J Clin Aesthet Dermatol. 2024;17(2):15–19.
J Clin Aesthet Dermatol. 2024;17(2):15–19.
by Zoe Diana Draelos, MD, FAAD; Pearl E. Grimes, MD, FAAD; Jacqueline Watchmaker, MD, FAAD;
and Diane B. Nelson, RN, MPH
Dr. Draelos is with Dermatology Consulting Services PLLC in High Point, North Carolina. Dr. Grimes is with The Grimes Center of Medical and Aesthetic Dermatology and the Vitiligo and Pigmentation Institute of Southern California in Los Angeles, California. Dr. Watchmaker is with Southwest Skin Specialists in Scottsdale, Arizona. Ms. Nelson is with Skinbetter Science, a Dermatological Beauty brand of L’Oréal USA, Inc., in Phoenix, Arizona.
FUNDING: This study was funded by skinbetter science, LLC, a Dermatological Beauty brand of L’Oréal USA, Inc..
DISCLOSURES: Drs. Draelos, Grimes and Watchmaker were investigators on this study; Diane Nelson is an employee of skinbetter science.
ABSTRACT: Objective. The ability of the skin to maintain homeostasis declines with age. Adaptogens support the capacity of the skin to respond to stress. We sought to evaluate the efficacy of a novel serum comprised of plant-based adaptogens for improving photoaged skin following twice-daily application.
Methods. A multi-center, 12-week trial was conducted in participants aged 45 to 65 years, Fitzpatrick Skin Type (FST) I to VI, with mild-to-severe photoaging based on a 10-point grading scale (3 [Minimum] to 7 [Maximum]). Visible improvements were assessed in erythema, pore size, skin dullness, skin texture, and uneven pigmentation utilizing a six-point grading scale (0=None to 5=Severe). Global skin quality was measured utilizing our Global Skin Quality Index (GSQI). Sebum measurements were obtained in a subset of participants. Patient satisfaction and tolerability were recorded throughout the study.
Results. Fifty-three participants were enrolled and completed the study. Mean age was 56 years and 66 percent were White, 17 percent were Black, 8 percent were Hispanic, 6 percent were Asian/Pacific Islander, and 81 percent had moderate photodamage. At Week 12, significant mean percent improvements from baseline were demonstrated in erythema (50%), dullness (44%), texture (52%), pore size (23%), and uneven pigmentation (21%; all p<.0001). Significant GSQI improvements from baseline were observed at Week 12 (39%; p<0.0001). Significant mean reductions from baseline in skin surface sebum were demonstrated at Week 12 (-38%; p<0.0001). All adverse events (AEs) were mild and transient.
Conclusion. A novel serum comprised of plant-based adaptogens, demonstrated improvements from baseline in the appearance of erythema, dullness, texture, pore size, uneven pigmentation, and global skin quality over 12 weeks. Participants reported high levels of satisfaction, with mild, transient AEs reported.
Keywords: Plants, adaptogens, homeostasis, cosmeceuticals, skin quality, photodamage, photoaging
