J Clin Aesthet Dermatol. 2020;13(3):12–14 
by Monica Janeczek, MD; Zachary Kozel, MD; Richa Bhasin, BS; Joy Tao, MD; David Eilers, MD; and James Swan, MD
Drs. Janeczek, Kozel, Tao, Eilers, and Swan are with the Hines VA Hospital in Hines, Illinois and the Division of Dermatology at Loyola University Chicago in Maywood, Illinois. Ms. Bhasin is with the Stritch School of Medicine at Loyola University of Chicago in Maywood, Illinois.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Inverse psoriasis is characterized by erythematous nonscaly plaques in intertriginous regions. Similarly, erythrasma, a superficial infection caused by Corynebacterium minutissimum (C. minutissimum), is also found in skin folds with red-brown lesions, making the distinction between psoriasis and erythrasma difficult. No studies have previously determined whether these two clinically similar cutaneous disorders can occur concurrently.
Methods. Thirty patients with inverse psoriatic plaques were examined using a standard Wood’s lamp to visualize porphyrins associated with C. minutissimum.
Results. Just over half (56.6%) of patients with inverse psoriatic plaques showed evidence of this bacterium. Specifically, 45.5 percent of inverse psoriatic lesions were found to be positive for C. minutissimum, with the highest prevalence of erythrasma located in the gluteal cleft.
Conclusion. Clinical suspicion for C. minutissimum should be high in patients with inverse psoriasis due to the organism’s potential to trigger or exacerbate psoriatic lesions. Further studies are indicated to determine the response to treatment in patients with this combination.
KEYWORDS: Inverse psoriasis, erythrasma, Corynebacterium minutissimum, plaques
Inverse psoriasis was considered to be an uncommon form of psoriasis; however, recent data suggests it is more common than believed, occurring in 21 to 30 percent of patients with psoriasis, with the groin being the most prevalent area of involvement.1 Inverse psoriasis is characterized by lesions affecting intertriginous regions, including but not limited to the inguinal, perineal, genital, intergluteal, axillary, umbilical, and inframammary regions.2 This presentation is in marked contrast to plaque psoriasis, which primarily presents on the extensor regions of the body, especially targeting the knees and elbows. In addition, inverse psoriasis lacks the raised, dry, silvery scale appearance typical of plaque psoriasis, instead presenting as shiny, smooth, erythematous patches targeting body folds.2,3 Due to this nonspecific appearance, inverse psoriasis is often misdiagnosed as intertrigo, erythrasma, seborrheic dermatitis, or other common skin conditions.1,4 The treatment of these lesions is also complicated by increased absorption and sensitivity in intertriginous regions; hence, current standard treatments include less potent topical steroids as short-term therapy, with topical immunomodulators, calcitriol, or calcipotriene as long-term therapy.5 Topical antifungal therapy is commonly used to suppress yeast flora, and, in our clinical experience, might trigger new or recurrent lesions. .
