire. Personal diaries were checked for treatment compliance, and digital imaging was repeated. The LED devices were returned to the clinic when patients exited the study.
Study endpoints. The primary efficacy endpoints were the change from baseline in inflammatory lesion counts, change from baseline in noninflammatory lesion counts, and the proportion of patients achieving a ≥1-grade reduction from baseline IGA Scale scores at Visit 3. Secondary efficacy endpoints were the change from baseline in investigator acne scarring assessment scores on the QGAS, change from baseline investigator tolerability assessment (erythema, edema, dryness, scaling, hypopigmentation, and hyperpigmentation), change from baseline in acne QoL questionnaire scores, change from baseline on the acne self-assessment questionnaire, and an analysis of the patient satisfaction questionnaire completed at Visit 3.
Statistical analysis. The sample size was calculated according to the intended analysis of the study primary efficacy endpoints using Student’s t-test for one sample with a right-tailed significance level (α) of 0.5 and power of 80%, with effect size to be detected according to mean difference of change with medium effect size of 0.5 and standard deviation of 1.0. The results were a required sample of 27 patients to complete the study for the results to be considered generalizable to the broader patient population. Assuming a potential 10% attrition, the starting sample size was determined to be 30 patients.
Primary endpoints. Primary study analysis of efficacy was performed on the per protocol population, defined as the patients who completed all 24 treatment sessions and all three study visits. The safety analysis population were all patients who received at least one treatment with the LED device. The change from mean baseline inflammatory and noninflammatory lesion counts at Visit 3 (primary endpoints) were compared using a Student’s t-test for correlated samples. Statistical significance was established at p<0.05. The change in proportion of patients who achieved a ≥1-grade reduction from baseline IGA scores at Visit 3 was calculated as the number of patients who achieved this goal divided by the total sample size. A calculated percentage of 50% or greater was established as supportive of a positive treatment effect of the LED device.
Secondary endpoints. The change from baseline in investigator tolerability assessments at Visit 3 (secondary endpoint) was assessed according to its categorical response scale. The change in assessments was analyzed using Fischer’s exact test or binomial tests for two proportions with p<0.5. Significantly greater improved grades support the safety of the LED device. The change from baseline in acne QoL scores at Visit 3 was presented descriptively (n, %) and categorized as improved, no change, or worsened. A significant difference in change was analyzed using Fischer’s exact test or binomial tests for two proportions with p<0.05. The change from baseline acne self-assessment questionnaire scores at Visit 3 were presented descriptively (n, %) and the change in the frequency of individual responses by category were categorized as satisfied, neutral, or dissatisfied. The significance in change between response categories across assessments was analyzed using Fischer’s exact test or binomial tests for two proportions with p<0.05. The patient satisfaction questionnaire ratings at Visit 3 for each individual question were presented descriptively (n, %) and were categorized as satisfied, neutral, or dissatisfied. As this outcome was a single assessment point, no comparison of change was possible.
Safety analysis. Reported AEs were categorized by type, severity, duration, and relatedness to study treatment. Patient tolerability self-assessment questionnaire responses were further evaluated for trends.
Ethics. This study protocol and related materials were approved by a commercial Institutional Review Board (Allendale IRB). All patients provided written informed consent prior to participating in any study-related activities. Patients also signed IRB-approved HIPAA and photography release forms. A parent or guardian provided signed consent for patients under 18 years of age. This study followed all applicable guidelines for the protection of human patients for research as outlined in the United States FDA 21 CFR Part 50, in accordance with the accepted standards for Good Clinical Practices, and the standard practices of Ablon Skin Institute and Research Center. Patients were permitted to withdraw from the study at any time and for any reason, and patients could be removed from the study for noncompliance or a medical condition that may compromise safety of the patient or jeopardize the objectives of the study.