Organisms are constantly challenged by internal and external stressors that threaten equilibrium, necessitating constant and rapid adjustments to achieve and maintain homeostasis.1,2 Optimal skin health relies on the ability of skin to achieve and maintain homeostasis through complex communication pathways.1,3,4 Skin cells utilize various signaling pathways that sense environmental fluctuations and respond via intracellular communication to promote adaptation to the changing environment.3–5 Effective activation of the complement system, involving inflammatory factors, the extracellular matrix (ECM), intracellular adaptive stress response signaling pathways, and cellular tissue structure morphology all play a critical role in supporting homeostasis of the skin.1,5 The innate ability of the skin to protect itself declines with age and continual exposure to environmental, physical, and emotional stress.1,4 Skin aging is a multifactorial process, with each layer differentially affected,7 leading to a loss of physiological integrity and impaired function, which in turn increases susceptibility to infection, low-grade chronic inflammation, and skin fragility.8–10
Plant-derived adaptogens are rich phytochemical compositions that have multitargeted effects in skin.5 Through adaptation and evolution, plant adaptogens have developed the ability to defend and protect themselves against environmental insults and other stressors.1,11 Adaptogens support the body’s resistance to negative stressors, helping to maintain and normalize functions and facilitate protective benefits.1,2,5,11 Adaptogens are increasingly used in skincare to manage certain dermatological conditions and in formulations designed to support aging skin, and have been shown to be beneficial in conditions including psoriasis, atopic dermatitis, and acne.5 Recent evidence suggests that combinations of adaptogens may have synergistic benefits to help increase resistance to stress, mitigate inflammation, and prevent premature aging.1
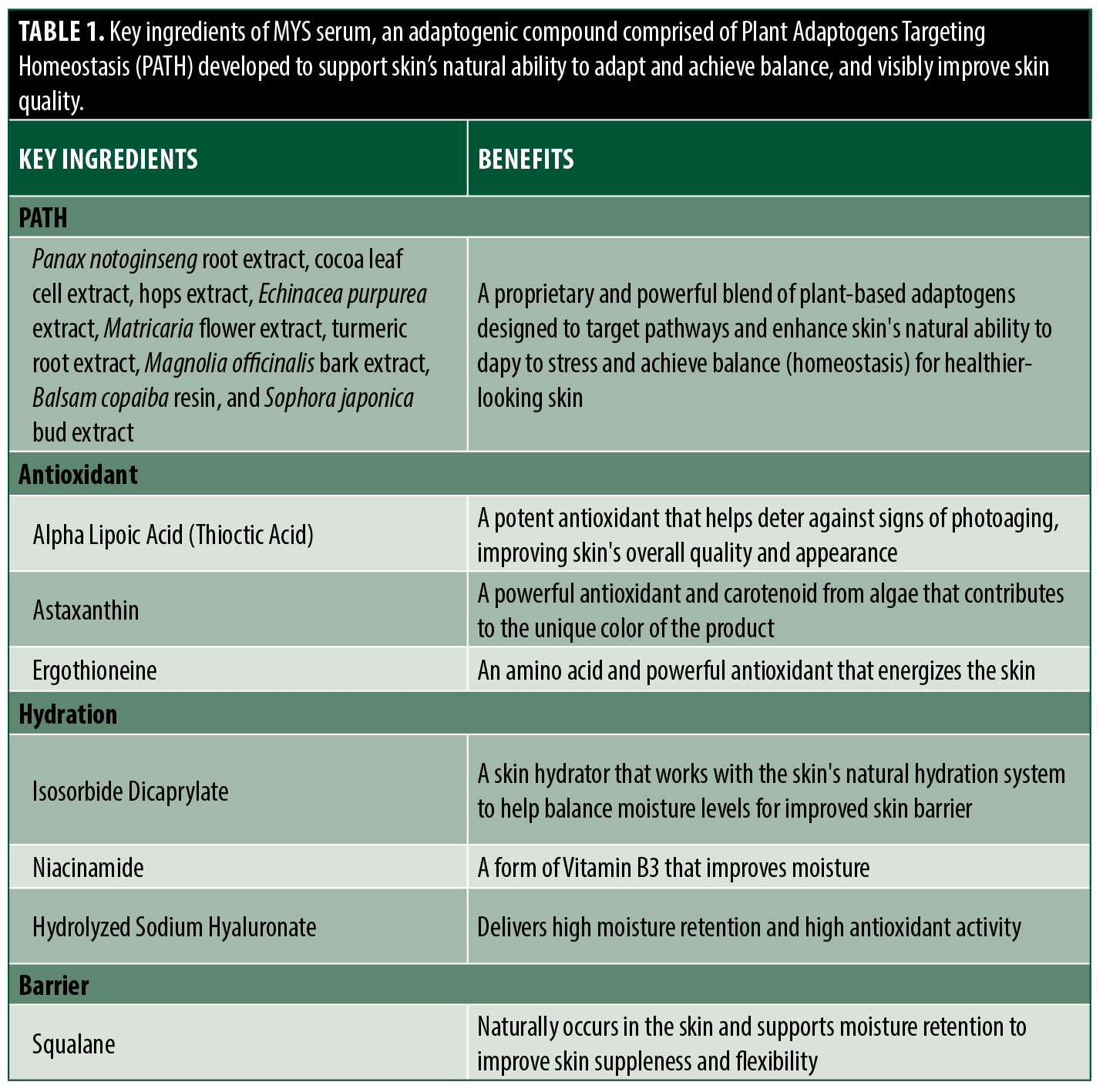
The incorporation of adaptogens into skincare presents a unique opportunity to optimize global skin health and quality. To this end, rigorous screening and analysis of a diverse group of plant extracts was conducted to identify and categorize their specific activity and impact on pathways in the skin.12 Owing to their individual and combined benefits, a novel serum comprised of Plant Adaptogens Targeting Homeostasis (PATH) was purposefully developed to support skin’s natural ability to adapt and achieve balance as well as visibly improve skin quality (Table 1). The adaptogenic compounds used in PATH have important multifunctional properties, such as inflammatory inhibitory activity, support of aging skin, and antioxidant capacity. Additionally, many of these agents have been found to reinforce skin barrier function.13–20 To help identify the mechanistic activity of this novel serum (MYS), gene expression analysis was conducted utilizing an in vitro epidermal skin model. Enhanced activity of key biomarkers was observed, suggesting support of the skin’s ability to respond to stress and achieve homeostasis.12 Herein, we describe the effects of MYS serum on the visible signs of skin quality in participants with photoaged skin. The objectives of this study were to evaluate tolerability, efficacy, and satisfaction in participants with mild-to-severe facial photoaging following twice-daily application of MYS serum over 12 weeks.