In addition to the difficulty of initial diagnosis and treatment, psoriasis and inverse psoriasis predispose the patient to secondary skin lesions of the affected area. Brook discovered multiple instances of infection complicating psoriatic lesions, with culture species ranging from Staphylococcus aureus to Prevotella species. Classifying such coinfection remains critical for proper management, due to the possibility of infection exacerbating symptoms and complicating treatment, ultimately preventing complete resolution of psoriatic lesions.6 Likewise, research has suggested that some cutaneous microflora, such as Malassezia and Candida, can trigger inflammatory responses, leading to the occurrence of psoriasis and further exacerbation of the disease process.7
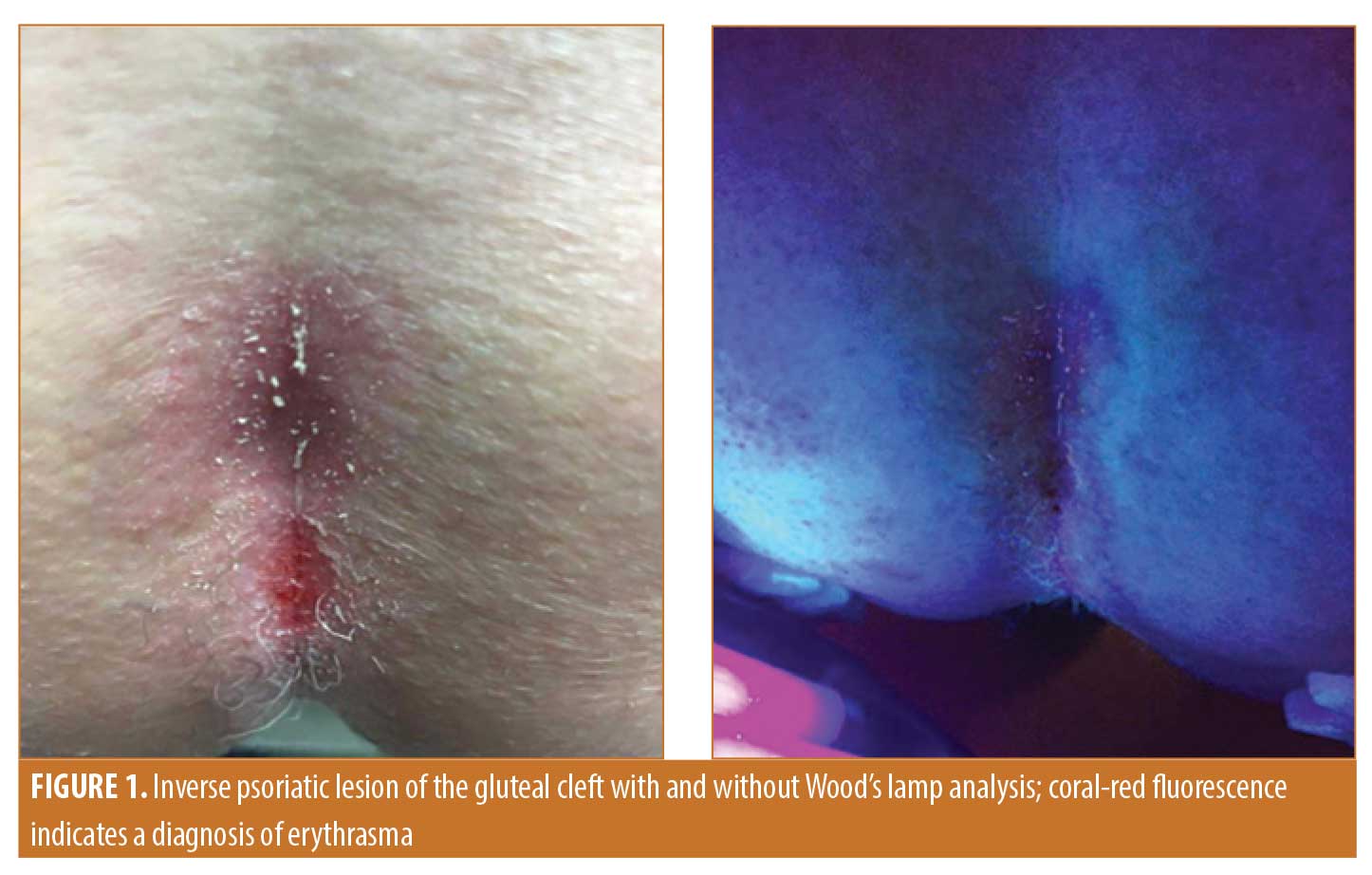
Corynebacterium minutissimum (C. minutissimum) is the causative agent of erythrasma, a superficial infection of the skin. Erythrasma presents as red-brown, well-demarcated lesions affecting intertriginous regions of the body with associated acute or chronic pruritus.8 Diagnosis is based on coral-red fluorescence following a Wood’s lamp examination and can be confirmed via gram stain.9 Due to their similar clinical appearance and proclivity for the same body locations, much of the literature surrounding inverse psoriasis and erythrasma centers on the clinical differentiation of the two conditions. However, to our knowledge, there are currently no studies that have focused on the possibility that many cases of inverse psoriasis might also contain C. minutissimum, which might trigger or exacerbate the inflammatory process. Determining the prevalence of erythrasma in inverse psoriasis might enable more effective treatment of psoriatic lesions, especially in refractory cases.
Materials and Methods
Study design. Thirty patients with inverse psoriasis were recruited to determine the coprevalence of erythrasma. Patients with a clear clinical presentation of inverse psoriasis, as determined by a board-certified dermatologist, were included regardless of lesion location or severity. Cases where the diagnosis was uncertain and cases in which patients were treated for erythrasma within one year of the study were excluded. A full-body skin examination was performed to identify all lesions, and the location of each lesion was documented accordingly. All psoriatic lesions were examined using a standard Wood’s lamp to visualize porphyrins associated with the presence of C. minutissimum. Coral-red fluorescence emitted by these porphyrins indicated a positive test result. Photos were taken of representative lesions with and without Wood’s lamp analysis to document results (Figure 1). This study was approved by the Hines VA Hospital Institutional Review Board, and informed consent was obtained from all participants included in the study.
Statistical analysis. The prevalence of erythrasma was determined in the entire patient cohort represented by the percentage of patients exhibiting at least one lesion with C. minutissimum. In addition, of the total number of psoriatic lesions in the patient cohort, the percentage of lesions positive for infection was also determined. Location of positive lesions was also assessed to identify areas that are more susceptible to C. minutissimum infection. Of the patients that exhibited this bacterium, the percentage of patients with multiple site infections, defined as showing at least two or more sites of infection, was also determined.