Results
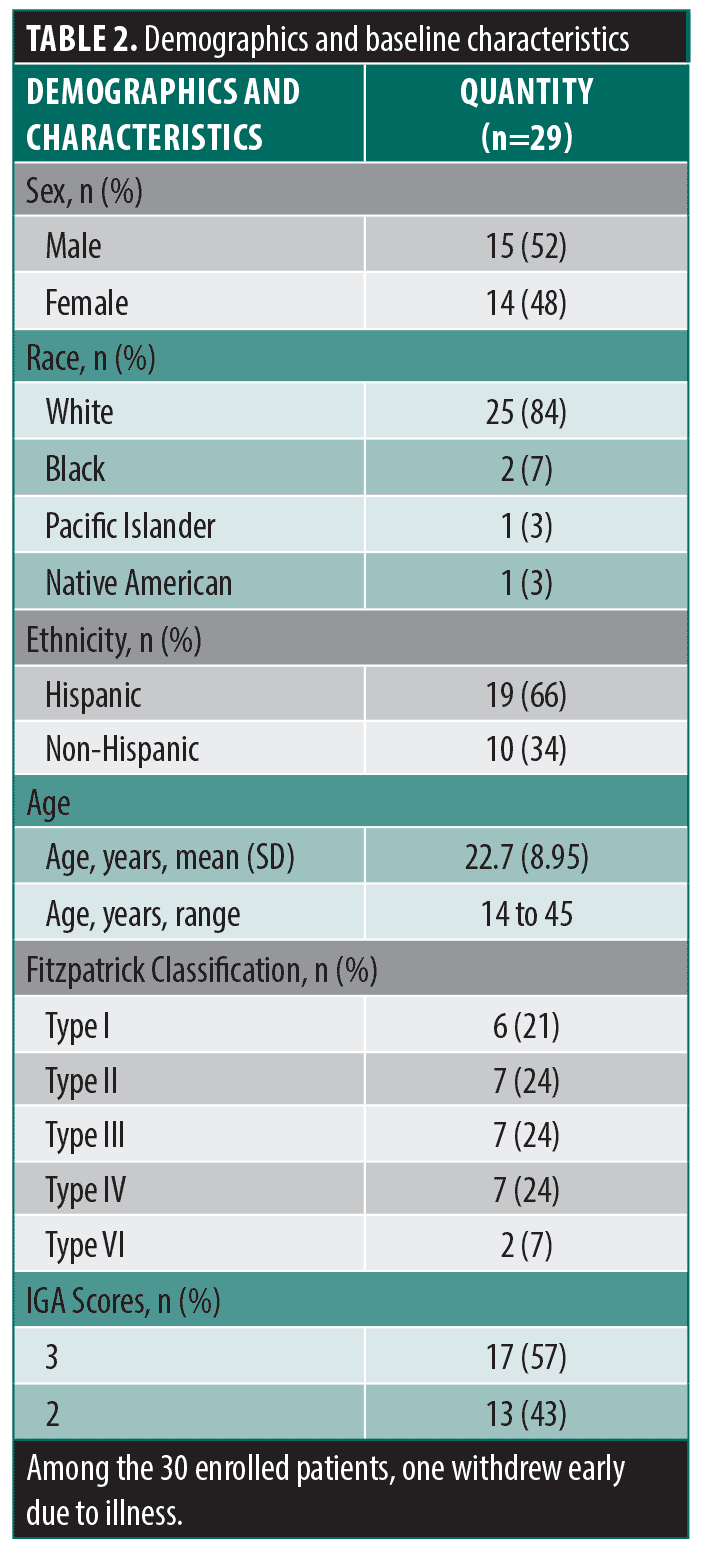
Among the enrolled patients, one was withdrawn early due to illness, and the remaining 29 completed the full course of study treatments and attended each study visit. Demographics and baseline characteristics are summarized in Table 2. Patients reported their acne began at a mean age of 13.5 (3.2) years (range, 9 to 24 years), which had persisted for a mean (SD) duration of 9.2 (9.1) years (range, 1 to 34 years).