Methods
This multi-center, open-label clinical trial was conducted across three dermatology research centers under Independent Review Board (IRB; Sterling IRB #10298) approval in conjunction with current Good Clinical Practices (cGCP) guidelines. The trial included participants aged 45 to 65 years with Fitzpatrick Skin Types (FST) I to VI and mild-to-severe photoaging based on a 10-point grading scale (3 [Minimum] to 7 [Maximum]). Participants applied MYS serum twice-daily (AM/PM) in conjunction with a standardized regimen that included a gentle cleanser (AM/PM), moisturizer (AM/PM) and a mineral-based sunscreen (SPF 56) for 12 weeks.
Exclusion criteria included dermatological disorders (e.g., moderate to severe acne vulgaris), melasma or PIH, severe rosacea, facial scarring. Pregnant or lactating participants or participants planning to become pregnant during the study period were also excluded. Participants were deemed eligible for inclusion in the study following a four-week washout period of any cosmetic product containing alpha- and beta-hydroxy acids (AHAs, BHAs), antioxidants, peptides, non-prescription retinoids, or prescription-strength hydroquinone. A three-month washout period was required of topical prescription products containing corticosteroids or retinoids; medications for rosacea or erythema; growth factors; and/or microdermabrasion, microneedling, chemical peels, or similar procedures. Participants who had any prior use of isotretinoin were excluded. Participants who had undergone energy-based treatments in the 12 months prior to the study or had undergone other cosmetic treatments (e.g., injectable neuromodulators or dermal fillers) in the previous six months were also excluded from study participation.
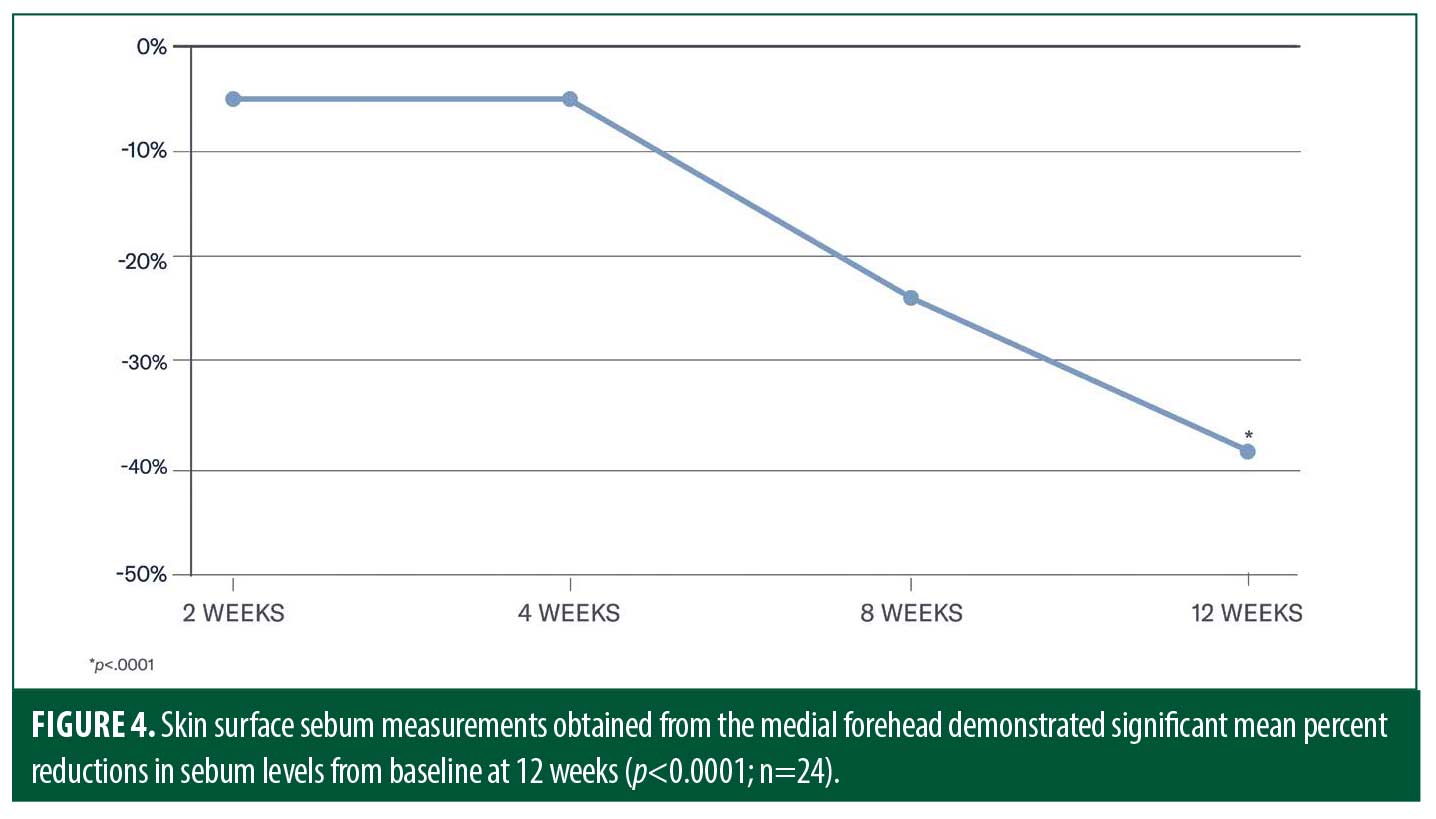
Investigators evaluated parameters associated with facial skin quality, including erythema, pore size, skin dullness, skin roughness/ texture (visual), and uneven pigmentation at baseline, Weeks 2, 4, 8, and 12 using a six-point grading scale (0=none to 5=very severe). Global skin quality was measured utilizing our Global Skin Quality Index (GSQI). The GSQI is based upon the total sum of scores according to investigator grading of individual parameters and aids in quantifying both the visual and topographical attributes of skin quality. Quantitative skin surface sebum measurements were obtained in a subset of participants (medial forehead; N=28) using non-invasive bioinstrumentation at a single research center (Sebumeter®, Courage + Kazaka Electronic GmbH, Köln, Germany) at baseline, Weeks 2, 4, 8, and 12. Participants were instructed to apply the moisturizer and sunscreen following their clinic visits at Weeks 2, 4, 8 and 12 so as not to interfere with instrument measurements.