Results
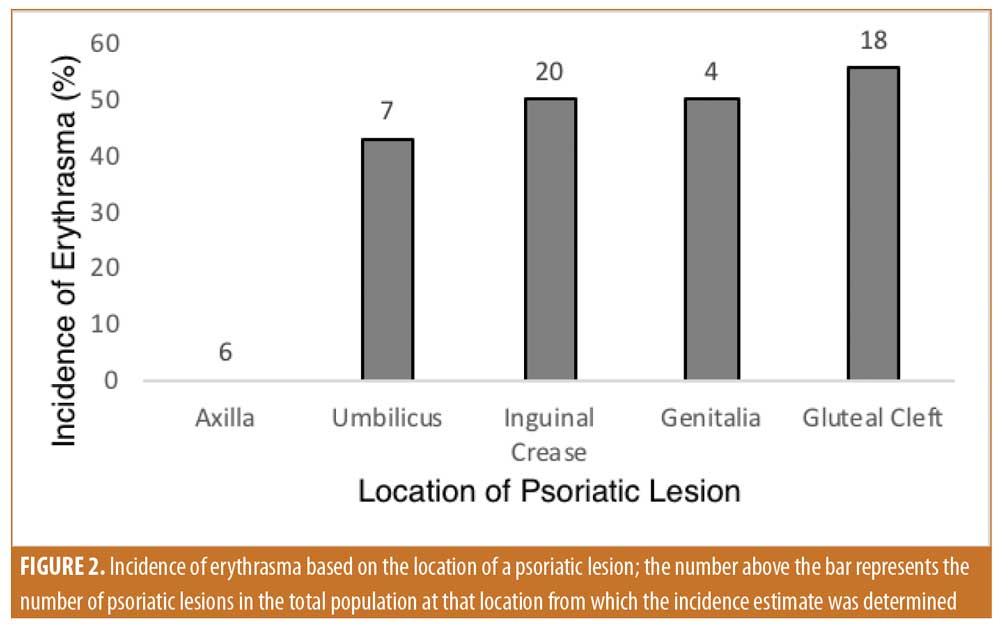
Of the 30 patients with inverse psoriasis, 17 patients had erythrasma, corresponding to a prevalence of 57 percent. Inverse psoriasis lesions were identified in the axilla, inguinal crease, gluteal cleft, genitalia, and umbilicus. Specifically, 6 patients showed lesions in the axilla, 20 showed lesions in the inguinal crease, 18 showed lesions in the gluteal cleft, 4 showed lesions on the genitalia, and 7 showed lesions in the umbilicus. It was determined that 25 inverse psoriatic plaques of 55 lesions (46%) in the entire cohort were positive for infection. Based on location, the highest rate of infection was found in the gluteal cleft (55%); followed by 50 percent in the inguinal crease, 50 percent in the genitalia, 43 percent in the umbilicus, and 0 percent in the axilla (Figure 2). Among the total population, 41 percent of patients with C. minutissimum presented with multiple sites of infection. The extent and intensity of erythrasma varied from site to site: some had diffuse coverage with coral-red fluorescence, while other sites had more focal but clear-cut areas of fluorescence.
Discussion
To our knowledge, there have been no reported cases of erythrasma with concurrent inverse psoriatic lesions in the literature. Both cutaneous disorders are difficult to diagnose individually and even more so in combination due to similar erythematous lesions observed in similar skin-fold regions. This study indicates a high prevalence of C. minutissimum in patients with inverse psoriasis, indicating that Wood’s lamp analysis should be considered in refractory cases of psoriasis or in the initial diagnostic work-up of inverse psoriatic lesions.
Clinical suspicion should be higher in patients with risk factors for erythrasma, including poor hygiene, obesity, diabetes mellitus, hyperhidrosis, advanced age, immunosuppression, and residence in warm climates.10 Currently, the diagnosis of inverse psoriasis is clinical, but can be confirmed with histopathologic examination. Fungal cultures are often taken to rule out dermatophyte infection or cutaneous candidiasis; however, C. minutissimum is often undetected by culture results.11,12 Although not part of the current diagnostic workup, we propose that the addition of Wood’s lamp analysis should be considered, especially when risk factors for infection are present. The analysis is considered safe and is quick, painless, and cost-effective to conduct.9
Once identified, erythrasma can usually be treated with topical 2% clindamycin solution or 2% erythromycin solution applied up to three times daily or Whitfield’s ointment (consisting of 12% benzoic acid and 6% salicylic acid) applied up to twice daily.8,13,14 In extensive involvement, oral erythromycin 250mg four times daily for 14 days can be used. Some topical antifungal agents, such as ciclopirox and miconazole, have been shown to have antibacterial properties and might also be useful.11,15,16
Based on an extensive literature review, it is not known if C. minutissimum triggers inflammation leading to psoriasis, if it exacerbates current symptoms, or if it has no contributory role in the disease process. Previous studies have shown that cutaneous microflora, such as Malassezia, can trigger or exacerbate psoriasis through the release of arachidonic acid and its metabolites via secreted lipases. These metabolites then lead to local inflammatory responses.7,14 Other organisms that have been shown to promote inflammation in psoriatic lesions include Streptococcus pyogenes, Staphylococcus aureus, and Candida.17 Moreover, there are no studies available to indicate the proper treatment of patients with concomitant erythrasma and inverse psoriasis. Further studies are necessary to determine whether treatment of erythrasma in patients with both inverse psoriasis and erythrasma impacts the therapeutic response of inverse psoriasis.