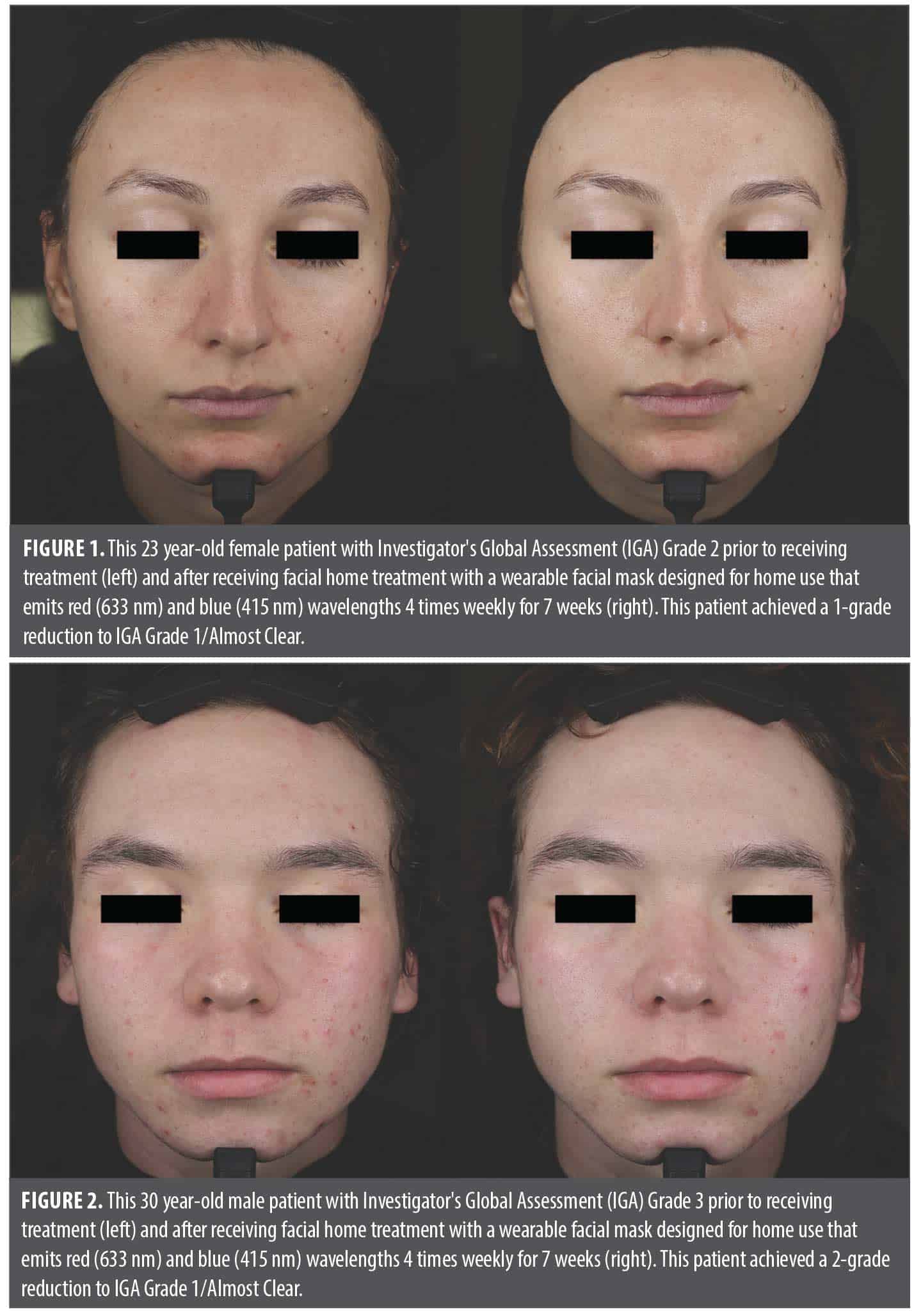
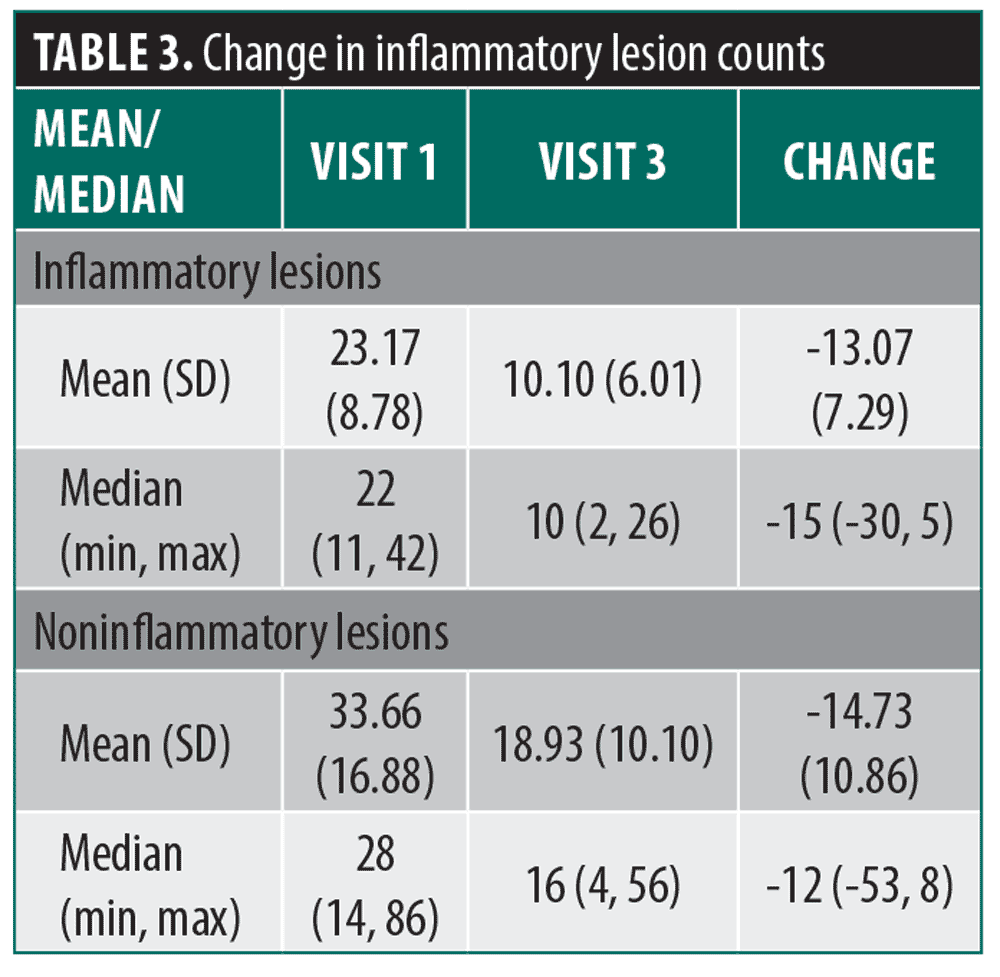
Primary endpoints. The inflammatory lesion count decreased by a mean (SD) of 13.07 (7.29) lesions from Visit 1 (Day 0) to Visit 3 (Day 49) (p<0.0001) and the noninflammatory lesion count decreased by a mean of 14.73 (10.86) lesions from Visit 1 to Visit 3 (p<0.0001) (Table 3). Most patients (n=25, 86%) achieved a ≥1-grade reduction (improvement) from baseline IGA scores at Visit 3 and several (n=4, 14%) achieved a 2-grade reduction. The proportion of patients achieving a 1-grade reduction or greater in baseline IGA scores exceeded the predetermined minimum success rate of 50% by 36%, supporting a positive treatment effect of the LED device. Pre- and post-treatment images of two representative patients are shown in Figure 1 and Figure 2.
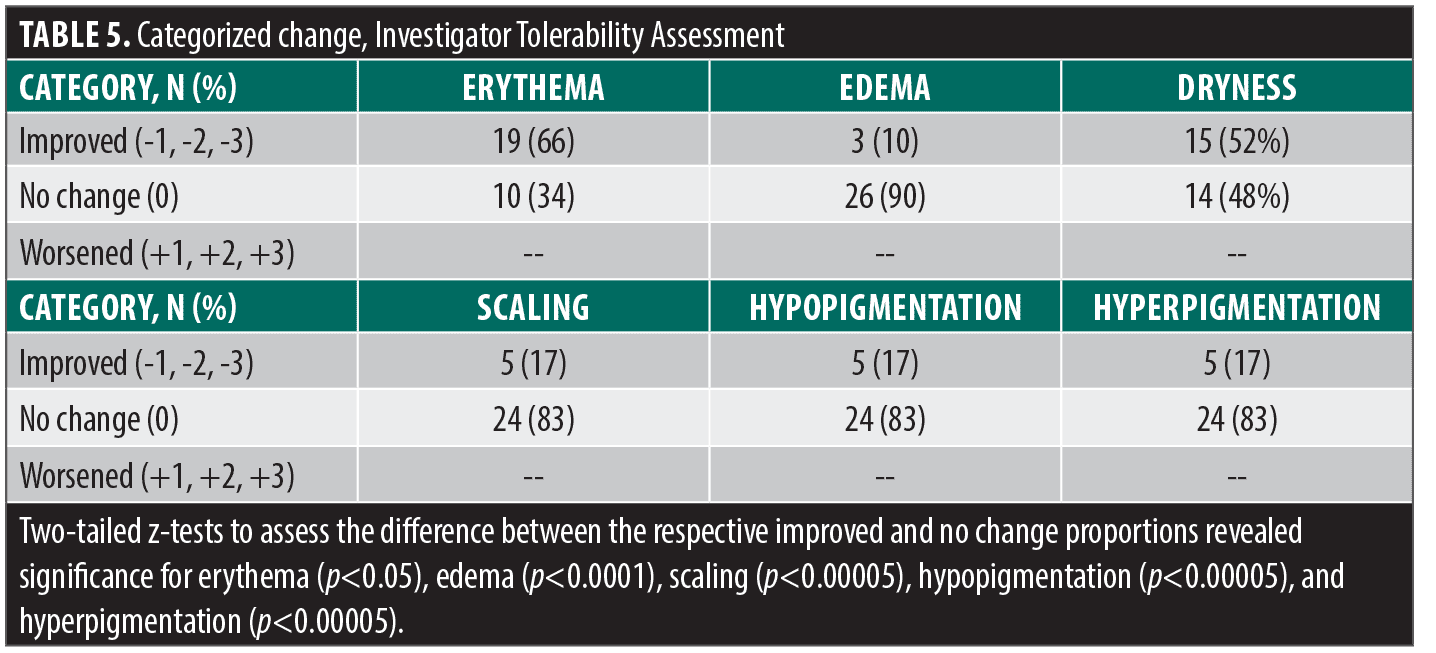
Secondary endpoints. Investigator tolerability assessments recorded local signs of erythema, edema, dryness, scaling, hypopigmentation and hyperpigmentation at Visit 1 and Visit 3 (Table 4). There were significant changes for erythema (p<0.00001) and dryness (p<0.00001). The change in categorized baseline investigator tolerability assessment grades are summarized in Table 5. There were no instances of worsening of any tolerability assessment grade.