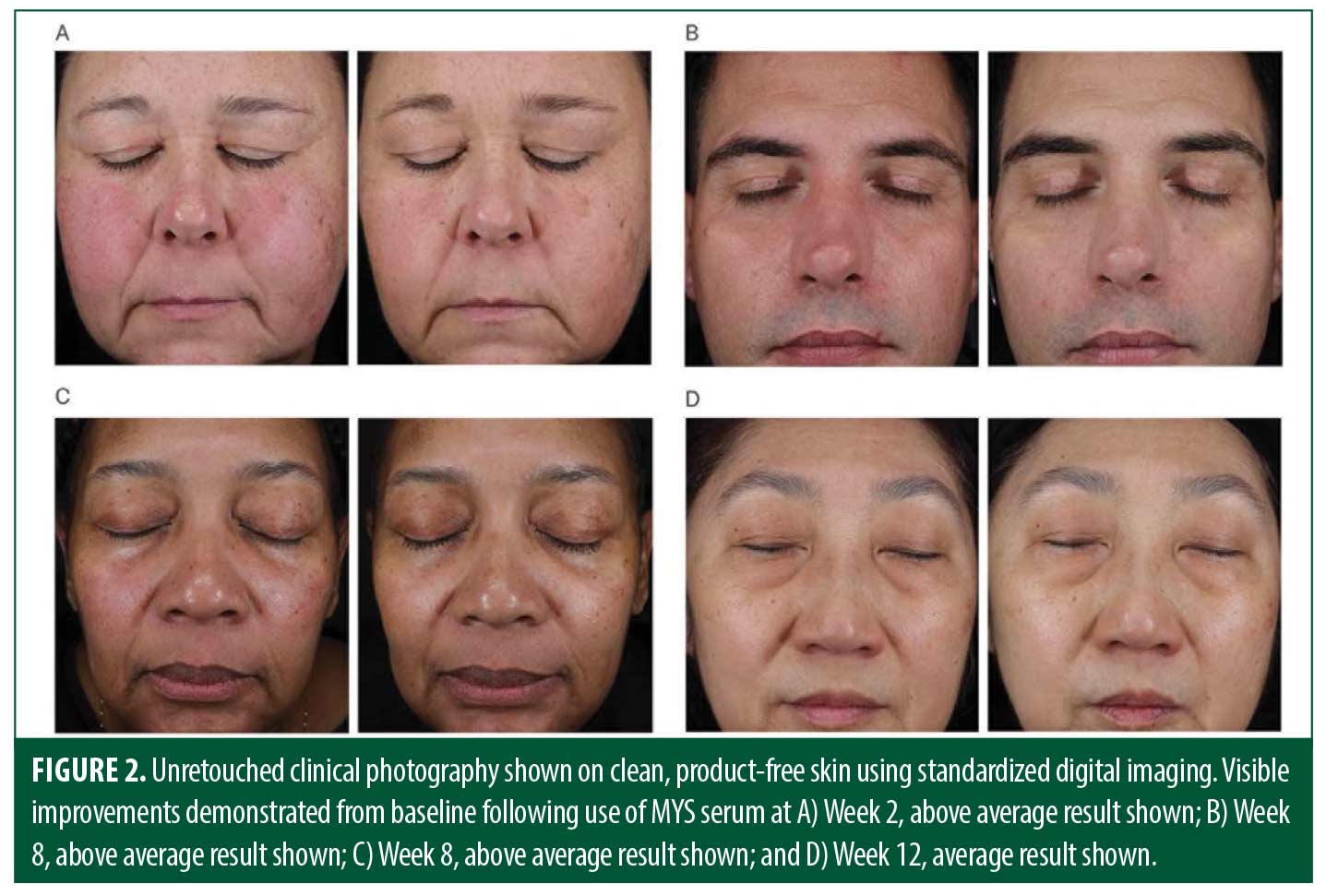
Facial digital images were taken at baseline, Weeks 2, 4, 8, and 12. Images were captured using a Canfield VISIA-CR Imaging System (Canfield Scientific Inc., Parsippany, New Jersey). Participants completed a self-assessment questionnaire at all post-baseline visits. The questionnaire assessed participants’ perception of the appearance of their facial skin, perceived effect of the study product, and their overall satisfaction with the study product. Evaluation and collection of AEs occurred throughout the study period.