Limitations. Limitations of this study include the potential presence of factors that could lead to false-positive or false-negative results in Wood’s lamp analysis. Specifically, the examination should not be conducted soon after bathing, as porphyrins are water-soluble and can be washed away, leading to a false-negative test result.15 In this study, we did not ask patients about this factor; thus, the prevalence of erythrasma might actually be higher due to possible false-negative results. Likewise, lint, soap residue, deodorant, and certain topical medications can emit a red-pink fluorescence, leading to false-positive test results.12 However, we think this possibility is unlikely, since the axillary sites were negative for fluorescence. In fact, the lack of erythrasma in the axillae might be due to common deodorant usage at this site. The detection of coral-red fluorescence is a subjective observation. An experienced dermatologist should be able to identify distinctions in the emitted color. This risk was minimized in this study by using an experienced dermatologist as the observer. Likewise, since it is clinically difficult to diagnose inverse psoriasis, it is possible that some patients might not have had this diagnosis. This was minimized by including diagnoses established by a dermatologist who specializes in psoriasis. In addition, all patients had an established diagnosis of inverse psoriasis on intertriginous surfaces.
Conclusion
Our results indicate a high prevalence of cutaneous C. minutissimum infection (erythrasma) in patients with inverse psoriasis. Clinicians should maintain a high level of suspicion for the presence of this organism in patients presenting with inverse psoriasis due to its potential to trigger or exacerbate psoriatic lesions. Wood’s lamp analysis should be considered in patients who present with symptoms of inverse psoriasis, especially when risk factors for C. minutissimum are present. Additional research with a larger cohort is needed to determine the true prevalence.
References
- Merola J, Qureshi A, Husni M. Underdiagnosed and undertreated psoriasis: Nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589.
- Wang G, Li C, Gao T, Liu Y. Clinical analysis of 48 cases of inverse psoriasis: a hospital-based study. Eur J Dermatol. 2005;15(3):176–178.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994.
- Vary JC, O’Connor KM. Common dermatologic conditions. Med Clin North Am. 2014;98(3):445–485.
- Khosravi H, Siegel MP, Van Voorhees AS, Merola JF. Treatment of inverse/intertriginous psoriasis: updated guidelines from the Medical Board of the National Psoriasis Foundation. J Drugs Dermatol. 2017;16(8):760–766.
- Brook I. Secondary bacterial infections complicating skin lesions. J Med Microbiol. 2002;51(10):808–812.
- Zomorodian K, Mirhendi H, Tarazooie B, et al. Distribution of Malassezia species in patients with psoriasis and healthy individuals in Tehran, Iran. J Cutan Pathol. 2008;35(11):1027–1031.
- Holdiness MR. Management of cutaneous erythrasma. Drugs. 2002;62(8):1131–1141.
- Asawanonda P, Taylor CR. Woods light in dermatology. Int J Dermatol. 1999;38(11):801–807.
- Holdiness MR. Erythrasma and common bacterial skin infections. Am Fam Physician. 2003;67(2):254.
- Chen JF, Liu YC, Wang W-M. Answer: can you identify this condition? Can Fam Physician. 2011;57(8): 903–904.
- Morales-Trujillo ML, Arenas R, Arroyo S. Interdigital erythrasma: clinical, epidemiologic, and microbiologic findings. Actas Dermosifiliogr. 2008;99(6):469–473.
- Gupta LK, Singhi MK. Wood’s lamp. Indian J Dermatol Venereol Leprol. 2004;70(2):131–135.
- Brunke S, Hube B. MfLIP1, a gene encoding an extracellular lipase of the lipid-dependent fungus Malassezia furfur. Microbiology. 2006;152(Pt 2):547–554.
- Van Cutsem JM, Thienpont D. Miconazole, a broad-spectrum antimycotic agent with antibacterial activity. Chemotherapy. 1972;17(6):392–404.
- Andrews MD, Burns M. Common tinea infections in children. Am Fam Physician. 2008;77(10): 1415–1420.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25(6):606–615.