There was a significant decrease (improvement) in the mean baseline response for each of the 19 items on the acne QoL questionnaire at Visit 3 and also for the total score across all items (Table 6). It is noteworthy that for nine questionnaire items, the mean Visit 3 response was 0 or “not at all” indicating for these items, patients were not impacted by their acne in those aspects of their daily life or self-image.
There were significant improvements in eight of the nine questions on the acne self-assessment questionnaire (Table 7) and substantial improvement in the number of “dissatisfied” responses to the remaining question (34% vs. 17%) at Visit 3; however, those responses did not achieve significance. When the responses were analyzed categorically (improvement, no change, or worsening), there was a significant improvement for each item except one which showed improvement in 38% of patients but did not achieve significance.
Safety endpoints. There were no reported local or systemic AEs that were probably or possibly related to the study device. Four unrelated AEs were reported by three patients. Unrelated illness in one led to their early withdrawal from the study. The results of the patient tolerability self-assessment questionnaire revealed one patient (3%) reported mild stinging at Visit 1 and two patients (7%) reported mild itching at Visit 1. These AEs were not reported in subsequent visits.
Discussion
The objective of this study was to further demonstrate the efficacy and safety of an LED mask as a stand-alone home treatment for adults and adolescents with mild-to-moderate facial acne. At the end of the 7-week treatment period, there was a significant improvement in each of the three primary efficacy endpoints, specifically, an improvement in inflammatory and noninflammatory facial acne lesion counts, and the proportion of patients achieving a ≥1-grade reduction from baseline IGA scores and exceeding the predetermined minimum success rate of 50%. The device was very well tolerated with only three reports of mild, transient treatment-related AEs. These results support the intended use of the 415 nm/633 nm LED device as a home treatment for adolescents and adults with mild-to-moderate facial acne.
The results of this study also revealed several other beneficial effects of this noninvasive LED acne treatment. There was a significant change in each of the 19 items in the acne QoL questionnaire, indicating a treatment-related improvement in every measured aspect of quality of life and self-esteem after 7 weeks of treatment. Importantly, patients responded with ‘not at all’ to nearly one-half of QoL-related questions at Week 7, indicating facial acne was no longer having an impact on that aspect of their daily life.