Results
Fifty-three (N=53) participants were enrolled and completed the study. The majority of participants were female (92%) with a mean age of 56 years and presented with a moderate amount of photodamage (81%). Sixty-four percent (64%) of participants were FST I-II, 21 percent were FST III-IV, 15 percent were FST V-VI. Sixty-six percent (66%) of participants were White, 17 percent were Black, 6 percent were Asian/Pacific Islander, and 6 percent identified as multi-race. Eight percent (8%) of enrolled participants were Hispanic.
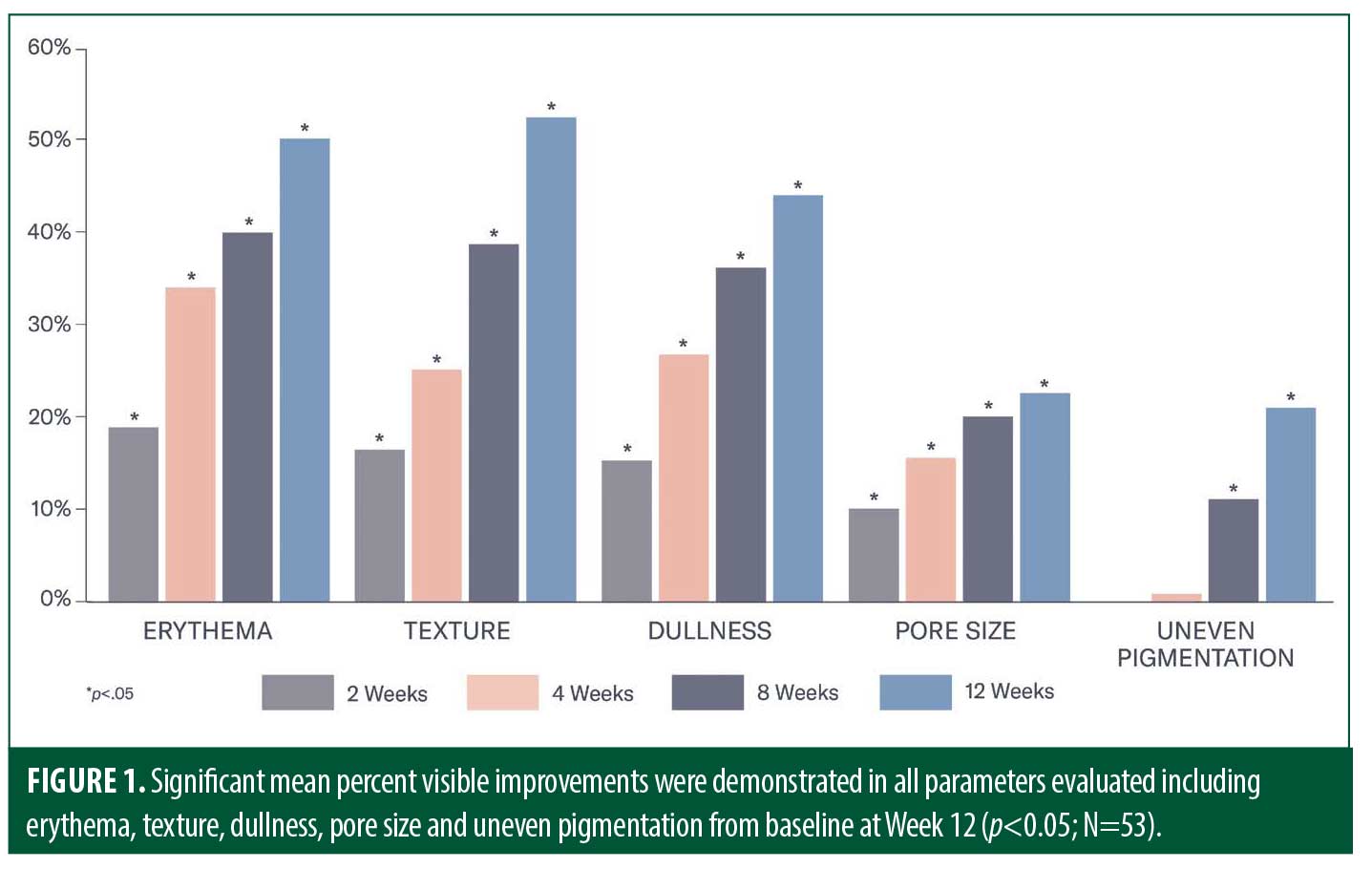
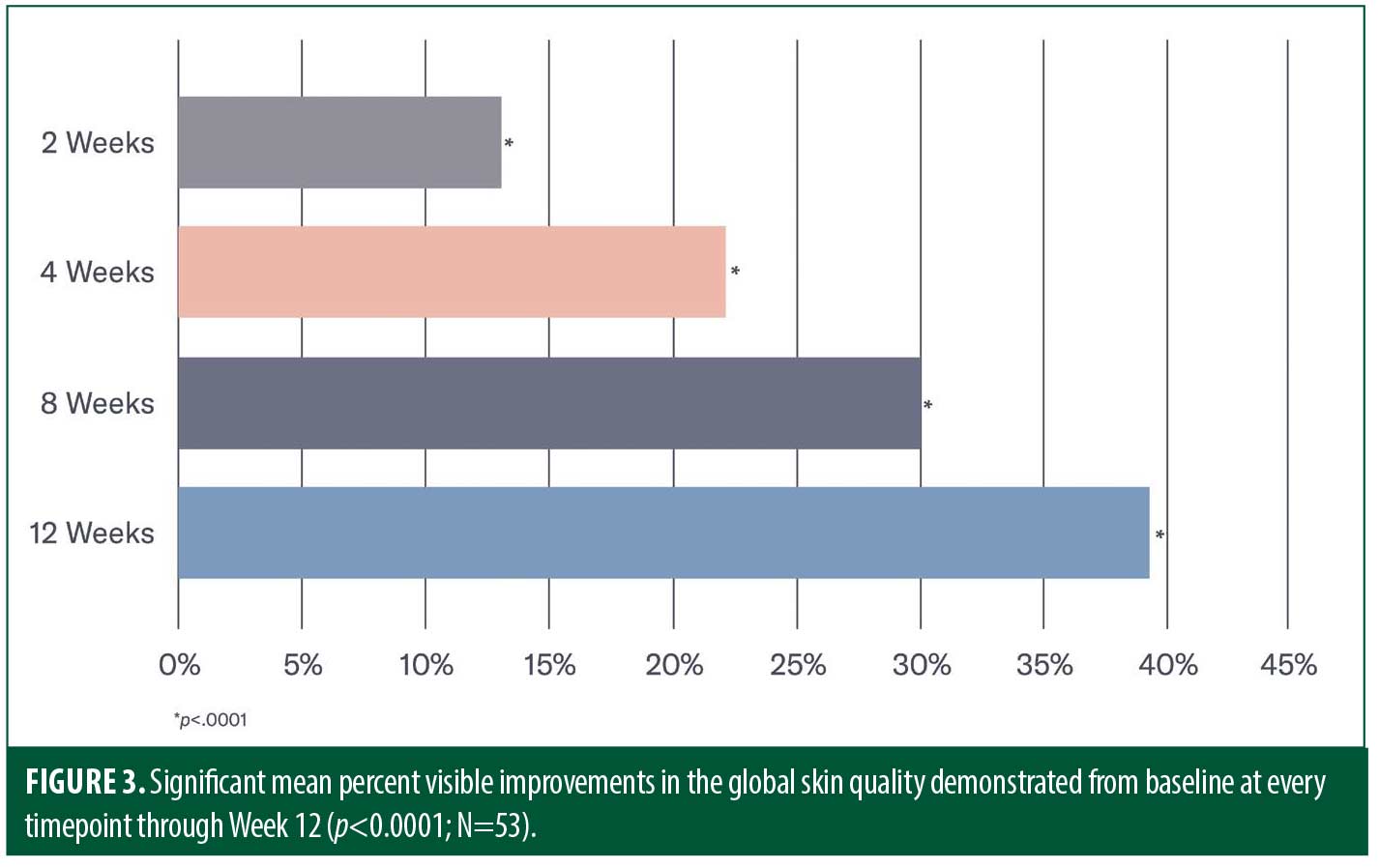
Efficacy. Early significant mean percent improvements from baseline occurred at Week 2 in the appearance of erythema, skin dullness, skin roughness/texture and pore size (all p<0.0001), with improvements in the appearance of uneven pigmentation demonstrated at Week 8 (p<0.001). At Week 12, significant mean percent improvements from baseline were demonstrated in all parameters including the appearance of erythema (50%), skin dullness (44%), skin roughness/texture (52%), pore size (23%) and uneven pigmentation (21%; all p<0.0001; Figures 1 and 2). Significant improvements from baseline according to the GSQI were observed as early as Week 2 (13%; p<.0001), improving to 39 percent at Week 12 (p<0.0001; Figure 3).
A total of 28 participants underwent Sebumeter measurements. Four participants’ sebum levels were zero at study initiation, resulting in 24 evaluable subjects. Skin surface sebum measurements obtained from the medial forehead demonstrated a 38-percent reduction from baseline at Week 12 (p<0.0001; n=24; Figure 4).
Participants reported high levels of satisfaction with MYS serum throughout the study period. As early as Week 2, 94 percent or more of the participants agreed that the overall quality of their skin had improved. By Week 12, 96 percent or more of participants reported that their skin looked less red, pores were less visible, skin texture appeared smoother, and their skin appeared brighter and healthier. Notably, 82 percent of participants reported wearing less makeup following use of MYS serum, and 87 percent of participants agreed that family or friends noticed or commented on the improved appearance of their skin after 12 weeks. Nearly all participants (98%) felt more confident in the appearance of their skin by the end of the study.
Tolerability. Adverse events were mild and transient in nature (n=12). Among these events were reports of breakouts, tingling, irritation, and peeling. No participant discontinued study product use or enrollment in the study due to an AE.
Discussion
Optimal skin health relies on the ability of skin to efficiently respond to stress. Skin innately strives to achieve and maintain homeostasis, but this ability naturally declines with age and with continued exposure to stressors.1 Aging and stress impair the ability of skin to sufficiently adapt to internal and external stressors, increasing risk of infection, chronic low-grade inflammation, and fragility, as well as reducing immune function. In addition, these changes impair optimal skin quality leading to the appearance of erythema, rough skin, skin dullness, enlarged pores and uneven pigmentation.1 While agents such as retinoids, AHAs, BHAs, and antioxidants are effective, they do not globally address cellular or epidermal homeostasis. In contrast, plant adaptogens, by definition, are uniquely able to communicate with multiple pathways in the skin to activate the complement system, ECM, intracellular adaptive stress response signaling pathways, and inhibit oxidative stress pathways, among others, to facilitate homeostasis.5,6
Adaptogenic compounds are multifunctional and target numerous communication pathways. The plant-based adaptogens that comprise PATH technology were systematically selected based on their ability to facilitate inflammatory inhibitory activity, support aging skin, afford antioxidant benefits, and reinforce skin barrier function. Prior to conducting this clinical trial, an in vitro gene expression study was performed in which MYS serum was analyzed for its effects on specific genes known to influence skin homeostasis: aryl hydrocarbon receptor (AhR), chloride channel accessory 2 (CLCA2), metallothionein 1A (MT1A), 1F (MT1F), and 1G (MT1G), and thioredoxin reductase 1 (TXNRD1). Utilizing an in vitro epidermal skin model, increased expression of AhR, TXNRD1, MT1A, MT1F, and MT1G, and reduced expression of CLCA2 were demonstrated after 48 hours of topical exposure to MYS serum. These findings indicate the ability of MYS serum to support skin’s adaptive response to stress and achieve homeostasis.12
We hypothesized that these benefits would translate to visible improvements in skin quality, a term often used to describe the global state of skin.1,23,24 Of note, there is no current universal definition of skin quality, nor is there a validated means for evaluating skin quality for topical treatments.22 Generally, skin quality encompasses numerous parameters, including skin tone evenness (pigmentation, erythema, and discoloration); skin surface evenness (pore size, lines and wrinkles, crepiness, scars, hair and skin clarity); skin firmness (elasticity, tightness, and hydration) and skin glow or radiance.21,22 In this study, we utilized GSQI to evaluate skin quality parameters defined as erythema, pore size, skin dullness, visual skin roughness/texture, and uneven pigmentation. Based on the GSQI, improvements from baseline were shown to be statistically significant at all study time points with improvements in skin quality demonstrated as early as Week 2. Additionally, significant mean percent improvements from baseline occurred by Week 2 in individual parameters, including erythema, skin dullness, visual skin roughness/texture, and pore size. Application of MYS serum led to significant reductions in sebum levels and significant improvements in the appearance of pores from baseline at 12 weeks. While pores are generally determined by heredity, evidence suggests that increased sebum production is correlated with facial pore development as well as acne severity.22 Homeostasis of sebaceous glands and sebum production is influenced, in part, by AhR, p53, and other signaling pathways.21
Importantly, participants reported high levels of satisfaction throughout the study period, with more than 82 percent of participants noting the use of less makeup and feeling more confident in the appearance of their skin after 12 weeks.
We enrolled an intentionally diverse study population to assess tolerability and efficacy of MYS serum across participants representing a variety of skin types and ethnicities. Sixty-six percent (66%) of participants were White, 17 percent were Black, 6 percent were Asian/Pacific Islander, and 6 percent identified as multi-race. Eight percent (8%) of enrolled participants were Hispanic. Although relatively small, the diversity reflected in the study population is similar to United States census data from 2022.25 Future studies may include a larger, more diverse population and a control group.