The results of this study further support the clinical use of combined blue (415 nm) and red (633 nm) light for the treatment of acne by simultaneously targeting C. acnes and sebaceous glands, respectively,14,22 although most previous clinical trials have used red and blue light sources sequentially.20,21,27 In one randomized, sham-controlled study, patients with mild-to-moderate acne were randomized to receive exposure to 420 nm blue and 660 nm red light for 2.5 minutes each twice-daily for four weeks.28 Inflammatory and noninflammatory acne lesions decreased by 77% and 54%, respectively, compared to no significant difference in the sham group. Notably, several of these devices have been effective when administered in the home setting.17,24,28-30
While not an objective of the present study, light therapy or photobiomodulation is also an effective means of achieving facial rejuvenation. A recent sham-controlled study assessed the benefits of a 633 nm LED mask for treating women seeking facial rejuvenation.31 Patients were randomized to receive two or three weekly applications with the LED mask for 12 weeks or a twice-weekly sham treatment. Both groups of LED-treated patients achieved significant improvements in forehead, glabellar, and periorbital wrinkles versus sham treatment. Treatment satisfaction was also significantly greater than sham treatment. Similar results have been demonstrated by others using an LED mask combining 630-nm and 850-nm LEDs.32 Another sham-controlled study assessed the effectiveness of a 660-nm LED administered twice-weekly for six weeks or three times weekly for four weeks.33 All patients showed significant improvements of hyperpigmentation, periorbital wrinkles, and overall appearance. A 630-nm LED mask decreased lateral rhytids, skin sagging, and skin roughness, increased facial skin firmness, density, and elasticity, and improved complexion after 28 days or twice-weekly treatment.34 These beneficial effects are likely due to increased expression of collagen and growth factors and decreased matrix metalloproteinases.35 Future studies that employ similar light therapy for treating acne should also assess changes in other parameters suggesting facial rejuvenation.
The major limitations of this study were the open-label study design and the lack of a sham control group.
Conclusion
The results of this study provide strong evidence that this LED device which combines blue (415 nm) and red (633 nm) is effective and safe for treating inflammatory and noninflammatory lesions in adolescents and adults with mild-to-moderate acne vulgaris resulting in enhanced self-perception and quality of daily life. The device is well tolerated and well suited as intended as a home therapy.
Acknowledgments
The author recognizes the editorial assistance of Dr. Carl S. Hornfeldt from Apothekon, Inc., during the preparation of this manuscript, and the statistical support of Elvira Cawthon, MS from Cawthon Clinical Consulting. This study was sponsored by GlobalMed Technologies; Napa, California.
References
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196.
- American Academy of Dermatology. Burden of skin disease briefs. Acne by the numbers. American Academy of Dermatology Association. 2017. Accessed: March 13, 2025. https://www.aad.org/asset/1hPRvLX6as5tVDTlOnnc0F.
- Layton AM, Thiboutot D, Tan J. Reviewing the global burden of acne: How could we improve care to reduce the burden? Br J Dermatol. 2021;184:219-225.
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56-59.
- Tunca M, Balik ZB, Uncu HB, Botsali A. Stigmatization and psychosocial burden in acne patients. J Cosmet Dermatol. 2025;24(2):e16678.
- Nandy P, Shrivastava T. Exploring the multifaceted impact of acne on quality of life and well-being. Cureus. 2024;16(1):e52727.
- Vilar GN, Santos LA, Sobral Filho JF. Quality of life, self-esteem and psychosocial factors in adolescents with acne vulgaris. An Bras Dermatol. 2015;90(5):622-629.
- Sharma R, Dogra N, Arora M. Psychosocial impact of acne vulgaris on the quality of life among adolescents versus adults. Clin Med (Lond). 2023;23(Suppl 6):35.
- Sutaria AH, Masood S, Saleh HM, Schlessinger J. Acne vulgaris. StatPearls Publishing; 2025. Accessed: March 13, 2025. https://www.ncbi.nlm.nih.gov/books/NBK459173.
- Santer M, Burden-Teh E, Ravenscroft J. Managing acne vulgaris: An update. Drug Ther Bull. 2023;62(1):6-10.
- Baldwin H. Oral antibiotic treatment options for acne vulgaris. J Clin Aesthet Dermatol. 2020;13:26-32.
- Valente Duarte de Sousa IC. An update on the pharmacological management of acne vulgaris: the state of the art. Expert Opin Pharmacother. 2024;25(16):2177-2190.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-973.e33.