Conclusion
A novel serum comprised of plant-based adaptogens demonstrated early and significant improvements from baseline in erythema, skin dullness, skin roughness/texture (visual), pore size, uneven pigmentation, and global skin quality in participants with mild-to-severe facial photodamage over 12 weeks. Adverse events were mild and transient, with no participant discontinuing the study owing to an AE. High levels of participant satisfaction were reported throughout the study period.
Acknowledgments
The authors would like to thank Lynne Kolton Schneider, PhD, for her editorial assistance on this manuscript.
References
- Pomatto LCD, Davies KJA. The role of declining adaptive homeostasis in ageing. J Physiol. 2017;595(24):7275–7309 .
- Chen Y, Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm Allergy. 2014;13:177–190.
- Eckhart L, Tschachler E, Gruber F. Autophagic control of skin aging et al. Front Cell Dev Biol. 2019;7:143.
- Liu X-X, Chen C-Y, Li L, et al. Bibliometric study of adaptogens in dermatology: pharmacophylogeny, phytochemistry, and pharmacological mechanisms. Drug Design, Devel Ther. 2023;17:341–361.
- Panossian AG, Efferth T, Shikov AN, et al. Evolution of the adaptogenic concept from traditional use to medical systems: pharmacology of stress- and aging-related diseases. Med Res Rev. 2021;41:630–703.
- Zorina A, Zorin V, Kudlay D, et al. Molecular mechanisms of changes in homeostasis of the dermal extracellular matrix: both involutional and mediated by ultraviolet radiation. Int J Mol Sci. 2022;23:6655.
- Lopez-Otin C, Blasco A, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217.
- Pilkington SM, Bulfone-Paus S, Griffiths CEM, et al. Inflammaging and the skin. J Investig Dermatol. 2021;141:1087–1095.
- Xia S, Zhang X, Zheng S, et al. An update on inflamm-aging: mechanisms, prevention, and treatment. J Immunol Res. 2016;8426874:1–12.
- Todorova V, Ivanov K, Delattre C, et al. Plant adaptogens – history and future perspectives. Nutrients. 2021;13:2861.et al; Nutrients. 2021;13(8):2861.
- Ray A, Gulati K, Anand R. Stress. adaptogens and their evaluation: an overview. J Pharma Reports. 2016;1:110.
- Draelos ZD, Grimes PE, Watchmaker J, Nelson DB. Gene Expression Analysis of a Topical Serum Comprised of Plant-based Adaptogens Developed to Support Homeostasis and Skin Quality. J Clin Aesthet Dermatol. 2024;17(2):43–46.
- Barbalho SM, de Sousa Gonzaga HF, de Souza GA, et al. Dermatological effects of Curcuma species: a systematic review. Clin Exp Dermatol. 2021;46(5):825–833.
- Hwang E, Park S-Y, Yin CS, et al. Antiaging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. J Ginseng Res. 2017;41:69–77.
- Lima EP, Goncalves OH, Ames FQ, et al. Anti-inflammatory and antioxidant activity of nanoencapsulated Curcuminoids extracted from Curcuma longa L. in a model of cutaneous inflammation. Inflammation. 2021;44(2):604–616.
- Park MY, Han SJ, Moon D, et al. Effects of red ginseng on the elastic properties of human skin. J Ginseng Res. 2019;44:738–746.
- Pham QL, Jang H-J, Kim K-B. Anti-wrinkle effect of fermented black ginseng on human fibroblasts. Int J Mol Med. 2017;39(3):681–686.
- Saboori S, Falahi E, Rad EY, et al. Effects of ginseng on C-reactive protein level: a systematic review and meta-analysis of clinical trials. Complement Ther Med. 2019;45:98–103.
- Vaughn AR, Branum A, Sivamani RK. Effects of turmeric (Curcuma longa) on skin health: a systematic review of the clinical evidence. Phytother Res. 2016;30(9):1243–1264.
- Yang Y, Ren C, Zhang Y, et al. Ginseng: an nonnegligible natural remedy for healthy aging. Aging Dis. 2017;8(6):708–720.
- Hou X, Wei Z, Zouboulis CC, et al. Aging in the sebaceous gland. Front Cell Dev Biol. 2022;10:909694.
- Liu Y, Jiang W, Tang Y, et al. An optimal method for quantifying the facial sebum level and characterizing facial sebum features. Skin Res Technol. 2023;29(9):e13454.
- Goldie K, Kerscher M, Fabi SG, et al. Skin Quality – A Holistic 360° View: Consensus Results. Clin Cosmet Investig Dermatol. 2021;14:643–654.
- Humphrey S, Brown SM, Cross SJ, et al. Defining skin quality: clinical relevance, terminology, and assessment. Dermatol Surg. 2021;47(7):974–981.
- https://www.census.gov/quickfacts/fact/table/US/PST045222.