- Hong JY, Seok J, Han HS, Park KY. Emerging innovations in acne management: a focus on nonpharmacological therapeutic devices. J Korean Med Sci. 2025;40(9):e118.
- Cotter EJ, Cotter LM, Riley CN, et al. Antimicrobial effects of blue light therapy against Cutibacterium acnes: optimal dosing and impact of serial treatments. JSES Int. 2023;8(2):328-334.
- Antoniou C, Dessinioti C, Sotiriadis D, et al. A multicenter, randomized, split-face clinical trial evaluating the efficacy and safety of chromophore gel-assisted blue light phototherapy for the treatment of acne. Int J Dermatol. 2016;55(12):1321-1328.
- Tremblay JF, Sire DJ, Lowe NJ, Moy RL. Light-emitting diode 415nm in the treatment of inflammatory acne: an open-label, multicentric, pilot investigation. J Cosmet Laser Ther. 2006;8(1):31-33.
- Ammad S, Gonzales M, Edwards C, Finlay AY, Mills C. An assessment of the efficacy of blue light phototherapy in the treatment of acne vulgaris. J Cosmet Dermatol. 2008;7(3):180-188.
- Li J, Li J, Zhang L, et al. Comparison of red light and blue light therapies for mild-to-moderate acne vulgaris: a randomized controlled clinical study. Photodermatol Photoimmunol Photomed. 2022;38(5):459-464.
- Lee SY, You CE, Park MY. Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg Med. 2007;39(2):180-188.
- Goldberg DJ, Russell BA. Combination blue (415 nm) and red (633 nm) LED phototherapy in the treatment of mild to severe acne vulgaris. J Cosmet Laser Ther. 2006;8:71-75.
- Pei S, Inamadar AC, Adya KA, Tsoukas MM. Light-based therapies in acne treatment. Indian Dermatol Online J. 2015;6(3):145-157.
- Jung YR, Kim SJ, Sohn KC, et al. Regulation of lipid production by light-emitting diodes in human sebocytes. Arch Dermatol Res. 2015;307(3):265-273.
- Papageorgiou P, Katsambas A, Chu A. Phototherapy with blue (415 nm) and red (660 nm) light in the treatment of acne vulgaris. Br J Dermatol. 2000;142(5):973-978.
- Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32(12):1458-1466.
- Martin AR, Lookingbill DP, Botek A, et al. Health-related quality of life among patients with facial acne — assessment of a new acne-specific questionnaire. Clin Exp Dermatol. 2001;26(5):380-385.
- Sadick NS. Handheld LED array device in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7:347-350.
- Kwon HH, Lee JB, Yoon JY, et al. The clinical and histological effect of home-use, combination blue-red LED phototherapy for mild-to-moderate acne vulgaris in Korean patients: A double-blind, randomized controlled trial. Br J Dermatol. 2013;168(5):1088-1094.
- Ash C, Harrison A, Drew S, Whittall R. A randomized controlled study for the treatment of acne vulgaris using high-intensity 414 nm solid state diode arrays. J Cosmet Laser Ther. 2015;17(4):170-176.
- Gold MH, Andriessen A, Biron J, Andriessen H. Clinical efficacy of self-applied blue light therapy for mild-to-moderate facial acne. J Clin Aesthet Dermatol. 2009;2(3):44-50.
- Bragato EF, Paisano AF, Pavani C, et al. Role of photobiomodulation application frequency in facial rejuvenation: randomized, sham-controlled, double-blind, clinical trial. Lasers Med Sci. 2025;40:170.
- Park SH, Park SO, Jung JA. Clinical study to evaluate the efficacy and safety of home-used LED and IRED mask for crow’s feet: a multi-center, randomized, double-blind, sham-controlled study. Medicine (Baltimore). 2025;104:e41596.
- Shurrab K, Alzghayar JN. Low-level laser therapy for skin rejuvenation: a safe and effective solution baked by data and visual evidence. J Cosmet Dermatol. 2024;23(10):3234-3324.
- Couturaud V, Le Fur M, Pelletier M, Granotier F. Reverse skin aging signs by red light photobiomodulation. Skin Res Technol. 2023;29(7):e13391.
- Lee YI, Lee SG, Ham S, et al. Exploring the safety and efficacy of organic light-emitting diode in skin rejuvenation and wound healing. Yonsei Med J. 2024;65(2):98-107.